Hygienic Behavior of Africanized Honey Bees Apis mellifera Directed towards Brood in Old and New Combs during Diurnal and Nocturnal Periods
Abstract
:1. Introduction
2. Experimental Section
2.1. Selection of Colonies to Supply Bees
2.2. Observation Hives
2.3. Marking Bees
2.4. Filming Behaviors
2.5. Provision of New and Old Combs to Study Hygienic Behavior
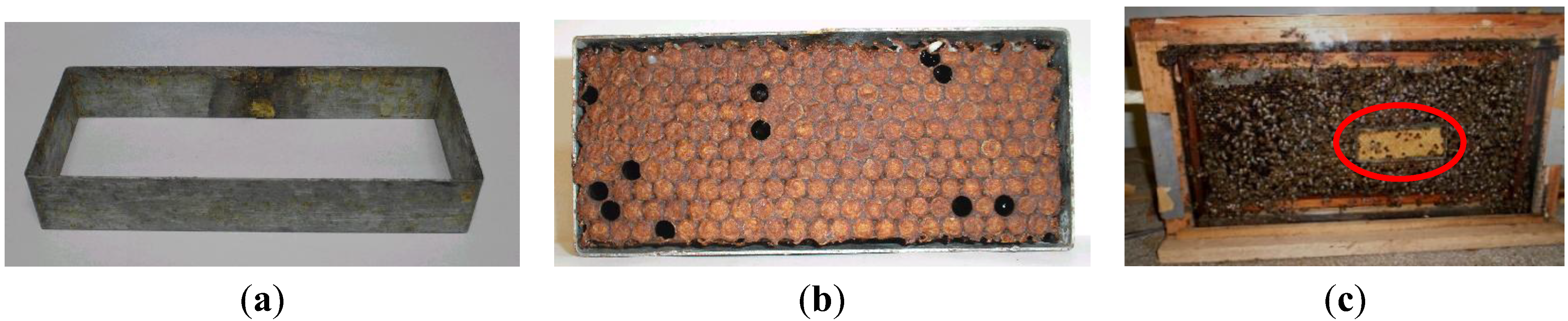
2.6. Hygienic Behavior at Different Times of Day
2.7. Statistical Analysis
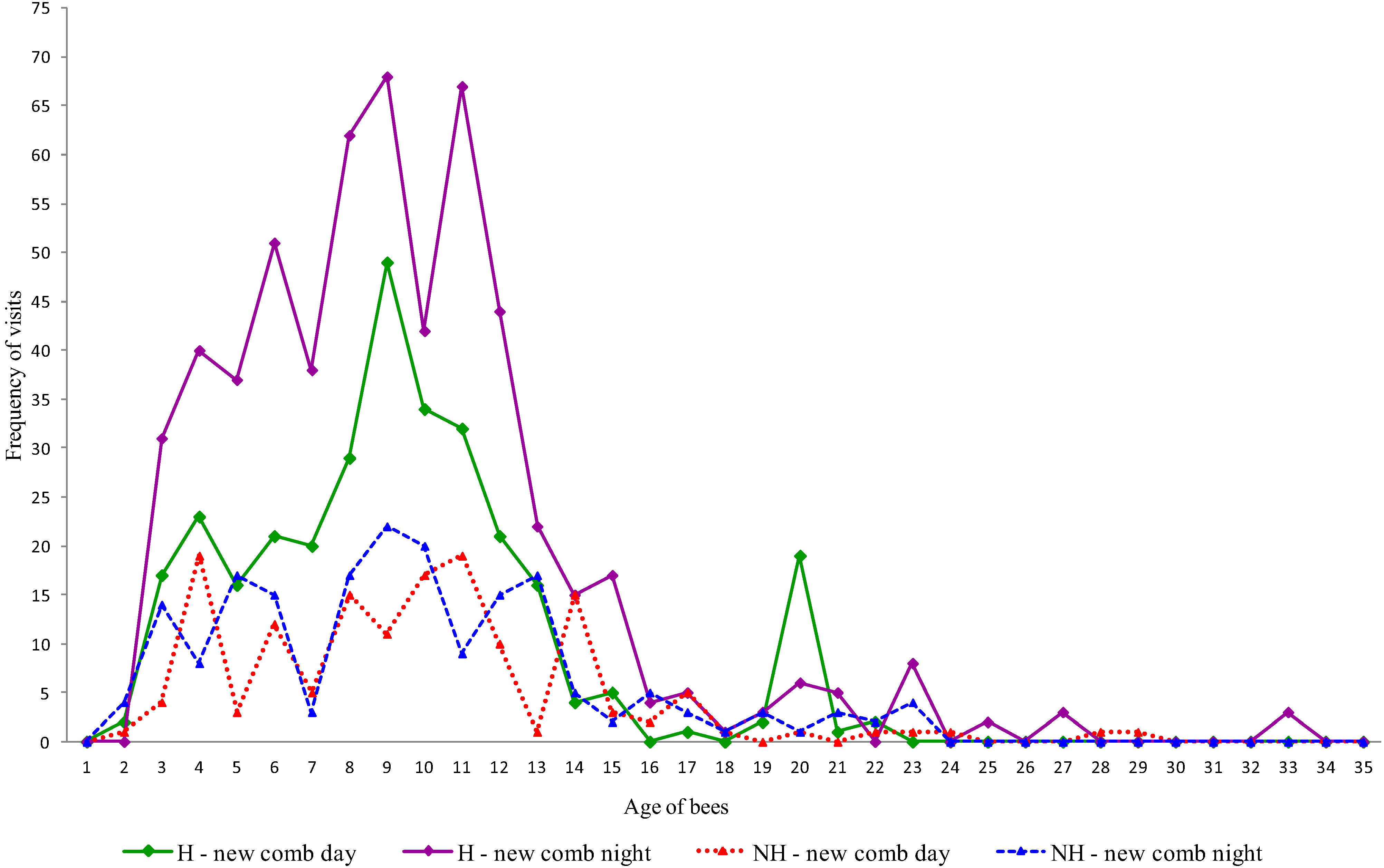
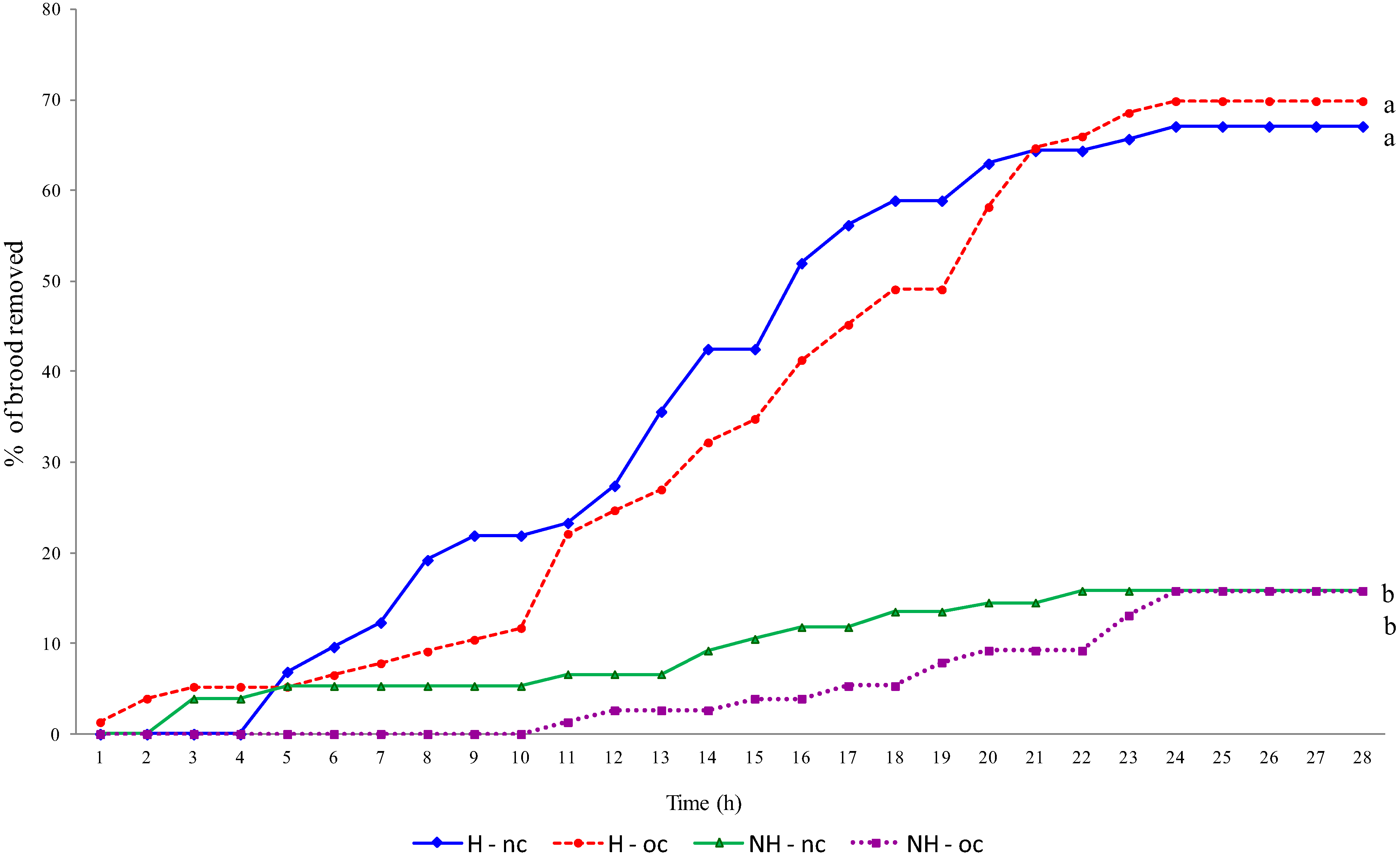
3. Results and Discussion

| UNCAPPING | ||||
|---|---|---|---|---|
| Strain/type of comb/period | N | Median | 25% | 75% |
| H/new/day | 177 | 19.0 a | 11.0 | 29.0 |
| H/new/night | 327 | 16.0 b | 11.0 | 30.7 |
| H/old/day | 333 | 38.0 c | 14.0 | 61.5 |
| H/old/night | 760 | 22.0 c | 10.0 | 57.0 |
| NH/new/day | 151 | 17.0 ª | 11.0 | 35.5 |
| NH/new/night | 151 | 20.0 ª | 12.0 | 32.0 |
| NH/old/day | 258 | 29.0 c | 14.0 | 48.0 |
| NH/old/night | 501 | 25.0 c | 14.0 | 43.0 |
| REMOVAL | ||||
|---|---|---|---|---|
| Strain/type of comb/period | N | Median | 25% | 75% |
| H/new/day | 339 | 390 ª | 229 | 657 |
| H/new/night | 863 | 388 ª | 210 | 644 |
| H/old/day | 395 | 311 b | 185 | 464 |
| H/old/night | 655 | 316 b | 179 | 533 |
| NH/new/day | 153 | 477 c | 253 | 808 |
| NH/new/night | 201 | 556 c | 269 | 811 |
| NH/old/day | 123 | 301 b | 179 | 448 |
| NH/old/night | 183 | 337 b | 190 | 475 |
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Gordon, D.M. The organization of work in social insect colonies. Nature 1996, 380, 121–124. [Google Scholar] [CrossRef]
- Johnson, B.R. Division of labor in honey bees: Form, function, and proximate mechanisms. Behav. Ecol. Sociobiol. 2010, 64, 305–316. [Google Scholar] [CrossRef]
- Winston, M.L. Atividades da operária, em função da sua idade. In A Biologia da Abelha; Tradução de Carlos A: Porto Alegre, Brazil, 2003; pp. 98–120. [Google Scholar]
- Visscher, P.K. The honey bee way of death: Necrophoric behavior in Apis mellifera colonies. Anim. Behav. 1983, 31, 1070–1076. [Google Scholar] [CrossRef]
