Research Contributing to Improvements in Controlling Florida’s Mosquitoes and Mosquito-borne Diseases
Abstract
:1. Introduction
2. The Florida Mosquito Control Research Program (FMCRP)
3. Improved Mosquito Impoundments Using Rotational Impoundment Management (RIM)
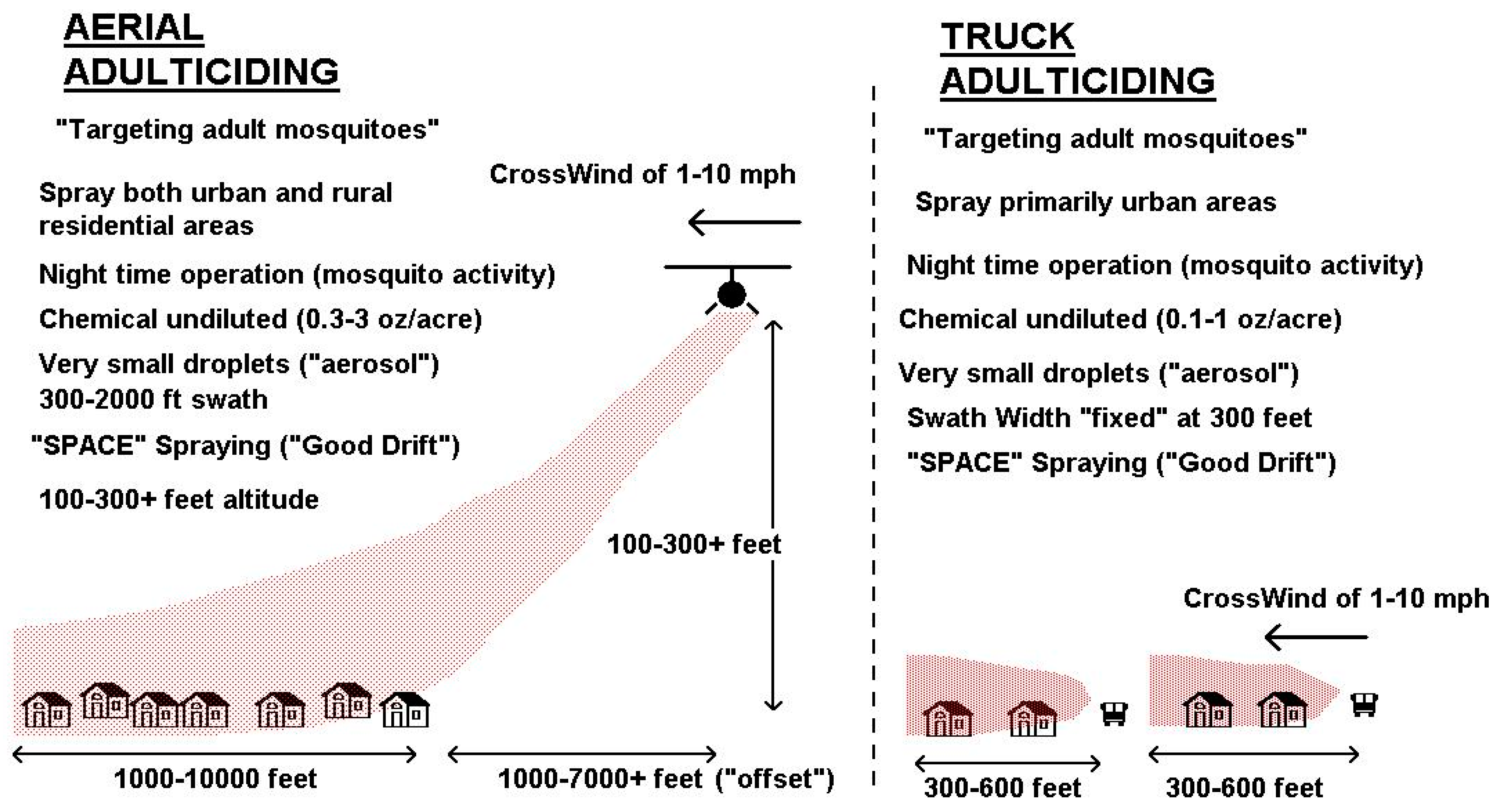
4. Improved Pesticide Application
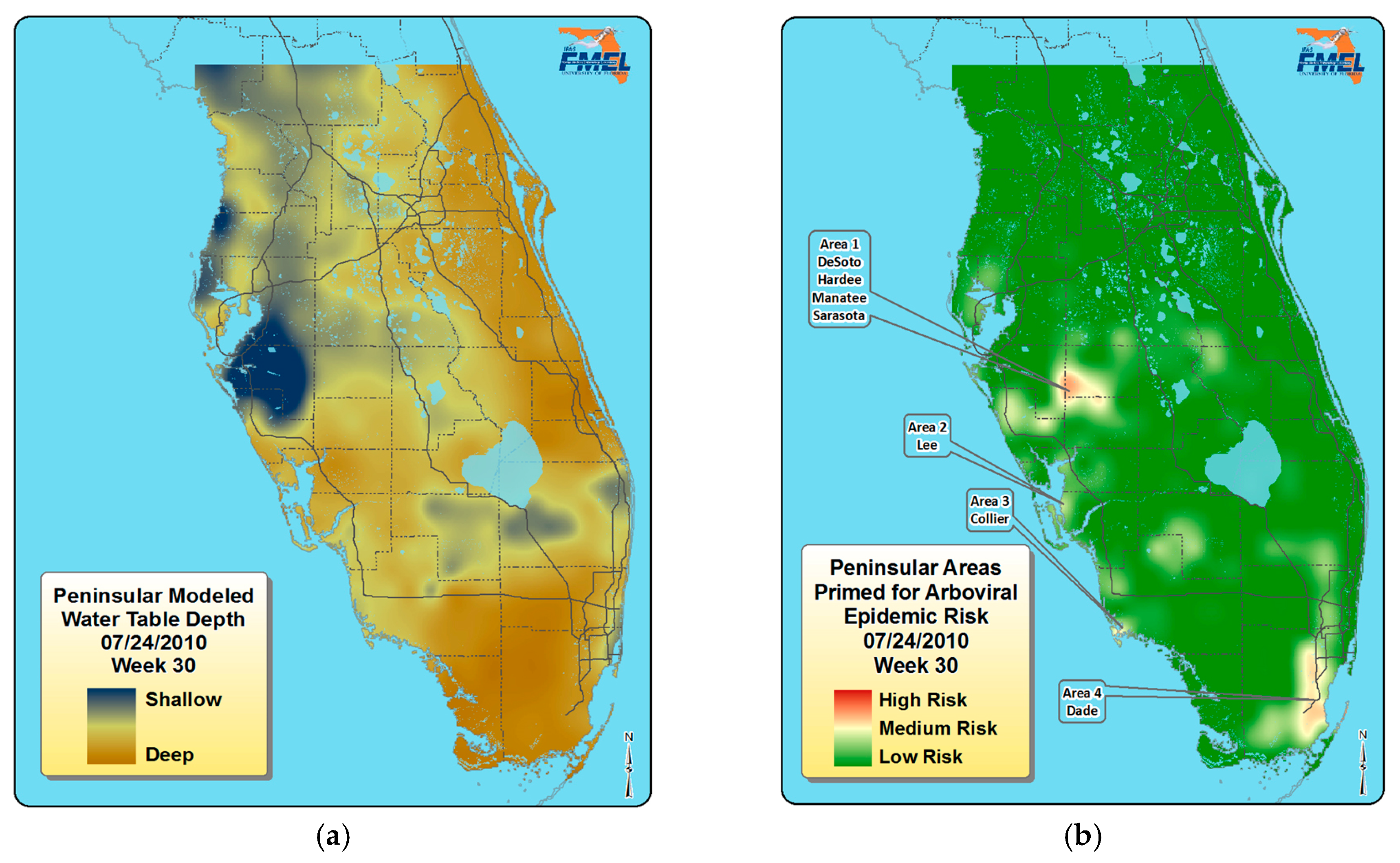
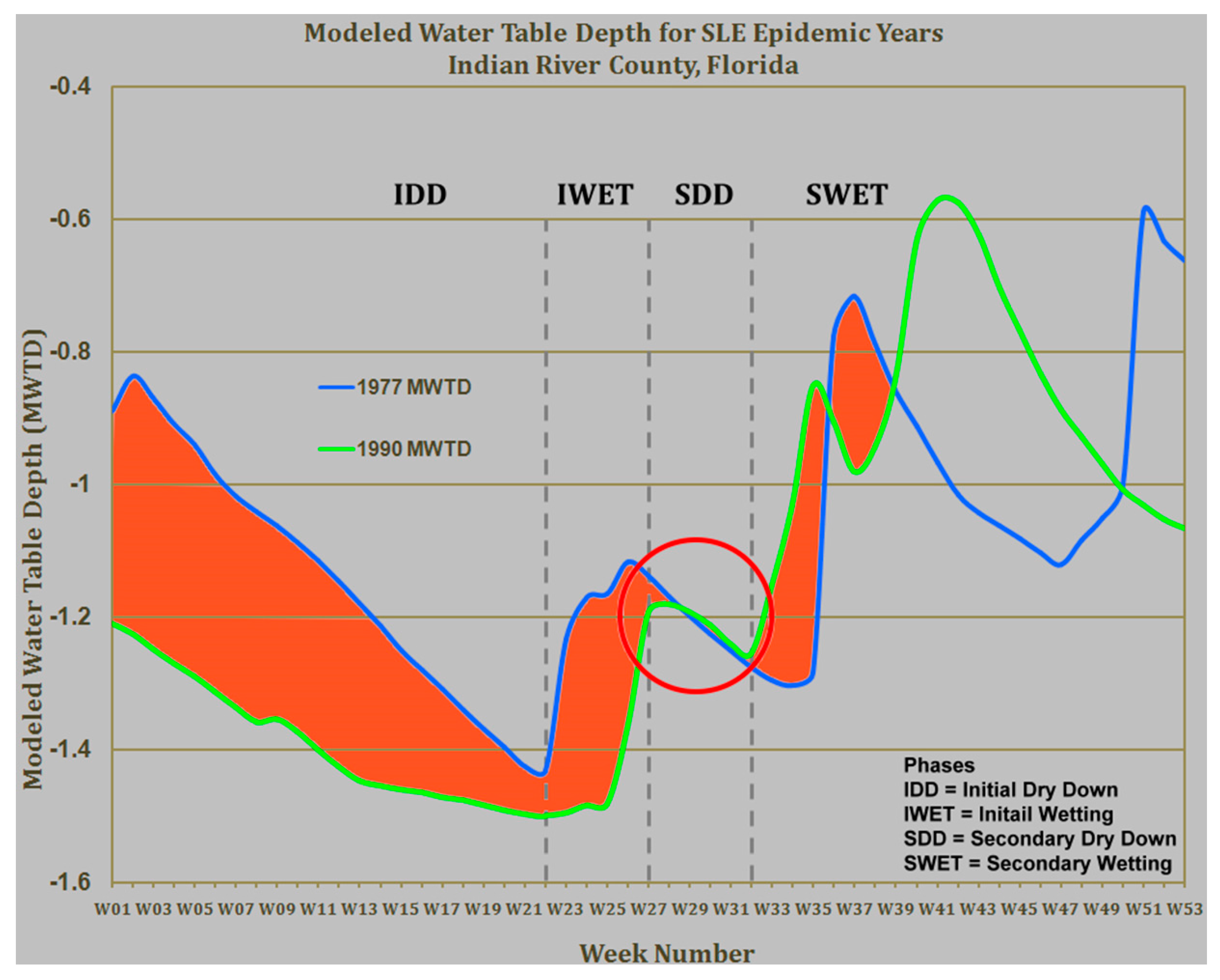
5. Florida Arbovirus Surveillance, Predicting Epidemics, and Mosquito Control
6. Challenges and Conclusions
Acknowledgments
Conflicts of Interest
References
- Crosby, M.C. The American Plague: The Untold Story of Yellow Fever, the Epidemic that Shaped Our History; Penguin Group: New York, NY, USA, 2006. [Google Scholar]
- Becker, N.; Petri, D.; Boase, C.; Lane, J.; Zgomba, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003. [Google Scholar]
- Marquardt, W.H. Biology of Disease Vectors, 2nd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Eldridge, B.F.; Edman, J. Medical Entomology: A Textbook on Public Health and Veterinary Problems Caused by Arthropods; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Zarowiecki, M; Loaiza, J.R.; Conn, J.E. Towards