Fate of Ingested Aristolactams from Aristolochia chilensis in Battus polydamas archidamas (Lepidoptera: Papilionidae)
Abstract
:1. Introduction
2. Experimental Section
2.1. Insects and Plants
2.2. Extraction and Isolation of Aristolactams
2.3. HPLC-DAD Analysis of Aristolactams

2.4. GC-MS Analysis of Aristolactams
3. Results and Discussion

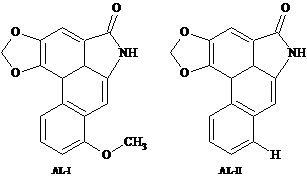
| Lactams | RT | KI | MS (m/z, %) |
|---|---|---|---|
| AL-II | 34.0 | 3112.7 | M+ 263 (95.5), 207.3 (38.9), 179.2 (35.8), 177.2 (43.1), 152.3 (75.3), 151.1 (38.5), 150.1 (100), 131.2 (40.0), 76.1 (28.9), 75.2 (55.4). |
| AL-I | 41.3 | 3383.9 | M+ 293 (95.6), 278.3 (96.7), 250.3 (54.0), 207.3 (38.6), 166.3 (69.2), 164.1 (100), 139.3 (59.8), 124.6 (40.1), 137.1 (55.7), 73.1 (48.1). |
| Lactams | Aristolactams in host plant samples (P1-P5) a | Frass | |||||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | Mean ± SD | ||
| AL-I(1) | 1,207 | 1,184 | 1,140 | 1,270 | 1,185 | 1,197.2 ± 47.4 | |
| AL-II(2) | 26.8 | 27.9 | 30.1 | 26.5 | 27.4 | 27.74 ± 1.43 | |
| Aristolactams in larvae samples (L1-L5) b | |||||||
| L1 | L2 | L3 | L4 | L5 | Mean ± SD | ||
| AL-I(1) | 291.5 | 289.5 | 278.5 | 285 | 290 | 286.9 ± 5.3 | 745.8 c |
| AL-II(2) | 70.5 | 76.5 | 75.5 | 78.1 | 74 | 74.92 ± 2.9 | – |
| Lactams | Amount in µg | |||||
|---|---|---|---|---|---|---|
| L1 | L2 | L3 | L4 | L5 | Mean ± SD | |
| AL-I | 58.3 (4.8%) | 57.9 (4.9%) | 55.7 (4.9%) | 57.0 (4.3%) | 57.0 (4.5%) | 57.38 ± 1.1 |
| AL-II | 14.1 (52.6%) | 15.3 (54.8%) | 16 (53.2%) | 15.9 (58.9%) | 14.8 (54.0%) | 15.22 ± 0.8 |
3.1. Discussion
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Feeny, P. Chemical constraints on the evolution of swallowtail butterflies. In Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions; Price, P.W., Lewinsohn, T.M., Fernandez, J.W., Benson, W.W., Eds.; Wiley: New York, NY, USA, 1991; pp. 315–340. [Google Scholar]
- Weintraub, J.D. Host plant association patterns and phylogeny in the tribe troidini. In Swallowtail Butterflies: Their Ecology and Evolutionary Biology; Scriber, J.M., Tsubaki, Y., Lederhouse, R.C., Eds.; Scientific Publishers: Gainesville, FL, USA, 1995; pp. 307–316. [Google Scholar]
- Silva-Brandão, K.L.; Freitas, A.V.L.; Brower, A.V.Z.; Solferini, V.N. Phylogenetic relationships of the new world troidini swallowtails (lepidoptera: papilionidae) based on COI, COII, and EF-1α genes. Mol. Phylogenet. Evol. 2005, 36, 468–483. [Google Scholar] [CrossRef]
- Peña, L.; Ugarte, A. Las Mariposas de Chile; Editorial Universitaria: Santiago, Chile, 1997; pp. 234–254. [Google Scholar]
- Urzúa, A.; Freyer, A.J.; Shamma, M. Aristolodione, a 4,5-Dioxoaporphine from Aristolochia chilensis. J. Nat. Prod. 1987, 50, 305–306. [Google Scholar] [CrossRef]
- Urzúa, A.; Rojas, V. Constituents of Aristolochia chilensis. Fitoterapia 1990, LXI, 190. [Google Scholar]
- Urzúa, A.; Santander, R.; Sotes, G. Aristolochic acids from Aristolochia bridgesii, a host-plant of Battus polydamas archidamas. J. Chil. Chem. Soc. 2009, 54, 437–438. [Google Scholar]
- Urzúa, A.; Salgado, G.; Cassels, B.K.; Eckhardt, G. Aristolochic acids in Aristolochia chilensis and the aristolochia-feeder Battus archidamas (lepidoptera). Coll. Czech. Chem. Comm. 1983, 48, 1513–1519. [Google Scholar] [CrossRef]
- Urzúa, A.; Rodríguez, R.; Cassels, B.K. Fate of ingested aristolochic acids in Battus archidamas. Biochem. Syst. Ecol. 1987, 15, 687–689. [Google Scholar] [CrossRef]
- Kumar, V.; Poonam; Prasad, A.K.; Parmar, V.S. Naturally occurring aristolactams, aristolochic acids and dioxoaporphines and their biological activities. Nat. Prod. Rep. 2003, 20, 565–583. [Google Scholar] [CrossRef]
- Bentley, K.W. β-Phenylethylamines and the isoquinoline alkaloids. Nat. Prod. Rep. 2006, 23, 444–463. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Li, Y.-C.; Wu, T.-S. Chemical constituents and pharmacology of the Aristolochia species. J. Tradit. Comp. Med. 2011, 2, 249–266. [Google Scholar]
- Priestap, H.A. Seven aristololactams from Aristolochia argentina. Phytochemistry 1985, 24, 849–852. [Google Scholar] [CrossRef]
- Urzúa, A.; Priestap, H.A. Aristolochic acids from Battus polydamas. Biochem. Syst. Ecol. 1985, 15, 687–689. [Google Scholar]