- Kolmes, S. Grooming specialists among worker honey bees Apis mellifera. Anim. Behav. 1989, 37, 1048–1049. [Google Scholar] [CrossRef]
- Moore, A.J.; Breed, M.D.; Moore, M.J. The guard honey bee: Ontogeny and behavioral variability of workers performing a specialized task. Anim. Behav. 1987, 35, 1159–1167. [Google Scholar] [CrossRef]
- Robinson, G.E.; Page, R.E., Jr. Genetic determination of nectar foraging, pollen foraging and nest-site scouting in honey bee colonies. Behav. Ecol. Sociobiol. 1989, 24, 317–323. [Google Scholar] [CrossRef]
- Rothenbuhler, W.C. Behavior genetics of nest cleaning in honey bees. IV. Responses to F1 and backcross generations to disease killed brood. Am. Zool. 1964, 4, 111–123. [Google Scholar]
- Rothenbuhler, W.C. Behavior genetics of nest cleaning in honey bees. I. Responses of four inbred lines to disease killed brood. Anim. Behav. 1964, 12, 578–583. [Google Scholar] [CrossRef]
- Gonçalves, L.S.; Kerr, W.E. Genética, seleção e melhoramento. Noções sobre genética e melhoramento em abelhas. In Anais do I Congresso Brasileiro de Apicultura; Anais: Florianopolis, Brazil, 1970; pp. 8–36. [Google Scholar]
- Büchler, R. Test de tolerancia de las colmenas frente a varroasis. Vida Apícola 1997, 82, 45–48. [Google Scholar]
- Spivak, M.; Downey, D.L. Field assays for hygienic behavior in honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 1998, 91, 64–70. [Google Scholar]
- Gilliam, M.; Taber, S., III; Richardson, G.V. Hygienic behaviour of honey bees in relation to chalk brood disease. Apidologie 1983, 14, 29–39. [Google Scholar] [CrossRef]
- Rath, W.; Drescher, W. Response of Apis cerana Fabr. towards brood infested with Varroa jacobsoni Oud and infestation rate of colonies in Thailand. Apidologie 1990, 21, 311–321. [Google Scholar] [CrossRef]
- Palacio, M.A.; Figini, E.E.; Ruffinengo, S.R.; Rodriguez, E.M.; del Hoyo, M.L.; Bedascarrasbure, E.L. Changes in a population of Apis mellifera L. selected for hygienic behaviour and its relation to brood disease tolerance. Apidologie 2000, 31, 471–478. [Google Scholar] [CrossRef]
