Periodic Physical Disturbance: An Alternative Method for Controlling Sitophilus zeamais (Maize Weevil) Infestation
Abstract
:1. Introduction
2. Materials and Methods
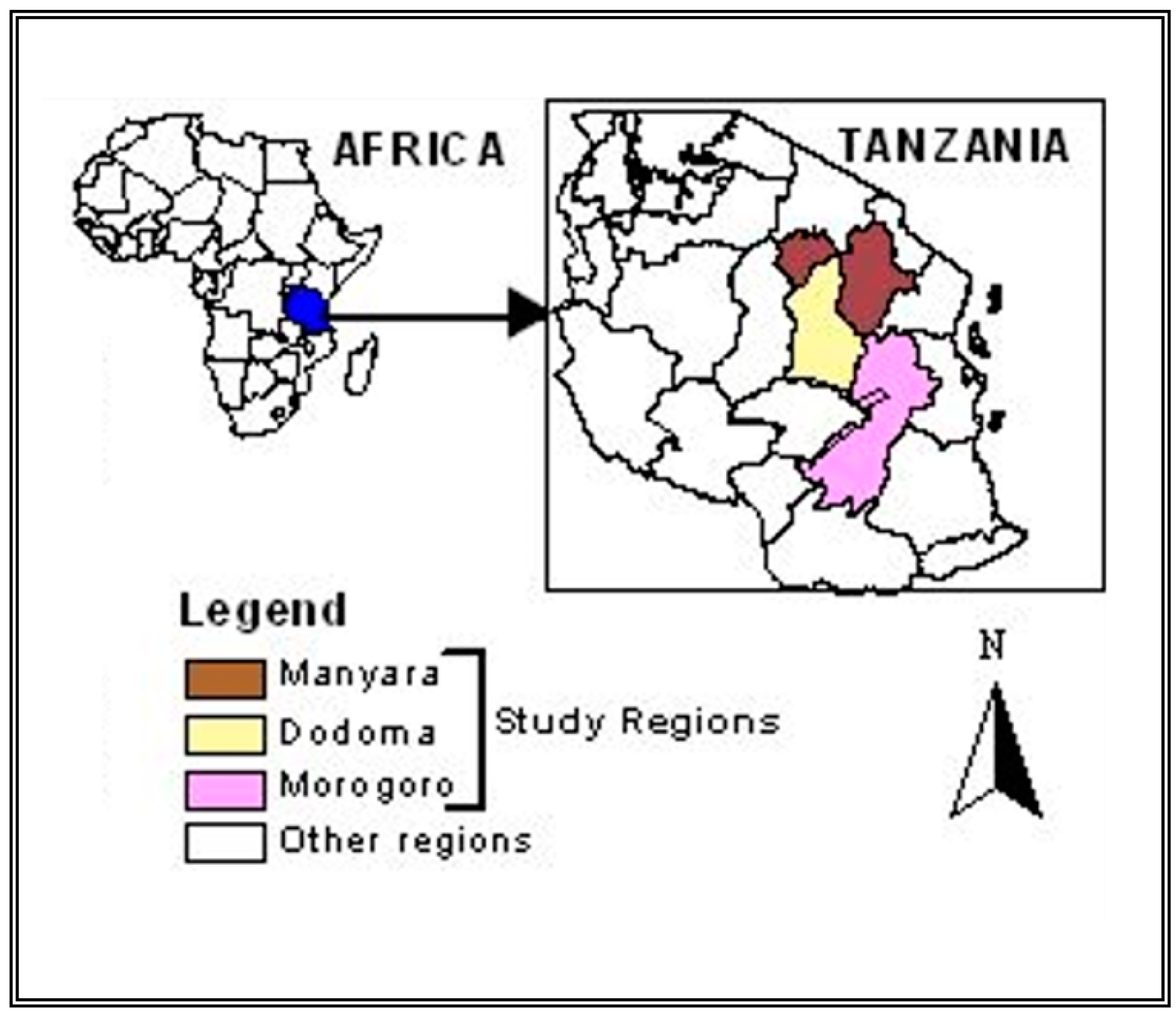
2.1. Study Area
2.2. Experimental Design
2.3. Determination of Live and Dead Insects (Mortality Rate)
2.4. Data Analysis
3. Results
3.1. Insect Mortality
3.2. Number of Live Insects
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IITA. International Institute of Tropical Agriculture (IITA)—Maize (Zea mays) Crop, 2016. Available online: http://www.iita.org/maize (accessed on 2 February 2016).
