Distribution, Pest Status and Fungal Associates of Euwallacea nr. fornicatus in Florida Avocado Groves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Area-Wide Survey for E. nr. fornicatus Beetles in the Avocado Production Region of Miami-Dade County, Florida
2.2. Fungal Isolations from Plant Tissue and Beetles
3. Results
3.1. Area-Wide Survey for E. nr. fornicatus Beetles in the Avocado Production Region of Miami-Dade County, Florida
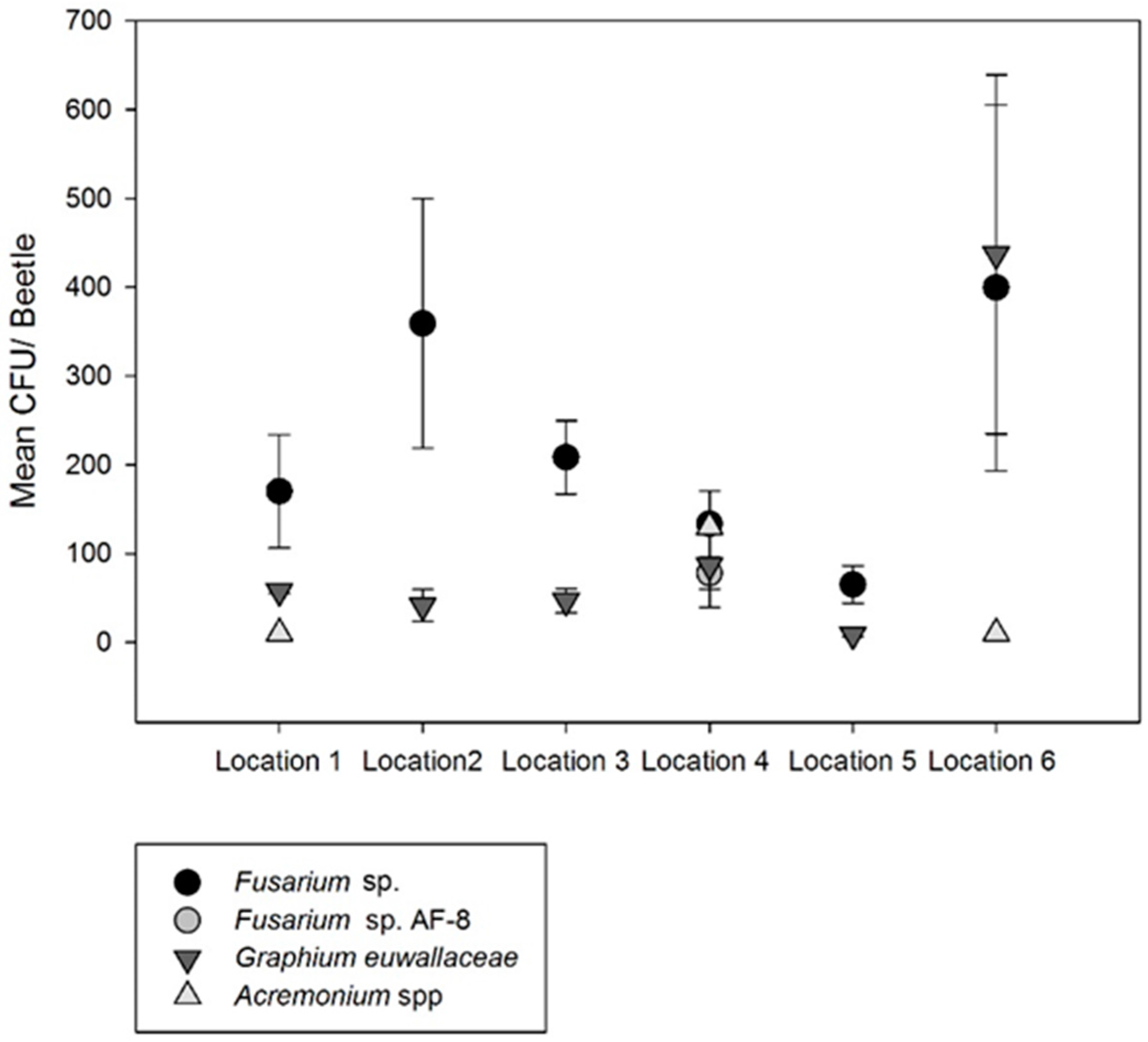
3.2. Fungal Isolations from Plant Tissue and Beetles
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carrillo, D.; Duncan, R.E.; Ploetz, J.N.; Campbell, A.F.; Ploetz, R.C.; Peña, J.E. Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol. 2014, 63, 54–62. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sink, S.; Ran, L.H.R.; Hulcr, J.; Kasson, M.T.; Ploetz, C.R.; Konkol, J.L.; Ploetz, J.N.; Carrillo, D.; Campbell, A.; et al. Discordant phylogenies suggest repeated host shifts in the Fusarium–Euwallacea ambrosia beetle mutualism. Fungal Genet. Biol. 2015, 82, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Eskalen, A. Host range of Fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in Southern California. Plant Dis. 2013, 97, 938–951. [Google Scholar] [CrossRef]
- Mendel, Z.; Protasov, A.; Sharon, M.; Zveibil, A.; Yehuda, S.B.; O’Donnell, K.; Rabaglia, R.; Wysoki, M.; Freeman, S. An Asian ambrosia beetle Euwallacea fornicatus and its novel symbiotic fungus Fusarium sp. pose a serious threat to the Israeli avocado industry. Phytoparasitica 2012, 40, 235–238. [Google Scholar] [CrossRef]
- Freeman, S.; Sharon, M.; Maymon, M.; Mendel, Z.; Protasov, A.; Aoki, T.; Eskalen, A.; O’Donnell, K. Fusarium euwallaceae sp. nov.—A symbiotic fungus of Euwallacea sp., an invasive ambrosia beetle in Israel and California. Mycologia 2013, 105, 1595–1606. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.C.; Twizeyimana, M.; Mayorquin, J.S.; Wang, D.H.; Na, F.; Kayim, M.; Kasson, M.T.; Thu, P.Q.; Bateman, C.; Rugman-Jones, P.; et al. Identification, pathogenicity and abundance of Paracremonium pembeum sp. nov. and Graphium euwallaceae sp. nov. two newly discovered mycangial associates of the polyphagous shot hole borer (Euwallacea sp.) in California. Mycologia 2016, 108, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, D.; Duncan, R.; Peña, J.E. Ambrosia beetles (Curculionidae: Scolytinae) that breed in avocado wood in Florida. Fla. Entomol. 2012, 95, 573–579. [Google Scholar] [CrossRef]
