Application of Nuclear Techniques to Improve the Mass Production and Management of Fruit Fly Parasitoids
Abstract
:1. Introduction
2. Background
3. Physiological Basis
4. Optimizing Radiation Dose and Age of Irradiating Fruit Fly Hosts
| Family | Parasitoid species | Host species | Stage (instar) | Irradiation Dose (Gy) | Host irradiation | Irradiator / conditions | Reference |
|---|---|---|---|---|---|---|---|
| Braconidae | Fopius arisanus | Anastrephaludens | Egg 1 | 27.5 | 1 mL egg | Gammacell 220 Co 60 | [38] |
| 3 mL of water | 2.3–3.0 Gy/min free oxygen | ||||||
| Diachasmimorpha longicaudata | Anastrephaludens | Egg 1 | 27.5 | 1 mL egg | Gammacell 220 Co 60 | [38] | |
| 3 mL of water | 2.5–3.0 Gy/min free oxygen | ||||||
| Anastrephaludens | Larva (3rd) | 20 | 100 larvae | Gammacell 220 Co 60 | [19] | ||
| naked | 2.5–3.0 Gy/min free oxygen | ||||||
| Anastrepha obliqua | Larva (3rd) | 30 | 100 larvae | Gammacell 220 Co 60 | [19] | ||
| naked | 2.5–3.0 Gy/min free oxygen | ||||||
| Anastrephaserpentina | Larva (3rd) | 20 | 100 larvae | Gammacell 220 Co 60 | [19] | ||
| naked | 2.5–3.0 Gy/min free oxygen | ||||||
| Anastrephasuspensa | Larva (3rd) | 20 | 200 larvae | 137 Cs source | [18] | ||
| naked | 1732 roentgens/min | ||||||
| Ceratitis capitata | Larva (3rd) | 60–65 | larvae | Gammabean 650 Co 60 type IR31 | [66,67] | ||
| naked | 226.9–287.83 Gy/h | ||||||
| Doryctobraconcrawfordi | Anastrephaludens | Larva (3rd) | 20 | 100 larvae | Gammacell 220 Co 60 | [39] | |
| naked | 2.5–3.0 Gy/min free oxygen | ||||||
| Doryctobraconaerolatus | Anastrepha suspensa | Larva (2nd) | 70 | Larvae mixed | Gammacell 1,000 Cs137 | [84] | |
| in diet | 8.9 Gy/min | ||||||
| Opiushirtus | Anastrephaludens | Larva (3rd) | 20 | 100 larvae | Gammacell 220 Co 60 | [39] | |
| naked | 2.5–3.0 Gy/min free oxygen | ||||||
| Utetesanastrephae | Anastrephaludens | Larva (3rd) | 20 | 100 larvae | Gammacell 220 Co 60 | [39] | |
| naked | 2.5–3.0 Gy/min free oxygen | ||||||
| Diachasmimorphatryoni | Ceratitis capitata | Larva (3rd) | 40 | 100 larvae | Gammacell 220 Co 60 | [19] | |
| naked | 2.5–3.0 Gy/min free oxygen | ||||||
| Psyttaliaconcolor | Ceratitis capitata | Larva (3rd) | 60 | Larvae in water | Theratron Co 60 | [41] | |
| type C-146; 107.33 cGy/min | |||||||
| Psytaliahumillis | Ceratitis capitata | Larva (3rd) | 70 | 1 Lt naked larvaein a plastic bag | Gammacell 220 Co 60 3.0 Gy/min | [81] | |
| Diachasmimorphakraussii | Bactroceratryoni | Larva (2nd–3rd) | 15.9-26.8 | Larvae with dietin Petri dishes. | Gamma Technology Research Irradiator Co 60 | [83] | |
| Eulophidae | Aceratoneuromyia indica | Anastrepha ludens | Larva (3rd) | 45 | 100 larvae | JS-120 Co 60 | [85] |
| naked | 4.22 Gy/min | ||||||
| Diapriidae | Copterahaywardi | Anastrepha ludens | Pupa 2 | 20 | 100 pupae | Gammacell 220 Co 60 | [39] |
| naked | 2.5–3.0 Gy/min free oxygen | ||||||
| Eurytomidae | Eurytomasivinski | Anastrepha ludens | Pupa 2 | 20 | 100 pupae | Gammacell 220 Co 60 | [39] |
| naked | 2.5–3.0 Gy/min free oxygen | ||||||
| Chalcidoidea | Dirhinus spp. | Anastrepha ludens | Pupa 2 | 20 | 100 pupae | Gammacell 220 Co 60 | [39] |
| naked | 2.5–3.0 Gy/min free oxygen |

| Host Species | Stage (instar) | Irradiation Dose | Host Irradiation | Irradiator/conditions | Reference |
|---|---|---|---|---|---|
| Ceratitis capitata | Larva (3rd) | 6,250.2 R (60 Gy) | 150–200 larvae with and without larval diet | Philips MG 160 Constant Potential X-ray System-Minus H:T. Generator Type 160 kV/4 kW Free oxygen | [45] |
| Anastrepha fraterculus | Larva (3rd) | 10,417 R (100 Gy) | 2,200 larvae mixed in diet | Philips MG 160 Constant Potential X-ray System-Minus H:T. Generator Type 160 kV/4 kW Free oxygen | [46] |
| Parasitoid species | Host species | Stage (instar) | Irradiation Dose (Gy) | Irradiator/conditions | Weekly Pupae Production | Reference |
|---|---|---|---|---|---|---|
| D. longicaudata | A. ludens | Larvae (3rd) | 45 | JS-120 Co 60 | 50 millions | [19,91] |
| 4.22 Gy /min | ||||||