- Food and Agriculture Organization of the United Nations Statistics Division (FAO). Maize Production in Sub-Saharan Africa 2013/2014 Production Year, 2014. Available online: http://faostat3.fao.org/browse/Q/QC/E (accessed on 20 January 2016).
- World Bank. Missing Food: The Case of Postharvest Grain Losses in Sub-Saharan Africa; The International Bank for Reconstruction and Development/the World Bank: Washington, DC, USA, 2011; Available online: http://siteresources.worldbank.org/INTARD/Resources/MissingFoods10_web.pdf (accessed on 10 January 2016).
- Africa Postharvest Losses Information System (APHLIS). Losses Tables; Estimated Postharvest Losses (%), 2014. Available online: http://www.aphlis.net/?form=home (accessed on 18 December 2015).
- Rugumamu, C.P. Assessment of post-harvest technologies and gender relations in maize loss reduction in Pangawe village eastern Tanzania. Tanzania J. Sci. 2012, 35, 67–76. [Google Scholar]
- Suleiman, R.; Rosentrater, K.A.; Bern, C.J. Evaluation of maize weevils Sitophilus zeamais Motschulsky infestation on seven varieties of maize. J. Stored Prod. Res. 2015, 64, 97–102. [Google Scholar] [CrossRef]
- Demissie, G.; Teshom, A.; Abakemal, D.; Tadesse, A. Cooking oils and “Triplex” in the control of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) in farm-stored maize. J. Stored Prod. Res. 2008, 44, 173–178. [Google Scholar] [CrossRef]
- Dal Bello, G.; Padin, S.; Lastra, C.L.; Fabrizio, M. Laboratory evaluation of chemical-biological control of the rice weevil (Sitophilus oryzae L.) in stored grains. J. Stored Prod. Res. 2000, 37, 77–84. [Google Scholar] [CrossRef]
- Nwosu, L.C.; Adedire, C.O.; Ogunwolu, E.O.; Ashamo, M.O. Relative susceptibility of 20 elite maize varieties to infestation and damage by the maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae). Int. J. Trop. Insect Sci. 2015, 35, 185–192. [Google Scholar] [CrossRef]
- Wilson, C.; Tisdell, C. Economics, Ecology and the Environment. Why Farmers Continue to Use Pesticides Despite Environmental, Health and Sustainability Cost. Working paper No. 53. The University of Queensland: Australia, 2004. Available online: http://espace.library.uq.edu.au/view/UQ:154961/WP53.pdf (accessed on 21 June 2015).
- Khan, A.R.; Selman, B.J. Nosema spp. (Microspora: Microsporida: Nosematidae) of stored-product Coleoptera and their potential as microbial control agents. Agric. Zool. Rev. 1989, 3, 193–223. [Google Scholar]
- Ngowi, A.V.F.; Mbise, T.J.; Ijani, A.S.M.; London, L.; Ajayi, O.C. Smallholder vegetable farmers in Northern Tanzania: Pesticides use practices, perceptions, and cost and health effects. Crop Prot. 2007, 26, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakash, S.A.; Mohan, S.; Ramaraju, K. Egg removal device for the management of three stored product pests. In Proceedings of the 10th International Working Conference on Stored Product Protection, Estoril, Portugal, 27 June–2 July 2010; Volume 425, pp. 673–677.