a new role for vector systematics in parasite control. Parasitology 2011, 138, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, W.J.; Black, W.C. Making a case for molecular population genetic studies of arthropod vectors. Parasitol. Today. 1995, 11, 27–30. [Google Scholar] [CrossRef]
- Wang, S.; Jacobs-Lorena, M. Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotech. 2013, 31, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Lees, R.S.; Knols, B.; Bellini, R.; Benedict, M.Q.; Bheecarry, A.; Bossin, H.C.; Chadee, D.D.; Charlwood, J.; Dabire, R.K.; Djogbenou, L.; et al. Review: Improving our knowledge of male mosquito biology in relation to genetic control programmes. Acta Trop. 2014, 132, s2–s11. [Google Scholar] [CrossRef] [PubMed]
- Reisen, W.K.; Reeves, W.C. Bionomics and ecology of Culex tarsalis and other potential mosquito vector species. In Epidemiology and Control of Mosquito-Borne Arboviruses in California, 1943–1987; Reeves, W.C., Asman, S.M., Hardy, J.L., Milby, M.M., Reisen, W.K., Eds.; California Mosquito and Vector Control Association: Sacramento, CA, USA, 1990; pp. 254–329. [Google Scholar]
- Reeves, W.C.; Asman, S.M.; Hardy, J.L.; Milby, M.M.; Reisen, W.K. Epidemiology and Control of Mosquito-Borne Arboviruses in California, 1943–1987; California Mosquito and Vector Control Association, Inc.: Sacramento, CA, USA, 1990. [Google Scholar]
- Reisen, W.K. University of California—California Department of Public Health—Mosquito and Vector Control Association of California: Partners in arbovirus surveillance. Available online: http://www.mvcac.org/amg/wp-content/uploads/2011-MVCAC-Volume-79.pdf (accessed on 15 July 2016).
- Hribar, L.J. Influence and impact of mosquito-borne diseases on the history of Florida, USA. Life Excit. Biol. 2013, 1, 53–58. [Google Scholar] [CrossRef]
- Patterson, G. The Mosquito Wars: A History of Mosquito Control in Florida; University Press of Florida: Gainesville, FL, USA, 2004. [Google Scholar]
- Connelly, C.R.; Carlson, D.B. Florida Coordinating Council on Mosquito Control. In Florida Mosquito Control: The State of the Mission as Defined by Mosquito Controllers, Regulators, and Environmental Managers; Connelly, C.R., Carlson, D.B., Eds.; University of Florida: Vero Beach, FL, USA, 2009. [Google Scholar]