- Kasson, M.T.; O’Donnell, K.; Rooney, A.P.; Sink, S.; Ploetz, R.C.; Ploetz, J.N.; Konkol, J.L.; Carrillo, D.; Freeman, S.; Mendel, Z.; et al. Aninordinate fondness for Fusarium: Phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal Genet. Biol. 2013, 56, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Cooperband, M.F.; Stouthamer, R.; Carrillo, D.; Eskalen, A.; Thibault, T.; Cossé, A.A.; Castrillo, L.A.; Vandenberg, J.D.; Rugman-Jones, P.F. Biology of two members of the Euwallacea fornicatus species complex (Coleoptera: Curculionidae: Scolytinae), recently invasive in the USA, reared on an ambrosia beetle artificial diet. Agric. For. Entomol. 2016, 18, 223–237. [Google Scholar] [CrossRef]
- Carrillo, D.; Narvaez, T.; Cossé, A.A.; Stouthamer, R.; Cooperband, M.F. Attraction of Euwallacea nr. fornicatus (Coleoptera: Curculionidae: Scolytinae) to lures containing quercivorol. Fla. Entomol. 2015, 98, 780–782. [Google Scholar]
- Carrillo, D.; IFAS-Tropical Research and Education Center, University of Florida, Homestead, FL, USA. Personal observation, 2015.
- De La Torre, C.; Tree Injection Systems LLC. Homestead, FL, USA. Personal Communication, 2016.
- United States Department of Agriculture, National Agricultural Statistics Service. Census of Agriculture 2012. Available online: https://quickstats.nass.usda.gov/results/2126FF9A-4DBC-3F8E-9BB8-CA1976617919 (accessed on 11 October 2016).
- United States Department of Agriculture, National Agricultural Statistics Service. Census of Agriculture 2012. Available online: https://quickstats.nass.usda.gov/results/AA8D651A-9946-3384-8098-D0D9FDEAC0FC (accessed on 11 October 2016).
- Kendra, P.E.; Narvaez, T.I.; Montgomery, W.S.; Carrillo, D. Ambrosia beetle communities in forest and agricultural ecosystems with laurel wilt disease. In Proceedings of the 53rd Annual Meeting of the Entomological Society of America, Minneapolis, MN, USA, 15–18 November 2015.
- Doyle, J.; Doyle, J.L. Genomic plant DNA preparation from fresh tissue—CTAB method. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Dole, S.A.; Jordal, B.H.; Cognato, A.I. Polyphyly of Xylosandrus Reitter inferred from nuclear and mitochondrial genes (Coleoptera: Curculionidae: Scolytinae). Mol. Phylogenetics Evol. 2010, 54, 773–782. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 95, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4239–4246. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenies. In PCR Protocols, a Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Hiom, S.J.; Wade, W.G. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 1998, 64, 795–799. [Google Scholar] [PubMed]
- Carrillo, D.; IFAS-Tropical Research and Education Center, University of Florida, Homestead, FL, USA. Personal observation, 2016.