- Banks, H.J. Impact, physical removal and exclusion for insect control in stored products. In Proceedings of the 4th International Work Conference Store Products Protection, Tel Aviv, Israel, 21–26 September 1986; pp. 165–184.
- Paliwal, J.; Jayas, D.S.; White, N.D.G.; Muir, W.E. Effect of pneumatic conveying of wheat on mortality of insects. Appl. Eng. Agric. 1999, 15, 65–68. [Google Scholar] [CrossRef]
- White, N.D.; Jayas, D.S.; Demianyk, C.J. Movement of grain to control stored-product insects and mites. Phytoprotection 1997, 78, 75–84. [Google Scholar] [CrossRef]
- Facknath, S. Effect of grain tumbling on infestation by some insect pests. Rev. Agric. Sucr. Maurice 1993, 72, 5–8. [Google Scholar]
- Quentin, M.E.; Spencer, J.L.; Miller, J.R. Bean tumbling as a control measure for the common bean weevil, Acanthoscelides obtectus. Entomol. Exp. Et Appl. 1991, 60, 105–109. [Google Scholar] [CrossRef]
- Bbosa, D. Pesticide Free Methods of Maize Weevil Control in Stored Maize for Developing Countries. Chapter 4: Effect of Storage Container Physical Disturbance on Maize Weevil Mortality. Master’s Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar]
- Muir, W.E.; Yacuik, G.; Sinha, R.N. Effects of temperature and insect and mite population of turning and transferring farm-stored wheat. Can. Agric. Eng. 1977, 19, 25–28. [Google Scholar]
- Joffe, A.; Clarke, B. The effect of physical disturbance or turning of stored maize on the development of insect infestations-II. Laboratory studies with Sitophilus oryzae (L.). S. Afr. J. Sci. 1963, 6, 65–84. [Google Scholar]
- Joffe, A. The effect of physical disturbance or turning of stored maize on the development of insect infestation-I. Grain elevation studies. S. Afr. J. Sci. 1963, 6, 55–64. [Google Scholar]
- Schuler, N.J.; Bern, C.J.; Loy, D.D.; Brumm, T.J.; Strohbehn, D.R. Mixing beef feed rotations containing distillers’ wet grains. Appl. Eng. Agric. 2014, 30, 199–204. [Google Scholar]
- Omotoso, O.T.; Oso, A.A. Insecticidal and insect productivity reduction capacities of Aloe vera and Bryophyllum pinnatum on Tribolium castaneum (Herbst). Afr. J. Appl. Zool. Environ. Bio. 2005, 7, 95–100. [Google Scholar]
- Bailey, S.W. The effects of percussion on insect pests of grain. J. Econ. Entomol. 1962, 55, 301–310. [Google Scholar] [CrossRef]
- Bailey, S.W. The effects of physical stress in the grain weevil, Sitophilus granarius. J. Stored Prod. Res. 1969, 5, 311–324. [Google Scholar] [CrossRef]
- Bryan, J.M.; Elvidge, J. Mortality of adult grain beetles in sample delivery systems used in terminal grain elevators. Can. Entomol. 1977, 109, 209–213. [Google Scholar] [CrossRef]
- Loschiav, S.R. Effect of disturbance of wheat on four species of stored-product insects. J. Econ. Entomol. 1978, 71, 888–893. [Google Scholar] [CrossRef]
- Ungsunantwiwat, A.; Mills, R.B. Influence of medium and physical disturbances during rearing on development and numbers of Sitophilus progeny. J. Stored Prod. Res. 1979, 15, 37–42. [Google Scholar] [CrossRef]
- Plarre, R.; Reichmuth, F. Impact. In Alternatives to Pesticides in Stored-Product IPM; Springer Science and Business Media: New York, NY, USA, 2000; pp. 401–417. [Google Scholar]
- Facknath, S. Combination of neem and physical disturbance for the control of four insect pests of stored products. Int. J. Trop. Insect Sci. 2006, 26, 16–27. [Google Scholar] [CrossRef]
- Bahr, I. Reduction of stored product insects during pneumatic unloading of ship cargoes. In Proceedings of the 5th International Working Conference on Stored Products Protection, Bordeaux, France, 9–14 September 1990.