- Linthicum, K.; Smith, J.; Tabachnick, W. Mosquito control research. In Florida Mosquito Control: The State of the Mission as Defined by Mosquito Controllers, Regulators, and Environmental Managers; Connelly, C.R., Carlson, D.B., Eds.; University of Florida: Vero Beach, FL, USA, 2009; pp. 193–223. [Google Scholar]
- Bidlingmayer, W.L. The influence of environmental factors and physiological stage on flight patterns of mosquitoes taken in the vehicle aspirator and truck, suction, bait and New Jersey light traps. J. Med. Entomol. 1974, 11, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P. The Florida contract mosquito control research program. Wing Beats 2009, 20, 18–21. [Google Scholar]
- Knight, R.L.; Walton, W.E.; O’Meara, G.F.; Reisen, W.K.; Wass, R. Strategies for effective mosquito control in constructed treatment wetlands. Ecol. Eng. 2003, 21, 211–232. [Google Scholar] [CrossRef]
- Provost, M.W. Tidal datum planes circumscribing salt marshes. Bull. Mar. Sci. 1976, 26, 558–563. [Google Scholar]
- Rey, J.R.; Rutledge, C.R. Mosquito Control Impoundments. Available online: http://desoto.ifas.ufl.edu/pdf/Insects/Mosquito%20Control%20Impoundments%20%20IN19200[1].pdf (accessed on 19 July 2016).