| D. longicaudata | A. suspensa | Larvae (3rd) | 40 | Gammacell 1,000 Cs 137 | ~150,000 | [12] |
| 12 Gy/min | ||||||
| D. krausi | C. capitata | Larvae (3rd) | 70 | Gammacell 220 Co 60 | ~1 million | [92] |
| 3 Gy/min | ||||||
| D. tryoni | C. capitata | Larvae (3rd) | 70 | Gammacell 220 Co 60 | ~1 million | [93] |
| 3 Gy/min | ||||||
| P. humillis | C. capitata | Larvae (3rd) | 70 | Gammacell 220 Co 60 | ~100,000 | [81] |
| 3 Gy/min | ||||||
| C. haywardi | A. ludens | Pupae | 40 | JS-120 Co 60 | 150,000 | [17,39] |
| 4.22 Gy/min |
5. Quality of Emerged Adult Parasitoids
6. Practical Applications
- (a)
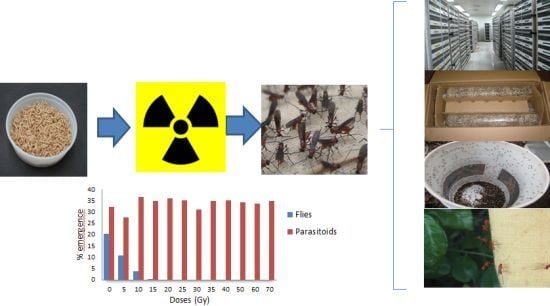
- Avoidance of host emergence: This is without doubt the most important consequence of host irradiation. Developmental suppression of non-parasitized hosts, which represent between 10%–50% of hosts under mass rearing conditions, is a key to increasing parasitoid production to the level of millions per week. Without irradiation it is practically impossible to maintain large-scale production without the risk of shipping and releasing pest flies.
- (b)
- (c)
- Pupae packing and shipment: Production laboratories are often located far from the targeted release areas. The transportation of exclusively parasitized pupae in plastic bags under hypoxic conditions improves security considerations and so facilitates transport, handling and makes post-transport quality evaluations simpler to perform [97].
- (d)
- Preparation for release: The sole emergence of adult parasitoids facilitates the design of methods to release millions of parasitoids in the field [12,13]. This is particularly true when devices such as those employed for aerial release need to be calibrated for a particular size of insect with unique environmental tolerances [93,97].
- (e)
- Parasitoid quality: Avoiding the separation of parasitoids and flies, allowing the packing and transport of only parasitoids in pupae rather than as adults, significantly increases their quality. Also, fewer dead host larvae and pupae during the production phase as a result of the exclusive development of parasitoids, improves sanitation and the quality of mass produced parasitoids while favoring a female-biased sex ratio [66,67,96].
- (f)
- Field evaluations: Parasitoid presence, behavior, survival and dispersal can be assessed by deploying devices baited with irradiated hosts (Figure 2) [13]. Irradiated hosts do not present an infestation risk in the field. The use of such devices is currently the only practical alternative to experiments conducted under laboratory or greenhouse conditions [98].
- (g)
- Export of parasitoids within quarantined pest pupae: The MOSCAFRUT production facility in Mexico has exported D. longicaudata parasitoids to different countries such as Argentina, Brazil, Colombia, Costa Rica and Peru [79,99]. This involved the transport of A. ludens as the host, a quarantined fly species in all the mentioned countries. The consignments were carried out using parasitoids in irradiated hosts, thus eliminating the risk of introducing an economically important species. In addition, the Campaña Nacional contra Moscas de la Fruta (National Campaign against Fruit Flies) in Mexico, transports millions of A. ludens pupae containing D. longicaudata weekly to various “low prevalence” agricultural production zones in northern Mexico. Since these are areas where eradication or suppression campaigns are ongoing, the inadvertent release of fertile flies would be disastrous [91]. However, due to host irradiation there have been no reports of fruit fly contamination in over 15 years. Another noteworthy case is the import and release of Psyttalia humilis (Silvestri) in California for the control of B. oleae. These parasitoids are produced using irradiated C. capitata [81] larvae transported from Guatemala. This species of fly is commercially important throughout the world and is a quarantine species in the United States. Without irradiation, the project in its present form would not be feasible.