- Cooperband, M.C.; Otis Laboratory, Plant Protection and Quarantine’s Science and Technology, Animal and Plant Health Inspection Service, United States Department of Agriculture, Buzzards Bay, MA, USA. Personal communication, 2013.
- Danthanarayana, W. The distribution and host-range of the shot-hole borer (Xyleborus fornicatus Eichh.) of tea. Tea Q. 1968, 39, 61–69. [Google Scholar]
- Freeman, S.; Sharon, M.; Dori-Bachash, M.; Maymon, M.; Belausov, E.; Maoz, Y.; Margalit, O.; Protasov, A.; Mendel, Z. Symbiotic association of three fungal species throughout the life cycle of the ambrosia beetle Euwallacea nr. fornicatus. Symbiosis 2016, 68, 115–128. [Google Scholar] [CrossRef]
- Bugg, T.D.; Ahmad, M.; Hardiman, E.M.; Singh, R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr. Opin. Biotechnol. 2011, 22, 394–400. [Google Scholar] [CrossRef] [PubMed]
- White, D.C.; Sutton, S.D.; Ringelberg, D.B. The genus Sphingomonas: Physiology and ecology. Curr. Opin. Biotechnol. 1996, 7, 301–306. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, Y.; Fenf, J. Analysis of both chitinase and chitosanase produced by Sphingomonas sp. CJ-5. J. Zhejiang Univ. Sci. 2007, 8, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Doty, S.L.; Oakley, B.; Xin, G.; Kang, J.W.; Singleton, G.; Khan, Z.; Vajzovic, A.; Staley, J.T. Diazotrophic endophytes of native black cottonwood and willow. Symbiosis 2009, 47, 23–33. [Google Scholar] [CrossRef]
- Vandamme, P.; Bernardet, J.F.; Segers, P.; Kersters, K.; Holmes, B. New Perspectives in the Classification of the Flavobacteria: Description of Chryseobacterium gen. nov., Bergeyella gen. nov., and Empedobacter nom. rev. Int. J. Syst. Evol. Microbiol. 1994, 44, 827–831. [Google Scholar] [CrossRef]
- Park, D.; Oh, H.; Jeong, W.; Kim, H.; Park, H.; Bae, K.S. A culture-based study of the bacterial communities within the guts of nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. J. Microbiol. Seoul 2007, 45, 394–401. [Google Scholar]
- Cho, M.J.; Kim, Y.H.; Shin, K.; Kim, Y.K.; Kim, Y.S.; Kim, T.J. Symbiotic adaptation of bacteria in the gut of Reticulitermes speratus: Low endo-β-1, 4-glucanase activity. Biochem. Biophys. Res. Commun. 2010, 395, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Swings, J.; Lambert, B.; Kersters, K.; Holmes, B. The genera Phyllobacterium and Ochrobactrum. In The Prokaryotes, a handbook on the biology of bacteria, ecophysiology, isolation, identification and applications, 3th ed.; Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer Science & Business Media: New York, NY, USA, 2006; Volume 5, pp. 747–750. [Google Scholar]
- Schäfer, A.; Konra, R.; Kuhnigk, T.; Kämpfer, P.; Hertel, H.; König, H. Hemicellulose-degrading bacteria and yeasts from the termite gut. J. Appl. Bacteriol. 1996, 80, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Cortés, N.; Quesada, M.; Watanabe, H.; Cano-Camacho, H.; Oyama, K. Endogenous plant cell wall digestion: A key mechanism in insect evolution. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 45–71. [Google Scholar] [CrossRef]
- Oliver, K.M.; Degnen, P.H.; Burke, G.R.; Moran, N.A. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 2010, 55, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Van der Walt, J.P.; Von Arx, J.A.; Ferreira, N.P.; Richards, P.D.G. Zygozyma, a new genus of the Lipornycetaceae. Syst. Appl. Microbiol. 1987, 9, 115–120. [Google Scholar] [CrossRef]