- Carlson, D.B.; O’Bryan, P.D. Mosquito production in a rotationally managed impoundment compared to other management techniques. J. Am. Mosq. Control Assoc. 1988, 4, 146–151. [Google Scholar] [PubMed]
- Carlson, D.B.; O’Bryan, P.D.; Rey, J.R. A review of current salt marsh management issues in Florida. J. Am. Mosq. Control Assoc. 1991, 7, 83–88. [Google Scholar] [PubMed]
- Rey, J.R.; Carlson, D.B.; Brockmeyer, R.E. Coastal wetland management in Florida: Environmental concerns and human health. Wetl. Ecol. Mgmt. 2012, 20, 197–211. [Google Scholar] [CrossRef]
- Brockmeyer, R.E., Jr.; Rey, J.R.; Virnstein, R.W.; Gilmore, R.G.; Earnest, L. Rehabilitation of impounded estuarine wetlands by hydrologic reconnection to the Indian River Lagoon, Florida (USA). Wetl. Ecol. Manag. 1996, 4, 93–109. [Google Scholar] [CrossRef]
- Carlson, D.B.; Indian River Mosquito Control District, Vero Beach, Florida, USA. Personal communication, 2016.
- Bonds, J.A.S. Ultra-low-volume space sprays in mosquito control: A critical review. Med. Vet. Entomol. 2012, 26, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Mount, G.A.; Biery, T.L.; Haile, D.G. A review of ultralow-volume aerial sprays of insecticide for mosquito control. J. Am. Mosq. Control Assoc. 1996, 12, 601–618. [Google Scholar] [PubMed]
- Mount, G.A. A critical review of ultralow-volume aerosols of insecticide applied with vehicle-mounted generators for adult mosquito control. J. Am. Mosq. Control Assoc. 1998, 14, 305–334. [Google Scholar] [PubMed]
- Latham, M.D. Aerial adulticiding to control adult mosquitoes in Florida: 10 years of improvements in understanding and technology. Asp. Appl. Biol. 2008, 84, 281–288. [Google Scholar]
- Latham, M.; Barber, J. Mosquito control in Florida with a focus on aerial adulticiding. Outlooks Pest Manag. 2007, 18, 178–183. [Google Scholar] [CrossRef]
- Bonds, J.A.S.; Greer, M.J.; Fritz, B.K.; Hoffmann, W.C. Aerosol sampling: Comparison of two rotating impactors for field droplet sizing and volumetric measurements. J. Am. Mosq. Control Assoc. 2009, 25, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Mount, G.A. Optimum droplet size for adult mosquito control with space sprays or aerosols of insecticides. Mosq. News 1970, 30, 70–75. [Google Scholar]
- Dukes, J.C.; Hallmon, C.F.; Shaffer, K.R.; Hester, P.G. Effects of pressure and flow rate on Cythion droplet size produced by three different ground ULV aerosol generators. J. Am. Mosq. Control. Assoc. 1990, 6, 279–282. [Google Scholar] [PubMed]
- Barber, J.; Greer, M.; Hewitt, A. A field measurement device for the aerosols used in mosquito control. Available online: http://johnwhock.com/wp-content/uploads/2013/06/FL-AM-Aerosol-Sampler-ASAE.pdf (accessed on 1 June 2016).