7. Conclusions
Acknowledgments
References
- Parella, M.P.; Heinz, K.M.; Nunney, L. Biological control through augmentative releases of natural enemies: A strategy whose time has come. Am. Entomol. 1992, 38, 172–180. [Google Scholar]
- Van Lenteren, J.C.; Bueno, V.H.P. Augmentative biological control of arthropods in Latin America. Biocontrol 2003, 48, 123–139. [Google Scholar] [CrossRef]
- Knipling, E.F. Principles of insect parasitism analyzed from new perspectives: Practical implications for regulating insect populations by biological means. In Agriculture Handbook Number 512; United States Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 1992; p. 659. [Google Scholar]
- Wharton, R.A. Classical biological control of fruit-infesting Tephritidae. In Fruit Flies: Their Biology, Natural Enemies and Control; Robinson, A.S., Hooper, G., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 303–313. [Google Scholar]
- Leyva, J.; Browning, W.V.; Gilstrap, F.E. Effect of host species size and color on parasitization of Anastrepha ludens (Diptera: Tephritidae) by Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Environ. Entomol. 1991, 20, 1469–1476. [Google Scholar]
- Aluja, M.; Birke, A. Habitat use by adults of Anastrepha obliqua (Diptera: Tephritidae) in a mixed mango and tropical plum orchard. Ann. Entomol. Soc. Am. 1993, 86, 799–812. [Google Scholar]
- Wong, T.T.Y.; Ramadan, M.M.; McInnis, D.O.; Mochizuki, N.; Nishimoto, J.I.; Herr, J.C. Augmentative releases of Diachasmimorpha tryoni (Hymenoptera: Braconidae) to suppress a Mediterranean fruit fly (Diptera: Tephritidae) population in Kula, Maui, Hawaii. Biol. Control 1991, 1, 2–7. [Google Scholar] [CrossRef]
- Wong, T.T.Y.; Ramadan, M.M.; Herr, J.C.; McInnis, D.O. Suppression of a Mediterranean fruit fly (Diptera: Tephritidae) population with concurrent parasitoid and sterile fly releases in Kula, Maui, Hawaii. J. Econ. Entomol. 1992, 85, 1671–1681. [Google Scholar]
- Vargas, R.I.; Peck, S.L.; McQuate, G.J.; Jackson, C.G.; Stark, J.D.; Armstrong, J.W. Potential for area wide integrated management of Mediterranean fruit fly (Diptera: Tephritidae) with a Braconid parasitoid and novel bait-spray. J. Econ. Entomol. 2001, 94, 817–825. [Google Scholar] [CrossRef]
- Vargas, R.I.; Long, J.; Miller, N.W.; Delete, K.; Jackson, C.G.; Uchida, G.K.; Bautista, R.C.; Harris, E.J. Releases of Psyttalia fletcheri (Hymenoptera: Braconidae) and sterile flies to suppress melon fly (Diptera: Tephritidae) in Hawaii. J. Econ. Entomol. 2004, 97, 1531–1539. [Google Scholar] [CrossRef]
- Harris, E.J.; Bautista, R.C.; Vargas, R.I.; Jang, E.V.; Eitam, A.; Leblanc, L. Suppression of melon fly (Diptera: Tephritidae) populations with releases of Fopius arisanus and Psyttalia fletcheri (Hymenoptera: Braconidae) in North Shore Oahu, HI, USA. Biocontrol 2010, 53, 593–599. [Google Scholar]
- Sivinski, J.; Calkins, C.O.; Baranowski, R.M.; Harris, D.; Brambila, J.; Díaz, J.; Burns, R.E.; Holler, T.; Dodson, G. Suppression of Caribbean fruit fly Anastrepha suspensa (Loew) (Diptera: Tephritidae) population through augmented releases of the parasitoid Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 1996, 6, 177–185. [Google Scholar] [CrossRef]
- Montoya, P.; Liedo, P.; Benrey, B.; Cancino, J.; Barrera, J.F.; Sivinski, J.; Aluja, M. Biological control of Anastrepha spp. (Diptera: Tephritidae) in mango orchards through augmentative releases of Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Biol. Control 2000, 18, 216–224. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. The state of the commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. Biocontrol 2011, 1, 1–20. [Google Scholar]
- Wong, T.T.Y.; Ramadan, M.M. Mass rearing biology of larval parasitoids (Hymenoptera: Braconidae) of Tephritid fruit flies in Hawaii. In Advances in Insect Rearing for Research and Pest Management; Anderson, T., Leppla, N., Eds.; Westview Press: Boulder, CO, USA, 1992; pp. 405–476. [Google Scholar]
- Bautista, R.C.; Mochizuki, N.; Spencer, J.P.; Harris, E.J.; Ichimura, D.M. Mass rearing of the Tephritid fruit fly parasitoid Fopius arisanus (Hymenoptera: Braconidae). Biol. Control 1999, 15, 137–144. [Google Scholar] [CrossRef]
- Cancino, J.; Montoya, P. Advances and perspectives in the mass rearing of fruit fly parasitoids in Mexico. In Proceedings of the 7th International Symposium on Fruit Fly on Economic Importance, Salvador, Bahia, Brazil, 10–15 September 2006; pp. 133–142.