- Fordyce, J.A. A model without a mimic: Aristolochic acids from the California pipevine swallowtail, Battus philenor hirsuta, and its host plant, Aristolochia californica. J. Chem. Ecol. 2000, 26, 2567–2578. [Google Scholar] [CrossRef]
- Klitzke, C.F.; Brown, K.S. The occurrence of aristolochic acids in neotropical troidine swallowtails (lepidoptera: papilionidae). Chemoecology 2000, 10, 99–102. [Google Scholar] [CrossRef]
- Priestap, H.A.; Velandia, A.; Johnson, J.; Barbieri, M. Secondary metabolite uptake by the Aristolochia-feeding papilionoid butterfly Battus polydamas. Biochem. Syst. Ecol. 2012, 40, 126–137. [Google Scholar] [CrossRef]
- Jou, J.-H.; Li, C.-Y.; Schelonka, E.P.; Lin, C.-H.; Wu, T.-S. Analysis of the analogues of aristolochic acid and aristolactam in the plant of Aristolochia genus by HPLC. J. Food Drug Anal. 2004, 12, 40–45. [Google Scholar]
- Zhang, C.; Wang, X.; Shang, M.; Yu, J.; Xu, Y.; Li, Z.; Lei, L.; Li, X.; Cai, S.; Namba, T. Simultaneous determination of five aristolochic acids and two aristololactams in Aristolochia plants by high-performance liquid chromatography. Biomed. Chromatogr. 2006, 20, 305–318. [Google Scholar]
- Yuan, J.; Nie, L.; Zeng, D.; Luo, X.; Tang, F.; Ding, L.; Liu, Q.; Guo, M.; Yao, S. Simultaneous determination of nine aristolochic acid analogues in medicinal plants and preparations by high-performance liquid chromatography. Talanta 2007, 73, 644–665. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Q.; Zhub, W.; Ding, L.; Tang, F.; Yao, S. Simultaneous analysis of six aristolochic acids and five aristolactams in herbal plants and their preparations by high-performance liquid chromatography-diode array detection-fluorescence detection. J. Chromatogr. A 2008, 1182, 85–92. [Google Scholar]
- Chen, H.J.; Li, X.; Chen, J.W.; Guo, S.; Cai, B.C. Simultaneous determination of eleven bioactive compounds in Saururus chinensis from different harvesting seasons by HPLC-DAD. J. Pharm. Biomed. Anal. 2010, 51, 1142–1146. [Google Scholar] [CrossRef]
- Priestap, H.A.; de los Santos, C.; Quirke, J.M. Identification of a reduction product of aristolochic acid: implications for the metabolic activation of carcinogenic aristolochic acid. J. Nat. Prod. 2011, 73, 1979–1986. [Google Scholar]
- Schmeiser, H.H.; Bieler, C.A.; Wiessler, M.; van Ypersele de Strihou, C.; Cosyns, J.-P. Detection of DNA adducts formed by aristolochic acid in renal tissue from patients with Chinese herbs nephropathy. Cancer Res. 1996, 5, 2025–2028. [Google Scholar]
- Zhou, S.; Koh, H.-L.; Gao, Y.; Gong, Z.-Y.; Lee, E.J.D. Herbal bioactivation: The good, the bad and the ugly. Life Sci. 2004, 74, 935–968. [Google Scholar] [CrossRef]
- Pinto, C.F.; Urzúa, A.; Niemeyer, H.M. Sequestration of aristolochic acids from meridic diets by larvae of Battus polydamas archidamas (papilionidae: troidini). Eur. J. Entomol. 2011, 108, 41–45. [Google Scholar]
- Sato, F.; Hashimoto, T.; Hachiya, A.; Tamura, K.; Choi, K-B.; Morishige, T.; Fujimoto, H.; Yamada, Y. Metabolic engineering of plant alkaloid biosynthesis. Proc. Natl. Acad. Sci. USA 2001, 98, 367–372. [Google Scholar]
- Alali, F.Q.; Tawaha, K.; Shehadeh, M.B.; Telfah, S. Phytochemical and biological investigation of Aristolochia maurorum L. Z. Naturforsch C 2006, 61, 685–691. [Google Scholar]
- Lajide, L.; Escoubas, P.; Mizutani, J. Antifeedant activity of metabolites of Aristolochia albida against the tobacco cutworm, Spodoptera litura. J. Agric. Food Chem. 1993, 41, 669–673. [Google Scholar] [CrossRef]
- Jeude, S.E. Quality vs. quantity: The effect of aristolochic acids on preference and performance of a non-specialist herbivore. Purs. J. Undergrad. Res. Univ. Tenn. 2011, 2, 109–119. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Urzúa, A.; Olguín, A.; Santander, R. Fate of Ingested Aristolactams from Aristolochia chilensis in Battus polydamas archidamas (Lepidoptera: Papilionidae). Insects 2013, 4, 533-541. https://doi.org/10.3390/insects4040533
Urzúa A, Olguín A, Santander R. Fate of Ingested Aristolactams from Aristolochia chilensis in Battus polydamas archidamas (Lepidoptera: Papilionidae). Insects. 2013; 4(4):533-541. https://doi.org/10.3390/insects4040533
Chicago/Turabian StyleUrzúa, Alejandro, Angel Olguín, and Rocío Santander. 2013. "Fate of Ingested Aristolactams from Aristolochia chilensis in Battus polydamas archidamas (Lepidoptera: Papilionidae)" Insects 4, no. 4: 533-541. https://doi.org/10.3390/insects4040533