- Dukes, J.; Zhong, H.; Greer, M.; Hester, P.; Hogan, D.; Barber, J.A. A comparison of two spray nozzle systems used to aerially apply the ultra-low-volume adulticide fenthion. J. Am. Mosq. Control Assoc. 2004, 20, 27–35. [Google Scholar] [PubMed]
- Dukes, J.; Zhong, H.; Greer, M.; Hester, P.; Hogan, D.; Barber, J.A.S. A comparison of two ultra-low-volume spray nozzle systems by using a multiple swath scenario for the aerial application of fenthion against adult mosquitoes. J. Am. Mosq. Control Assoc. 2004, 20, 36–44. [Google Scholar] [PubMed]
- Hoffmann, W.C.; Walker, T.W.; Martin, D.E.; Barber, J.A.B.; Gwinn, T.; Smith, V.L.; Szumlas, D.; Lan, Y.; Fritz, B.K. Characterization of truck-mounted atomization equipment typically used in vector control 1. J. Am. Mosq. Control Assoc. 2007, 23, 321–329. [Google Scholar] [CrossRef]
- Linley, J.R.; Parsons, R.E.; Winner, R.A. Evaluation of ULV naled applied simultaneously against caged adult Aedes taeniorhynchus and Culicoides furens. J. Am. Mosq. Control Assoc. 1988, 4, 326–332. [Google Scholar] [PubMed]
- Moore, J.C.; Dukes, J.C.; Clark, J.R.; Malone, J.; Hallmon, C.F.; Hester, P.G. Downwind drift and deposition of malathion on human targets from ground ultra-low volume mosquito sprays. J. Am. Mosq. Control Assoc. 1993, 9, 138–142. [Google Scholar] [PubMed]
- Curtis, G.A.; Beidler, E.J. Influence of ground ULV droplet spectra on adulticide efficacy for Aedes taeniorhynchus. J. Am. Mosq. Control Assoc. 1996, 12, 368–371. [Google Scholar] [PubMed]
- Barber, J.A.; Greer, M.; Latham, M.; Stout, G. Canopy effects droplet size distribution and meteorological change. J. Am. Mosq. Control. Assoc. 2008, 24, 177–181. [Google Scholar] [CrossRef]
- Curtis, G.A.; Mason, J.A. Evaluation of equipment modifications and dosage rates of ground ULV applications of naled against Aedes taeniorhynchus in a Florida citrus grove. J. Am. Mosq. Control Assoc. 1988, 4, 345–350. [Google Scholar] [PubMed]
- Rathburn, C.B., Jr.; Dukes, J.C. A comparison of the mortality of caged adult mosquitoes to the size, number and volume of ULV spray droplets sampled in an open and a vegetated area. J. Am. Mosq. Control Assoc. 1989, 5, 173–175. [Google Scholar] [PubMed]
- Barber, J.A.S.; Greer, M.; Coughlin, J. Field tests of malathion and permethrin applied via a truck-mounted cold fogger to both open and vegetated habitats. J. Am. Mosq. Control Assoc. 2007, 23, 55–59. [Google Scholar] [CrossRef]
- Zhong, H. Aerial ultra-low-volume application of insecticide to control adult mosquitoes. In Encyclopedia of Pest Management; Pimentel, D., Ed.; CRC Press: Boca Raton FL, USA, 2007; Volume 2, pp. 4–6. [Google Scholar]
- Hester, P.G.; Shaffer, K.R.; Tietze, N.S.; Zhong, H.; Griggs, N.L. Efficacy of ground-applied ultra-low-volume malathion on honey bee survival and productivity in open and forest areas. J. Am. Mosq. Control Assoc. 2001, 17, 2–7. [Google Scholar] [PubMed]
- Zhong, H.; Dukes, J.; Greer, M.; Hester, P.; Shirley, M.; Anderson, B. Ground deposition impact of aerially applied fenthion on the fiddler crabs, Uca pugilator. J. Am. Mosq. Control Assoc. 2003, 19, 47–52. [Google Scholar] [PubMed]