- Sivinski, J.; Smittle, B. Effect of gamma radiation on the development of the Caribbean fruit fly Anastrepha suspensa and the subsequent development of its parasite Diachasmimorpha longicaudata (Ashmead). Entomol. Exp. Appl. 1990, 55, 295–297. [Google Scholar] [CrossRef]
- Cancino, J.; Ruíz, L.; López, P.; Sivinski, J. The suitability of Anastrepha spp. and Ceratitis capitata larvae as hosts of Diachamimorpha longicaudata and Diachasmimorpha tryoni: Effects of host age and radiation dose and implications for quality control in mass rearing. Biocontrol Sci. Tech. 2009, 19, 81–94. [Google Scholar]
- Lawrence, P.O.; Baranowski, R.M.; Greany, P.D. Effect of host age on development of Biosteres (=Opius) longicaudatus, a parasitoid of the Caribbean fruit fly, Anastrepha suspens. Fla. Entomol. 1976, 59, 33–39. [Google Scholar] [CrossRef]
- Zenil, M.; Liedo, P.; Williams, T.; Valle, J.; Cancino, J.; Montoya, P. Reproductive biology of Fopius arisanus (Hymenoptera: Braconidae) on Ceratitis capitata and Anastrepha spp. (Diptera: Tephritidae). Biol. Control 2004, 29, 169–178. [Google Scholar] [CrossRef]
- Hamed, M.; Nadeem, S.; Riaz, A. Use of gamma radiation for improving the mass production of Trichogramma chilonis and Chrysoperla carnea. Biocontrol Sci. Tech. 2009, 19, 43–48. [Google Scholar] [CrossRef]
- Wang, E.; Lu, D.; Liu, X.; Li, Y. Evaluating the use of nuclear techniques for colonization and production of Trichogramma chilonis in combination with releasing irradiated moths for control of cotton bollworm, Helicoverpa armigera. Biocontrol Sci. Tech. 2009, 19, 235–242. [Google Scholar] [CrossRef]
- Fatima, B.; Ahmad, N.; Memon, R.M.; Bux, M.; Ahmad, Q. Enhancing biological control of sugarcane shoot borer, Chilo infuscatellus (Lepidoptera: Pyralidae) through use of radiation to improve laboratory rearing and field augmentation of egg and larval parasitoids. Biocontrol Sci. Tech. 2009, 19, 277–290. [Google Scholar]
- Hendrichs, J.; Bloem, K.; Hoch, G.; Carpenter, J.E.; Greany, P.; Robinson, A.S. Improving the cost-effectiveness, trade and safety of biological control for agricultural insect pests using nuclear techniques. Biocontrol Sci. Tech. 2009, 19, 3–22. [Google Scholar] [CrossRef]
- Mawela, K.V.; Kfir, R.; Krüger, K. Host Suitability of UV-irradiated eggs of three Lepidoptera species for rearing Trichogramma lutea Girault (Hymenoptera: Trichogrammatidae). J. Appl. Entomol. 2010, 134, 737–744. [Google Scholar] [CrossRef]
- Tuncbilek, A.S.; Canpolat, U.; Sumer, F. Suitability of irradiated and cold-stored eggs of Ephestia kuehniella (Pyralidae: Lepidoptera) and Sitotroga cerealella (Gelechidae: Lepidoptera) for stockpiling the egg-parasitoid Trichogramma evanescens (Trichogrammatidae: Hymenoptera) in diapause. Biocontrol Sci. Tech. 2009, 19, 127–138. [Google Scholar]
- Zapater, M.C.; Andiarena, C.E.; Camargo, G.P.; Bartolini, N. Use of irradiated Musca domestica pupae to optimize mass rearing and commercial shipment of the parasitoid Spalangia endius (Hymenoptera: Pteromalidae). Biocontrol Sci. Tech. 2009, 19, 261–270. [Google Scholar] [CrossRef]
- Paranhos, B.J.; Walder, J.M.M.; Papadopoulos, N.T. A simple method to study parasitism and field biology of the parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) on Ceratitis capitata (Diptera: Tephritidae). Biocontrol Sci. Tech. 2003, 13, 631–639. [Google Scholar] [CrossRef]
- Papathanos, P.A.; Bossin, H.C.; Benedict, M.Q.; Catteruccia, F.; Malcolm, C.A.; Alphey, L.; Crisanti, A. Sex separation strategies: past experience and new approaches. Malar. J. 2009, 8, S5. [Google Scholar]
- Jackson, C.G.; Chan, H.T.; Tanuchi, M.H.; Churchill, D.B.; Bilsland, D.M. Pneumatic air separation for the sorting of parasitized and unparasitized fruit fly (Diptera: Tephritidae) puparia. J. Econ. Entomol. 1996, 89, 353–358. [Google Scholar]
- Purcell, M.F.; Schroeder, W.J. Effect of silwet L.77 and diazinon on three fruit flies and associated endoparasitoids. J. Econ. Entomol. 1996, 89, 1566–1570. [Google Scholar]
- Morgan, P.B.; Smittle, B.J.; Patterson, R.S. Use of irradiated pupae to mass culture the microhymenopterous pupal parasitoid Spalangia endius Walker (Hymenoptera: Pteromalidae) I. Musca domestica L. (Diptera: Muscidae). J. Entomol. Sci. 1986, 21, 222–227. [Google Scholar]