- Ng, H.F.; Wilcke, W.F.; Morey, R.V.; Meronuck, R.A.; Lang, J.P. Mechanical damage and corn storability. Trans. ASAE 1998, 41, 1095–1100. [Google Scholar] [CrossRef]
- Sharifi, S.; Mills, R.B. Radiographic studies of Sitophilus zeamais mots. In wheat kernels. J. Stored Prod. Res. 1971, 7, 195–206. [Google Scholar] [CrossRef]

| Region | District | Ward | Village | Number of Farmers | Number of Containers |
|---|---|---|---|---|---|
| Dodoma | Chamwino | Ikawa | Makoja | 3 | 36 |
| Morogoro | Kilosa | Mabwerebwere | Muungano | 3 | 36 |
| Manyara | Babati | Gallapo | Gallapo Mjini | 1 | 12 |
| Gallapo Kati | 1 | 12 | |||
| Chalo B | 1 | 12 |
| Storage Time (days) | Initial Number of S. zeamais | |||||
|---|---|---|---|---|---|---|
| Dodoma | Morogoro | Manyara | ||||
| Control | Disturbed | Control | Disturbed | Control | Disturbed | |
| 30 | 89 | 53 | 28 | 21 | 75 | 30 |
| 60 | 52 | 54 | 25 | 27 | 73 | 41 |
| 90 | 74 | 51 | 23 | 20 | 120 | 86 |
| Storage Time (days) | Control | Treatment | ||||
|---|---|---|---|---|---|---|
| Dodoma | Morogoro | Manyara | Dodoma | Morogoro | Manyara | |
| 30 | 10 ± 12 a | 43 ± 13 a | 32 ± 9 a | 91 ± 4 a | 96 ± 4 a | 98 ± 4 a |
| 60 | 8 ± 2 b | 24 ± 17 b | 6 ± 1 b | 95 ± 1 a | 89 ± 6 b | 100 ± 1 a |
| 90 | 6 ± 5 b | 21 ± 11 b | 10 ± 17 b | 99 ± 1 a | 98 ± 3 a | 100 ± 0 a |
| Storage Time (days) | Control (stationary) | Disturbed (shaken) | ||||
|---|---|---|---|---|---|---|
| Dodoma | Morogoro | Manyara | Dodoma | Morogoro | Manyara | |
| 30 | 20 ± 8 c | 9 ± 2 c | 12 ± 4 c | 10 ± 2 a | 2 ± 1 a | 3 ± 1 a |
| 60 | 68 ± 31 b | 49 ± 35 b | 77 ± 44 b | 2 ± 1 b | 5 ± 1 a | 0 ± 0 a |
| 90 | 109 ± 22 a | 119 ± 35 a | 152 ± 36 a | 0 ± 0 b | 0 ± 0 a | 0 ± 0 a |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleiman, R.; Rosentrater, K.A.; Chove, B. Periodic Physical Disturbance: An Alternative Method for Controlling Sitophilus zeamais (Maize Weevil) Infestation. Insects 2016, 7, 51. https://doi.org/10.3390/insects7040051
Suleiman R, Rosentrater KA, Chove B. Periodic Physical Disturbance: An Alternative Method for Controlling Sitophilus zeamais (Maize Weevil) Infestation. Insects. 2016; 7(4):51. https://doi.org/10.3390/insects7040051
Chicago/Turabian StyleSuleiman, Rashid, Kurt A. Rosentrater, and Bernard Chove. 2016. "Periodic Physical Disturbance: An Alternative Method for Controlling Sitophilus zeamais (Maize Weevil) Infestation" Insects 7, no. 4: 51. https://doi.org/10.3390/insects7040051