- Zhong, H.; Latham, M.; Payne, S.; Brock, C. Minimizing the impact of the mosquito adulticide naled on honey bees, Apis mellifera (Hymenoptera: Apidae): Aerial ultra-low-volume application using a high-pressure nozzle system. J. Econ. Entomol. 2004, 97, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Hribar, L.J.; Daniels, J.C.; Feken, M.A.; Brock, C.; Trager, M.D. Aerial ultra-low-volume application of naled: Impact on nontarget imperiled butterfly larvae (Cyclargus thomasi bethunebakeri) and efficacy against adult mosquitoes (Aedes taeniorhynchus). Environ. Entomol. 2010, 39, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Qualls, W.A.; Xue, R.D.; Zhong, H. Impact of bifenthrin on honeybees and Culex quinquefasciatus. J. Am. Mosq. Control Assoc. 2010, 26, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Latham, M. Aspects to consider for vector control. In Proceedings of the International Conference on Pesticide Application for Drift Management, Waikoloa, HI, USA, 27–29 October 2004; pp. 97–101.
- Latham, M.D.; Manatee County Mosquito Control District, Palmetto, Florida, USA. Personal communication, 2016.
- Chamberlain, R.W.; Sudia, W.D.; Coleman, P.H.; Beadle, L.D. Vector studies in the St. Louis encephalitis epidemic, Tampa Bay area, Florida, 1962. Am. J. Trop. Med. Hyg. 1964, 13, 456–461. [Google Scholar] [PubMed]
- Day, J.F. Predicting St. Louis encephalitis virus epidemics: Lessons from recent, and not so recent, outbreaks. Annu. Rev. Entomol. 2001, 46, 111–138. [Google Scholar]
- Day, J.F.; Tabachnick, W.J.; Smartt, C.T. Factors that influence the transmission of West Nile virus in Florida. J. Med. Entomol. 2015, 52, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Day, J.F. Disease surveillance, outbreaks and control in Florida. In Florida Mosquito Control: The State of the Mission as Defined by Mosquito Controllers, Regulators, and Environmental Managers; Connelly, C.R., Carlson, D.B., Eds.; University of Florida: Vero Beach, FL, USA, 2009; pp. 131–144. [Google Scholar]
- Day, J.F.; Shaman, J. Mosquito-borne arboviral surveillance and the prediction of disease outbreaks. In Flavivirus Encephalitis; Ruzek, D., Ed.; InTech: Rijeka, Croatia, 2011; pp. 105–130. [Google Scholar]
- Day, J.F.; Shaman, J. Severe winter freezes enhance St. Louis encephalitis virus amplification and epidemic transmission in peninsular Florida. J. Med. Entomol. 2009, 46, 1498–1506. [Google Scholar] [PubMed]
- Shaman, J.; Day, J.F.; Stieglitz, M.; Zebiak, S.; Cane, M. An ensemble seasonal forecast of human cases of St. Louis encephalitis in Florida based on seasonal hydrologic forecasts. Clim. Chang. 2006, 75, 495–511. [Google Scholar] [CrossRef]
- Day, J.F.; Curtis, G.A. When it rains, they soar—And that makes Culex nigripalpus a dangerous mosquito. Am. Entomol. 1994, 40, 162–167. [Google Scholar] [CrossRef]
- Shaman, J.; Day, J.F.; Stieglitz, M. Drought-induced amplification and epidemic transmission of West Nile virus in southern Florida. J. Med. Entomol. 2005, 42, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Day, J.F.; Shaman, J. Using hydrologic conditions to forecast the risk of focal and epidemic arboviral transmission in peninsular Florida. J. Med. Entomol. 2008, 45, 458–465. [Google Scholar] [CrossRef]
- Detinova, T.S. Age-grouping Methods in Diptera of Medical Importance; World Health Organization: Geneva, Switzerland, 1962. [Google Scholar]
- Polovodova, V.P. The determination of the physiological age of female Anopheles by the number of gonotrophic cycles completed. Med. Parazitol. Parazit. Bolezn. 1949, 18, 352–355. [Google Scholar]