- Roth, J.P.; Fincher, G.T.; Summerlin, J.W. Suitability of irradiated or freeze-killed horn fly (Diptera: Muscidae) pupae as hosts for hymenopteran parasitoids. J. Econ. Entomol. 1991, 84, 94–98. [Google Scholar]
- Ramadan, M.M.; Wong, T.T.Y. Effect of gamma radiation on Biosteres longicaudatus (Ashmead) (Hymenoptera: Braconidae), a larval parasitoids of Dacus dorsalis Hendel (Diptera. Tephritidae). Proc. Hawaii Entomol. Soc. 1989, 29, 111–113. [Google Scholar]
- Cancino, J.; Ruíz, L.; Gómez, Y.; Toledo, J. Irradiación de larvas de Anastrepha ludens (Loew) (Diptera: Tephritidae) para inhibir la emergencia de moscas en la cría del parasitoide Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae). Folia Entomol. Mex 2002, 41, 195–208. [Google Scholar]
- Hallman, J.G.; Loaharanu, P. Generic ionizing radiation quarantine treatments against fruit flies (Diptera: Tephritidae) proposed. J. Econ. Entomol. 2002, 95, 893–901. [Google Scholar] [CrossRef]
- Cancino, J.; Ruíz, L.; Pérez, J.; Harris, E. Irradiation of Anastrepha ludens (Diptera: Tephritidae) eggs for the rearing of the fruit fly parasitoids, Fopius arisanus and Diachasmimorpha longicaudata (Hymenoptera: Braconidae). Biocontrol Sci. Tech. 2009, 19, 167–177. [Google Scholar] [CrossRef]
- Cancino, J.; Ruíz, L.; Sivinski, J.; Gálvez, F.O.; Aluja, M. Rearing of five hymenopterous larval-prepupal (Braconidae, Figitidae) and three pupal (Diapriidae, Chacidoidea, Eurytomidae) native parasitoids of the genus Anastrepha (Diptera: Tephritidae) on irradiated A. ludens larvae and pupae. Biocontrol Sci. Tech. 2009, 19, 193–209. [Google Scholar] [CrossRef]
- Cancino, J.; Ruíz, L.; Hendrichs, J.; Bloem, K. Evaluation of sequential exposure of irradiated hosts to maximize the mass rearing of fruit fly parasitoids. Biocontrol Sci. Tech. 2009, 19, 95–109. [Google Scholar] [CrossRef]
- Hepdurgun, B.; Turanli, T.; Zümreoglu, A. Parasitism rate and sex ratio of Psyttalia (=Opius) concolor (Hymenoptera: Braconidae) reared on irradiated Ceratitis capitata larvae (Diptera: Tephritidae). Biocontrol Sci. Tech. 2009, 19, 157–165. [Google Scholar] [CrossRef]
- Mastrangelo, T.; Parker, A.G.; Jessup, A.; Pereira, R.; Orozco-Dávila, D.; Islam, A.; Dammalage, T.; Walder, J.M.M. A new generation of X-ray irradiators for insect sterilization. J. Econ. Entomol. 2010, 103, 85–94. [Google Scholar] [CrossRef]
- Mehta, K.; Parker, A. Characterization and dosimetry of a practical X-ray alternative to self-shielded gamma irradiators. Radiat. Phys. Chem. 2011, 80, 107–113. [Google Scholar] [CrossRef]
- Mehta, K. Radiation sources supporting the use of natural enemies for biological control of agricultural pests. Biocontrol Sci. Tech. 2009, 19, 335–362. [Google Scholar] [CrossRef]
- Viscarret, M.M.; Conte, C.A.; Carabajal, L.Z.; López, S.N.; Segura, D.F.; Muntaabski, I.; Lanzavecchia, S.B.; Cladera, J.L. Rearing of the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae) on X-ray irradiated larvae of Ceratitis capitata (Diptera: Tephritidae). Biocontrol Sci. Tech. 2012, in press. [Google Scholar]
- Bachmann, G.; Carabajal, L.Z.; Conte, C.A.; Devoscovi, F.; Milla, F.H.; Cladera, J.L.; Segura, D.F.; Viscarret, M.M. Radiation doses to safely release the parasitoid Diachasmimorpha longicaudata reared on Anastrepha fraterculus larvae. In Proceedings of the 8th International Symposium on Fruit Fly on Economic Importance, Valencia, Spain, 26 September–1 October 2010; p. 377.
- Follet, P.A.; Armstrong, J.W. Revised irradiation doses to control melon fly, Mediterranean fruit fly and oriental fruit fly (Diptera: Tephritidae) and generic doses for tephritid fruit flies. J. Econ. Entomol. 2004, 97, 1254–1262. [Google Scholar] [CrossRef]
- Mastrangelo, T.; Walder, J.M.M. Use of isotopes in insects. In Radioisotopes-Applications in Bio-Medical Science; Singh, N., Ed.; In Tech-Open Access Company: Manhattan, NY, USA, 2011; pp. 67–92. [Google Scholar]
- Duccoff, H.S. Causes of death in irradiated adult insects. Biol. Rev. 1972, 47, 211–231. [Google Scholar] [CrossRef]
- Tillinger, N.A.; Hoch, G.; Schopf, A. Effects of parasitoids associated factors of the endoparasitoid Glyptapanteles liparidis (Hymenoptera: Braconidae). Eur. J. Entomol. 2004, 101, 243–249. [Google Scholar]