- Van der Walt, J.P.; Wingfield, M.J.; Yamada, Y. Zygozyma smithiae sp. n. (Lipomycetaceae), a new ambrosia yeast from Southern Africa. Antonie Van Leeuwenhoek 1990, 58, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.P.; Robnett, C.J.; Yarrow, D. Two new anamorphic yeasts: Candida germanica and Candida neerlandica. Antonie Van Leeuwenhoek 2001, 80, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.O.; Nguyen, N.H.; Blackwell, M. Yeasts isolated from plant-associated beetles and other insects: Seven novel Candida species near Candida albicans. FEMS Yeast Res. 2008, 8, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.H.; Suh, D.Y.; Yoo, H.D.; Oh, M.H.; Kim, S.H. Yeast Associated with the Ambrosia Beetle, Platypus koryoensis, the Pest of Oak Trees in Korea. Mycobiology 2015, 43, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Landell, M.F.; Brandão, L.R.; Barbosa, A.C.; Ramos, J.P.; Safar, S.V.; Gomes, F.C.; Sousa, F.M.; Morais, P.B.; Broetto, L.; Leoncini, O.; et al. Hannaella pagnoccae sp. nov., a tremellaceous yeast species isolated from plants and soil. Int. J. Syst. Evol. Microbiol. 2014, 64, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, S.L.; Pagnocca, F.C.; da Paixão Melo, W.G.; Nagamoto, N.S.; Komura, D.L.; Rodrigues, A. Yeasts found on an ephemeral reproductive caste of the leaf-cutting ant Atta sexdens rubropilosa. Antonie Van Leeuwenhoek 2014, 106, 475–487. [Google Scholar] [CrossRef] [PubMed]

| Location Coordinates | Isolation Source | Isolate ID | Sample | Frequency n/N | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||
| Location 1 25°27′53″ N 80°31′6″ W | Plant tissue | Fusarium sp. | x | x | x | x | x | 5/5 | |||||
| Beetle | Fusarium sp. | x | x | x | x | 4/5 | |||||||
| G. euwallaceae | x | x | 2/5 | ||||||||||
| Location 2 25°32′16″ N 80°29′11″ W | Plant tissue | Fusarium sp. | x | x | x | x | x | ||||||
| Beetle | Fusarium sp. | x | x | x | x | x | 5/5 | ||||||
| G. euwallaceae | x | x | x | x | 4/5 | ||||||||
| Location 3 25°32′28″ N 80°25′44″ W | Plant tissue | Fusarium sp. | x | x | x | x | x | ||||||
| Beetle | Fusarium sp. | x | x | x | 3/5 | ||||||||
| G. euwallaceae | x | x | x | 3/5 | |||||||||
| A. masseei | x | 1/5 | |||||||||||
| Location 4 25°29′8″ N 80°32′11″ W | Plant tissue | Fusarium sp. | x | x | x | x | x | ||||||
| Beetle | Fusarium sp. | x | x | 2/5 | |||||||||
| AF-8 | x | x | 2/5 | ||||||||||
| G. euwallaceae | x | x | 2/5 | ||||||||||
| Acremonium sp. | x | 1/5 | |||||||||||
| Elaphocordyceps sp. | x | 1/5 | |||||||||||
| Location 5 25°35′50″ N 80°30′16″ W | Plant tissue | Fusarium sp. | x | x | x | x | x | 5/5 | |||||
| Beetle | Fusarium sp. | x | x | x | x | x | 5/5 | ||||||
| G. euwallaceae | x | x | x | x | x | 5/5 | |||||||
| A. morum | x | 1/5 | |||||||||||
| Location 6 25°31′31″ N 80°30′16″ W | Plant tissue | Fusarium sp. | x | x | x | 3/5 | |||||||
| AF-8 | x | 1/5 | |||||||||||
| AF-6 | x | 1/5 | |||||||||||
| Beetle | Fusarium sp. | x | x | x | x | x | x | x | x | x | 9/10 | ||
| G. euwallaceae | x | x | x | x | x | x | 5/10 | ||||||
| Acremonium sp. | x | 1/10 | |||||||||||
| Elaphocordyceps sp. | x | 1/10 | |||||||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo, D.; Cruz, L.F.; Kendra, P.E.; Narvaez, T.I.; Montgomery, W.S.; Monterroso, A.; De Grave, C.; Cooperband, M.F. Distribution, Pest Status and Fungal Associates of Euwallacea nr. fornicatus in Florida Avocado Groves. Insects 2016, 7, 55. https://doi.org/10.3390/insects7040055
Carrillo D, Cruz LF, Kendra PE, Narvaez TI, Montgomery WS, Monterroso A, De Grave C, Cooperband MF. Distribution, Pest Status and Fungal Associates of Euwallacea nr. fornicatus in Florida Avocado Groves. Insects. 2016; 7(4):55. https://doi.org/10.3390/insects7040055
Chicago/Turabian StyleCarrillo, Daniel, Luisa F. Cruz, Paul E. Kendra, Teresa I. Narvaez, Wayne S. Montgomery, Armando Monterroso, Charlotte De Grave, and Miriam F. Cooperband. 2016. "Distribution, Pest Status and Fungal Associates of Euwallacea nr. fornicatus in Florida Avocado Groves" Insects 7, no. 4: 55. https://doi.org/10.3390/insects7040055