- Tabachnick, W.J. Arthropods and pathogens: Issues for emerging diseases. In Emerging Infections; Krause, R.M., Ed.; Academic Press: New York, NY, USA, 1998; pp. 411–429. [Google Scholar]
- Tabachnick, W.J. Reflections on the Anopheles gambiae genome sequence, transgenic mosquitoes and the prospect for controlling malaria and other vector borne diseases. J. Med. Entomol. 2003, 40, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, W.J. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J. Exp. Biol. 2010, 213, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Tabachnick, W.J.; Day, J.F. Impact of climate change on vector-borne arboviral episystems. In Viral Infections and Global Change; Singh, S.K., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2014; pp. 21–34. [Google Scholar]
- Tabachnick, W.J. Climate change and arboviruses: Lessons from the evolution of the yellow fever and dengue viruses. Annu. Rev. Virol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef] [PubMed]
- Ooi, E.E.; Goh, K.T.; Gubler, D.J. Dengue prevention and 35 years of vector control in Singapore. Emerg. Infect. Dis. 2006, 12, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.C.; Zielinski-Gutierrez, E.; Scott, T.W.; Rosenberg, R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 2008, 5, e68. [Google Scholar] [CrossRef] [PubMed]
- Araújo, H.R.; Carvalho, D.O.; Ioshino, R.S.; Costa-da-Silva, A.L.; Capurro, M.L. Aedes aegypti control strategies in Brazil: Incorporation of new technologies to overcome the persistence of dengue epidemics. Insects 2015, 6, 576–594. [Google Scholar] [CrossRef] [PubMed]
- Alfonso-Sierra, E.; Basso, C.; Beltrán-Ayala, E.; Mitchell-Foster, K.; Quintero, J.; Cortés, S.; Manrique-Saide, P.; Guillermo-May, G.; Caprara, A.; Carvalho de Lima, E.; et al. Innovative dengue vector control interventions in Latin America: What do they cost? Pathog. Glob. Health 2016, 110, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.R.; Donegan, S.; McCall, P.J. Is dengue vector control deficient in effectiveness or evidence?: Systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2016, 10, e0004551. [Google Scholar] [CrossRef] [PubMed]


| Topic | Total Publications Since 1960 | PHEREC | FMEL | % of Total by FMEL and PHEREC |
|---|---|---|---|---|
| Culex nigripalpus | 197 | 2 | 65 | 34 |
| Florida mosquito ecology | 77 | 1 | 26 | 35 |
| Florida mosquito control | 392 | 42 | 73 | 29 |
| Florida St. Louis encephalitis | 90 | 1 | 42 | 48 |
| Florida West Nile | 116 | 1 | 37 | 33 |
| Florida Aedes aegypti | 232 | 2 | 83 | 37 |
| Florida mosquito pesticides | 141 | 30 | 15 | 32 |
| Florida Aedes | 436 | 20 | 123 | 33 |
| Florida Culex | 235 | 26 | 109 | 57 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabachnick, W.J. Research Contributing to Improvements in Controlling Florida’s Mosquitoes and Mosquito-borne Diseases. Insects 2016, 7, 50. https://doi.org/10.3390/insects7040050
Tabachnick WJ. Research Contributing to Improvements in Controlling Florida’s Mosquitoes and Mosquito-borne Diseases. Insects. 2016; 7(4):50. https://doi.org/10.3390/insects7040050
Chicago/Turabian StyleTabachnick, Walter J. 2016. "Research Contributing to Improvements in Controlling Florida’s Mosquitoes and Mosquito-borne Diseases" Insects 7, no. 4: 50. https://doi.org/10.3390/insects7040050