- Faruki, S.I.; Dar, D.R.; Khan, A.R.; Khafun, M. Effects of ultraviolet (254 nm) irradiation on egg hatching and adult emergence of the flour beetles, Tribolium castaneum, T. confusum and the almond mouth, Cadra cautella. J. Insect Sci. 2007, 7, 1–7. [Google Scholar]
- Balock, J.W.; Burditt, A.K.; Christenson, L.D. Effects of gamma radiation on various stages of three fruit fly species. J. Econ. Entomol. 1963, 56, 42–46. [Google Scholar]
- Varanda, E.A.; Takahashi, C.S.; Soares, A.E.E. Effect of gamma radiation on eggs, larvae and pupae of Melittobia hawaiiensis detection of a body color mutation. Rev. Bras. Genet. 1985, 3, 439–448. [Google Scholar]
- Torres-Rivera, Z.; Hallman, G.J. Low-doses irradiation treatment against Mediterranean fruit fly (Diptera: Tephritidae). Fla. Entomol. 2007, 90, 343–346. [Google Scholar] [CrossRef]
- Thompson, S.N.; Hagen, R.S. Nutrition of entomophagous insects and other arthropods. In Handbook of Biological Control; Fisher, T.W., Bellows, T., Caltagirone, L., Dahlsten, D., Huffaker, C., Gordh, G., Eds.; Academic Press: Riverside, CA, USA, 1999; pp. 544–630. [Google Scholar]
- Van Driesche, R.G.; Murray, T.J. Parameters used in laboratory host range tests. In Assesing Host Ranges of Parasitoid and Predators; van Driesche, R.G., Reardon, R., Eds.; USDA-FHTET: Morgantown, WV, USA, 2004; pp. 56–67. [Google Scholar]
- Walder, J.J.M.; Lopez, L.A.; Costa, M.L.Z.; Sesso, N.J.; Tonin, G.; Carvalho, M.L.; Lara, P.P. Criacao e liberacao do parasitoide Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) para controle de moscas-das-frutas no estado de Sao Paulo. Laranja 1995, 16, 149–153. [Google Scholar]
- Vinson, S.B.; Iwantsch, G.F. Host suitability for insect parasitoids. Annu. Rev. Entomol. 1980, 25, 397–419. [Google Scholar] [CrossRef]
- Beckage, N.E. Modulation of immune response to parasitoids by polydnaviruses. Parasitology 1998, 116, 57–64. [Google Scholar] [CrossRef]
- Bokono-Ganta, A.; Ramadan, M.M.; Wang, X.; Messing, R. Biological performance and potential Fopius ceratitivorous (Hymenoptera: Braconidae), an egg-larval parasitoid of tephritid fruit flies newly imported to Hawaii. Biol. Control 2005, 33, 238–247. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Ekesi, S.; Hanna, R. Evaluation of the impact of Diachasmimorpha longicaudata to Bactrocera invadens and five African fruit fly species. J. Appl. Entomol. 2008, 132, 789–797. [Google Scholar] [CrossRef]
- Jervis, M.A.; Copland, M.J.W. The life cycle. In Insect Natural Enemies; Jervis, M.A., Kidd, N., Eds.; Chapman and Hall: London, UK, 1996; pp. 63–160. [Google Scholar]
- Carabajal, L.Z.; Papeschi, A.G.; Cladera, J.L. Immature stages of development in the parasitoid wasp, Diachasmimorpha longicaudata. J. Insect Sci. 2010, 10, 1–13. [Google Scholar]
- Brodeur, N.E.; Guy, B. Functional ecology of immature parasitoids. Ann. Rev. Entomol. 2004, 49, 27–49. [Google Scholar] [CrossRef]
- Scaglia, M.; Chaud-Netto, J.; Brochetto-Braga, M.R.; Ceregato, S.A.; Gobbi, N.; Rodríguez, A. Oviposition sequence and offspring of mated and virgin females of Cotesia flavipens (Hymenoptera: Braconidae) parasitizing Diatrea saccharalis larvae (Lepidoptera: Crambidae). J. Venom. Anim. Toxins Incl. Trop. Dis. 2005, 11, 283–298. [Google Scholar]
- Gil, R. Biologia e comportamento de Diachasmimorpha longicaudata (Ashmead) (Hymenoptera: Braconidae) criado sobre larvas de Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) irradiadas e não irradiadas con radiação gamma. M.Sc. Thesis, Universidade Estatal Paulista “Julius Mesquita Filho”, Sao Paulo, Brazil, 2003. [Google Scholar]
- Valle, G. Aspectos biológicos e morfológicos de Diachasmimorpha longicaudata (Ashmead, 1905) (Hymenoptera: Braconidae) criado em larvas irradiadas de Ceratitis capitata (Wiedemann, 1824) (Diptera: Tephritidae). Ph.D. Thesis, Centro de Energia Nuclear na Agricultura da Universidade de Sao Paulo, Sao Paulo, Brazil, 2006. [Google Scholar]
- Van Alphen, J.J.M.; Thunnissen, I. Host selection and sex allocation by Pachycrepoideus vindemmiae Rondani (Pteromalidae) as a facultative hyperparasitoid of Asobaratabida Nees (Braconidae: Alysiinae) and Leptopilina heterotema (Cynipoidea: Eucolidae). Neth. J. Zool. 1983, 33, 497–574. [Google Scholar]