- Spivak, M.; Reuter, G.S. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef]
- Wilson-Rich, N.; Spivak, M.; Fefferman, N.H.; Starks, P.T. Genetic, individual, and group facilitation of disease resistance in insects societies. Annu. Rev. Entomol. 2009, 54, 405–423. [Google Scholar] [CrossRef]
- Gonçalves, L.S. A influência do comportamento das abelhas africanizadas na população, capacidade de defesa e resistência à doenças. In Anais do I Encontro Sobre Abelhas; Anais: Ribeirão Preto, Brazil, 1994; pp. 69–79. [Google Scholar]
- Milne, C.P. Laboratory test of honey bee hygienic behavior and resistance to European Foulbrood. Amer. Bee J. 1985, 125, 578–580. [Google Scholar]
- Milne, C.P. Estimates of heritabilities and genetic correlation between two components of honey bee (Hymenoptera: Apidae) hygienic behavior: Uncapping and removing. Ann. Entomol. Soc. Am. 1985, 78, 841–844. [Google Scholar]
- Moritz, R.F.A. A re-evaluation of the two locus model for hygienic behaviour in honeybees (Apis mellifera L.). J. Hered. 1988, 79, 257–262. [Google Scholar]
- Kefuss, J.; Taber, S., III; Vanpoucke, J.; Rey, F. A practical method to test for disease resistance in honey bees. Am. Bee J. 1996, 136, 31–32. [Google Scholar]
- Thakur, R.K.; Bienenfeld, K.; Keller, R. Varroa defense behaviour in Apis mellifera carnica. Am. Bee J. 1997, 137, 143–148. [Google Scholar]
- Gramacho, K.P. Fatores que Interferem no Comportamento Higiênico das Abelhas Apis mellifera. Ph.D. thesis, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brasil, 1999. [Google Scholar]
- Lapidge, K.L.; Oldroyd, B.P.; Spivak, M. Seven suggestive quantitative loci influence hygienic behavior of honey bees. Naturwissenschaften 2002, 89, 565–568. [Google Scholar]
- Oxley, P.; Spivak, M.; Oldroyd, B. Six quantitative trait loci influence task thresholds for hygienic behavior in honeybees (Apis mellifera). Mol. Ecol. 2010, 19, 1452–1461. [Google Scholar] [CrossRef]
- Arechavaleta-Velasco, M.E.; Hunt, G.J.; Spivak, M.; Camacho-Rea, C. Loci de rasgos binarios que influyen en la expresión del comportamiento higiénico de las abejas melíferas. Rev. Mex. Cienc. Pecu. 2011, 2, 283–298. [Google Scholar]