- Menezes, E.; Sivinski, J.; Holler, T.; Aluja, M.; Jerónimo, F.; Ramírez, E. Development of Coptera haywardi (Hymenoptera: Diapriidae) in irradiated and unirradiated pupae of the Caribbean fruit fly and the Mediterranean fruit fly (Diptera: Tephritidae). Fla. Entomol. 1998, 81, 567–570. [Google Scholar] [CrossRef]
- Nussbaumer, C.; Schopf, A. Development of the solitary larval endoparasitoid Glyptapanteles parthetriae (Hymenoptera: Braconidae) in its host Lymantria dispar (Lepidotera: Lymantriidae). Eur. J. Entomol. 2000, 97, 355–361. [Google Scholar]
- Harvey, J.A.; Strand, M.R. The Development strategies of endoparasitoid wasps vary with host feeding ecology. Ecology 2002, 83, 2439–2451. [Google Scholar] [CrossRef]
- Ovruski, S.M. Immature stages of Aganaspis pelleranoi (Brethes) (Hymenoptera: Cynipoidea: Eucolidae), a parasitoid of Ceratitis capitata (Wied.) and Anastrepha spp. (Diptera: Tephritidae). J. Hym. Res. 1994, 3, 233–239. [Google Scholar]
- Nation, J.L.; Smittle, B.J.; Milne, K. Rdiation-induced changes in melanization and phenoloxidase in Caribbean fruit fly larvae (Diptera: Tephritidae) as the basis for a simple test of irradiation. Ann. Entomol. Soc. Am. 1995, 88, 201–205. [Google Scholar]
- Puanmanee, K.; Wongpiyasatid, A.; Sutantewong, M.; Hormchan, P. Gamma irradiation effect of guava fruit fly Bactrocera correcta (Bezzi) (Diptera: Tephritidae). Kasetsart J. (Nat. Sci.) 2010, 44, 830–836. [Google Scholar]
- Bautista, R.C.; Harris, E.J.; Vargas, R.I. The fruit fly parasitoid Fopius arisanus: Reproductive attributes of pre-released females and the use of added sugar as a potential food suplement in the fruit in the field. Entomol. Exp. Appl. 2001, 101, 247–255. [Google Scholar]
- Vargas, R.I.; Leblanc, L.; Putoa, R.; Eitam, A. Impact of introduction of Bactrocera dorsalis (Ditpera: Tephritidae) and classical biological control releases of Fopius arisanus (Hymenoptera: Braconidae) on economically important fruit flies in French Polynesia. J. Econ. Entomol. 2007, 100, 670–679. [Google Scholar] [CrossRef]
- Wharton, R.A.; Gilstrap, F.E. Key and status of opine braconid (Hymenoptera) parasitoid used in biological control of Ceratitis and Dacus s.l. (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 1983, 76, 721–742. [Google Scholar]
- Ovruski, S.M.; Aluja, M.; Sivinski, J.; Wharton, R. Hymenopteran parasitoids on fruit-infesting Tephritidae (Diptera) in Latin America and the Southern United States: Diversity, distribution, taxonomic status and their use in fruit fly biological control. Integr. Pest Manage. Rev. 2000, 5, 81–107. [Google Scholar] [CrossRef]
- Ovruski, S.M.; Colin, C.; Soria, A.; Oroño, L.E.; Schliserman, P. Introducción y producción en laboratorio de Diachasmimorpha tryoni y Diachasmimorpha longicaudata (Hymenoptera: Braconidae) para el control de Ceratitis capitata (Diptera: Tephritidae) en la Argentina. Rev. Soc. Entomol. Argent. 2003, 62, 49–59. [Google Scholar]
- Kimani-Njogu, S.W.; Trostte, M.K.; Wharton, R.; Woolley, J.B.; Raspi, A. Biosystematics of the Psyttalia concolor species complex (Hymenoptera: Braconidae: Opinae): The indentity of populations attacking Ceratitis capitata (Diptera: Tephritidae) in coffee in Kenya. Biol. Control 2001, 20, 167–174. [Google Scholar] [CrossRef]
- Yokoyama, V.; Cáceres, C.E.; Kuenen, L.P.S.; Wang, X.; Rendón, P.A.; Johnson, M.W.; Daane, K.M. Field performance and fitness on an olive fruit fly parasitoid Psyttalia humilis (Hymenoptera: Braconidae), mass reared on irradiated Medfly. Biol. Control 2010, 54, 90–99. [Google Scholar] [CrossRef]
- Hepdurgun, B.; Turanli, T.; Zümreoglu, A. Control of the olive fruit fly, Bactrocera oleae (Diptera: Tephritidae) through mass trapping and mass releases of the parasitoid Psittalia concolor (Hymenoptera: Braconidae) reared on irradiated Mediterranean fruit fly. Biocontrol Sci. Tech. 2009, 19, 211–224. [Google Scholar] [CrossRef]
- Harris, A.R.; Pratt, C.F.; Jessup, A.J.; Banos, C.; Lindhout, K.; Gurr, G.M.; Reynolds, O.L. Rearing the biological control agent Diachasmimorpha kraussii (Fullaway) (Hymenoptera: Braconidae) on irradiated larvae of the Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). In Proceedings of the 8th International Symposium on Fruit Fly on Economic Importance, Valencia, Spain, 26 September–1 October 2010; pp. 229–251.
- Palenchar, J.; Holler, T.; Moses-Rowley, A.; McGoven, R.; Sivisnki, J. Evaluation of irradiated Caribbean fruit fly (Diptera: Tephritidae) larvae for laboratory rearing of Doryctobracon aerolatus (Hymenoptera: Braconidae). Fla. Entomol. 2009, 92, 535–537. [Google Scholar] [CrossRef]
- Cancino, J. Departamento de Control Biológico, Programa Moscafrut, Metapa de Dominguez, Chiapas, Mexico. Personal comunication, 2012. [Google Scholar]
- Ovruski, S.M.; Schliserman, P.; De Coll, O.R.; Peñaloza, C.; Oroño, L.E.; Colín, C. The establishment of Aceratoneuromyia indica (Hymenoptera: Eulophidae) in three biogeographical regions of Argentina. Fla. Entomol. 2006, 89, 270–273. [Google Scholar] [CrossRef]
- Boucek, Z.; Narendran, T. Indian chalcid wasps (Hymenoptera) of the genus Dirhinus parasitic on synanthropic and other diptera. Syst. Entomol. 1981, 6, 229–251. [Google Scholar] [CrossRef]
- Mena-Correa, J.; Sivinski, J.; Gates, M.W.; Ramírez-Romero, R.; Aluja, M. Biology of Eurytoma sivinskii, an unusual Eurytomid (Hymenoptera) parasitoid of fruit fly (Diptera: Tephritidae) pupae. Fla. Entomol. 2008, 91, 598–603. [Google Scholar]
- Sivinski, J.; Vulinec, K.; Menezes, E.; Aluja, M. The bionomics of Coptera haywardi (Ogloblin) (Hymenoptera: Diapriidae) and other pupal parasitoids of Tephritid fruit flies (Diptera). Biol. Control 1998, 11, 193–202. [Google Scholar] [CrossRef]
- Linsley, G. Protection of natural ecosystems: Impact of radiation from waste disposal practices. IAEA Bull. 1989, 4, 28–31. [Google Scholar]
- Montoya, P.; Cancino, J.; Zenil, M.; Santiago, G.; Gutierrez, J.M. The augmentative biological control component of the Mexican national campaign against Anastrepha spp. fruit flies. In Area-Wide Control of Insect Pests, from Research to Field Implementation; Vreysen, M.J.B., Robinson, A.S., Hendrichs, J., Eds.; Springer: Dordrecht, The Netherlands, 2007; p. 789. [Google Scholar]
- Rendón, P.; Sivinski, J.; Holler, T.; Bloem, K.; Lopez, M.; Martinez, A.; Aluja, M. The effects of sterile males and two braconid parasitoids, Fopius arisanus (Sonan) and Diachasmimorpha krausi (Fullaway) (Hymenoptera), on caged populations of Mediterranean fruit flies, Ceratitis capitata (Wied.) (Diptera: Tephritidae) at various sites in Guatemala. Biol. Control 2006, 36, 224–231. [Google Scholar] [CrossRef]
- Baeza, G.; Sivinski, J.; Holler, T.; Aluja, M. The effects of chilling on the fecundity and life span of mass reared parasitoids (Hymenoptera: Braconidae) of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Biocontrol Sci. Tech. 2002, 12, 205–215. [Google Scholar] [CrossRef]
- Messing, R.H.; Klungness, L.M.; Purcell, M.; Wong, T.T.Y. Quality control parameters used in augmentative biological control of tephritid fruit flies in Hawaii. Biol. Control 1993, 3, 140–147. [Google Scholar] [CrossRef]
- Purcell, M.F.; Daniels, K.M.; Whitehand, L.C.; Messing, R.H. Improvement of quality control methods for augmentative releases of the fruit fly parasitoids, Diachasmimorpha longicaudata and Psyttalia fletcheri (Hymenoptera: Braconidae). Biocontrol Sci. Tech. 1994, 4, 155–166. [Google Scholar] [CrossRef]
- Cancino, J.; Cancino, J.L.; Martínez, M.; Liedo, P. Quality control parameters of wild and mass reared Diachasmimorpha longicaudata (Ashmead), a fruit fly parasitoid. In Quality Control for Mass-reared Arthropods. In Proceedings of the Eighth and Ninth Workshop of the Working Group on Quality Control of Mass-Reared Arthropods, Gainesville, FL, USA, 2002; pp. 84–94.
- Sivinski, J.; Jerónimo, F.; Holler, T. Development of aerial releases of Diachasmimorpha tryoni (Cameron) (Hymenoptera: Braconidae), a parasitoid that attacks the Mediterranean fruit fly, Ceratitis capitata (Weidemann) (Diptera: Tephritidae), in the Guatemalan highlands. Biocontrol Sci. Tech. 2000, 10, 15–25. [Google Scholar] [CrossRef]
- Paranhos, B.J.; Costa, M.L.Z.; Ovruski, S.M.; Alves, R.M.; Blummer, L.; Walder, J.M.M. Offspring in response to parental female densities in the fruit fly parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae: Opiine). Fla. Entomol. 2008, 91, 628–635. [Google Scholar]
- Narváez, A.; Cancino, J.; Canal, N.D.; Wyckhuys, K.A.G. Effect of different dietary resources on longevity, carbohydrate metabolism, and ovarian dynamics in two fruit fly parasitoids. Arthropod Plant Interact 2012. [Google Scholar]
- Steinberg, S.; Cayol, J.P. Synergism between biological control and sterile insect technique: Can commercial mass production of biocontrol agents and sterile insects be integrated within the same industrial entity? Biocontrol Sci. Tech. 2009, 19, 272–138. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cancino, J.; Ruíz, L.; Viscarret, M.; Sivinski, J.; Hendrichs, J. Application of Nuclear Techniques to Improve the Mass Production and Management of Fruit Fly Parasitoids. Insects 2012, 3, 1105-1125. https://doi.org/10.3390/insects3041105
Cancino J, Ruíz L, Viscarret M, Sivinski J, Hendrichs J. Application of Nuclear Techniques to Improve the Mass Production and Management of Fruit Fly Parasitoids. Insects. 2012; 3(4):1105-1125. https://doi.org/10.3390/insects3041105
Chicago/Turabian StyleCancino, Jorge, Lía Ruíz, Mariana Viscarret, John Sivinski, and Jorge Hendrichs. 2012. "Application of Nuclear Techniques to Improve the Mass Production and Management of Fruit Fly Parasitoids" Insects 3, no. 4: 1105-1125. https://doi.org/10.3390/insects3041105