- Spivak, M.; Gilliam, M. Facultative expression of hygienic behavior of honey bees in relation to disease resistance. J. Apicult. Res. 1993, 32, 147–157. [Google Scholar]
- Morais, M.M.; Francoy, T.M.; Borissevitch, Y.; Gonçalves, L.S. A scientific note about spectroscopic analysis of honey bee brood comb cappings. Comparison of hygienic and non-hygienic bees lines in honey bees. Apidologie 2010, 41, 531–533. [Google Scholar] [CrossRef]
- Arathi, H.S.; Burns, I.; Spivak, M. Ethology of hygienic behaviour in the honey bee Apis mellifera L. (Hymenoptera: Apidae): Behavioural repertoire of hygienic bees. Ethology 2000, 106, 365–379. [Google Scholar] [CrossRef]
- Arathi, H.S.; Spivak, M. Influence of colony genotypic composition on the performance of hygienic behaviour in the honeybee Apis mellifera L. Anim. Behav. 2001, 62, 57–66. [Google Scholar] [CrossRef]
- Palacio, M.A. Relación Apis mellifera—Aschosphaera apis Estudio del Comportamiento Higiénico. Ph.D. thesis, Facultad de Ciências Agrárias, Universidad Nacional de Mar del Plata, Mar del Plata, Argentina, 2005. [Google Scholar]
- Gramacho, K.P.; Gonçalves, L.S. Estudo Comparativo dos Métodos de Congelamento e Perfuração de Crias para Avaliação do Comportamento Higiênico em Abelhas Africanizadas. In Congresso Latinoiberoamericano de Apicultura; Anais: Cordoba, Argentina, 1994; p. 45. [Google Scholar]
- Invernizzi, C.; Corbella, E. Edad de las obreras que realizan comportamiento higienico y otros comportamientos en las abejas Apis mellifera. Rev. Etol. 1999, 1, 79–87. [Google Scholar]
- Guerra, J.C.V., Jr.; Gonçalves, L.S.; de Jong, D. Africanized honey bees (Apis mellifera) are more efficient at removing worker brood artificially infested with the parasitic mite Varroa jacobsoni Oudemans than are Italian bees or Italian/Africanized hybrids. Genet. Mol. Biol. 2000, 23, 89–92. [Google Scholar] [CrossRef]
- Gramacho, K.P.; Spivak, M. Differences in olfactory sensitivity and behavioural responses among honey bees bred for hygienic behavior. Behav. Ecol. Sociobiol. 2003, 54, 472–479. [Google Scholar] [CrossRef]
- Palacio, M.A.; Rodriguez, E.; Goncalves, L.S.; Bedascarrasbure, E.; Spivak, M. Hygienic behaviors of honey bees in response to brood experimentally pin-killed or infected with Ascosphaera apis. Apidologie 2010, 41, 1–11. [Google Scholar] [CrossRef]
- Panasiuk, B.; Skowronek, W.; Bienkowska, M.; Gerula, D.; Wegrzynovicz, P. Age of worker bees performing hygienic behavior in a honeybee colony. J. Apicul. Sci. 2010, 54, 109–115. [Google Scholar]
- Moore, D.; Angel, J.E.; Cheeseman, I.M.; Fahrbach, S.E.; Robinson, G.E. Timekeeping in the honey bee colony: Integration of circadian rhythms and division of labor. Behav. Ecol. Sociobiol. 1998, 43, 147–160. [Google Scholar] [CrossRef]
- Pereira, R.A. Monitoramento das Atividades Individuais de Abelhas Africanizadas Relacionadas ao Comportamento Higiênico. Ph.D. thesis, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brasil, 2008. [Google Scholar]
- Morais, M.M.; Francoy, T.M.; Pereira, R.A.; de Jong, D.; Gonçalves, L.S. Africanized honey bees are efficient at detecting, uncapping and removing dead brood. Genet. Mol. Res. 2009, 8, 718–724. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pereira, R.A.; Morais, M.M.; Francoy, T.M.; Gonçalves, L.S. Hygienic Behavior of Africanized Honey Bees Apis mellifera Directed towards Brood in Old and New Combs during Diurnal and Nocturnal Periods. Insects 2013, 4, 521-532. https://doi.org/10.3390/insects4040521
Pereira RA, Morais MM, Francoy TM, Gonçalves LS. Hygienic Behavior of Africanized Honey Bees Apis mellifera Directed towards Brood in Old and New Combs during Diurnal and Nocturnal Periods. Insects. 2013; 4(4):521-532. https://doi.org/10.3390/insects4040521
Chicago/Turabian StylePereira, Rogério A., Michelle M. Morais, Tiago M. Francoy, and Lionel S. Gonçalves. 2013. "Hygienic Behavior of Africanized Honey Bees Apis mellifera Directed towards Brood in Old and New Combs during Diurnal and Nocturnal Periods" Insects 4, no. 4: 521-532. https://doi.org/10.3390/insects4040521




