Efficacy of Topical Application, Leaf Residue or Soil Drench of Blastospores of Isaria fumosorosea for Citrus Root Weevil Management: Laboratory and Greenhouse Investigations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Citrus Leaves and Plants
2.2. Insects
2.3. Fungal Blastospore Preparation and Viability
2.4. Topical Spray Application
2.5. Detached Leaf Bioassay
2.6. Soil Drench Experiment
2.7. Statistical Analysis
3. Results
3.1. Topical Spray Application
3.2. Detached Leaf Bioassay
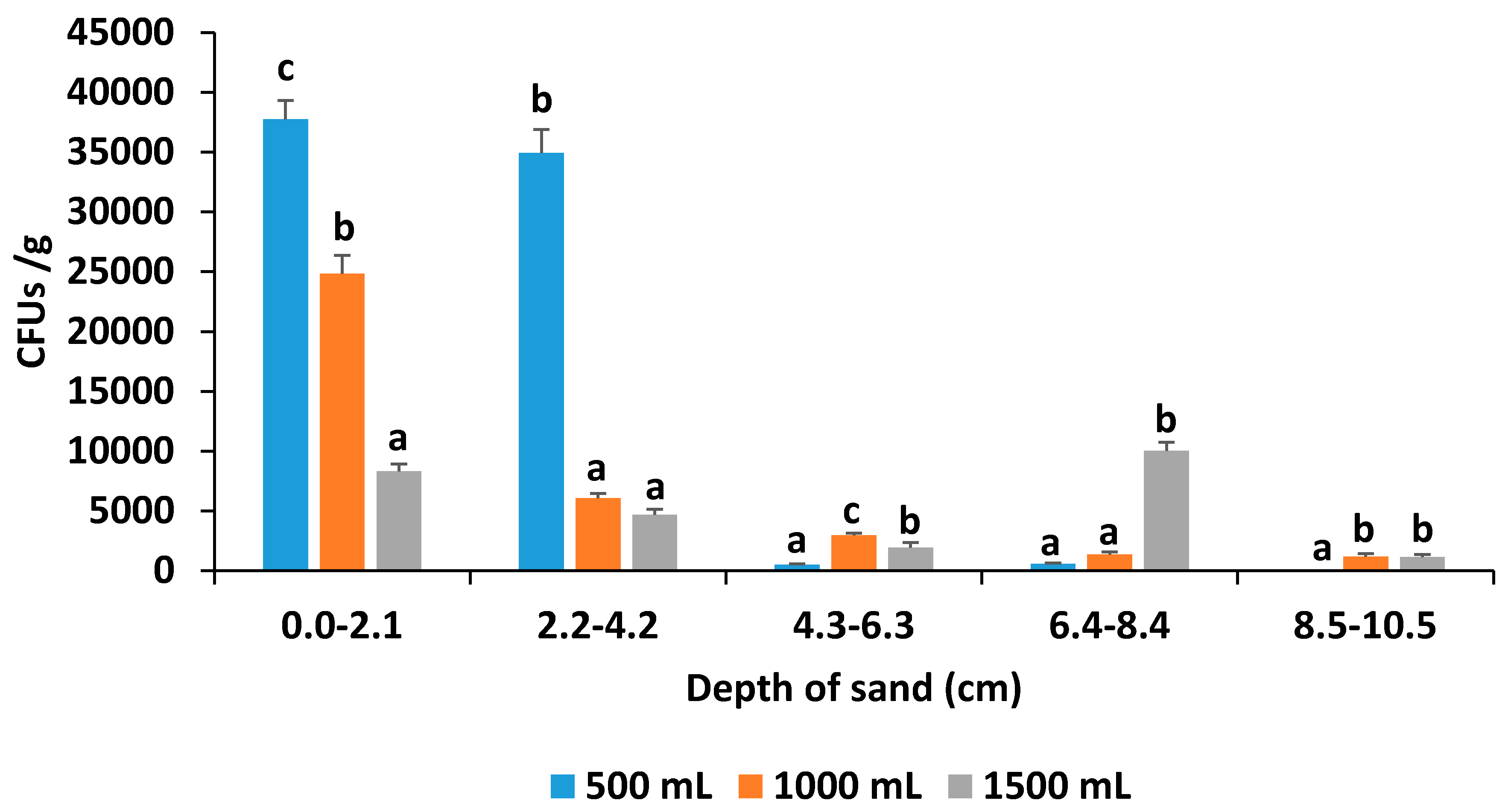
3.3. Soil Drench Experiment
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DRW | Diaprepes root weevil |
| IPM | Integrated pest management |
References
- Woodruff, R.E. A Puerto Rican weevil new to the United States (Coleoptera: Curculionidae). Fla. DPI Entomol. Circ. 1964, 30, 1–2. [Google Scholar]
- Duncan, L.W.; Rogers, M.E.; McCoy, C.W.; Futch, S.H.; Graham, J.H. Florida citrus pest management guide: Citrus root weevils. Available online: http://edis.ifas.ufl.edu/cg006 (accessed on 28 December 2015).
- Rogers, S.; Graham, J.H.; McCoy, C.W. Insect-plant interactions: Preliminary studies of Diaprepes root weevil injuries and Phytophthora infections. Proc. Fla. State Hort. Soc. 1996, 109, 57–62. [Google Scholar]
- Graham, J.H.; Bright, D.B.; McCoy, C.W. Phytophthora-Diaprepes weevil complex: Phytophthora spp. relationship to citrus rootstocks. Plant Dis. 2003, 87, 85–90. [Google Scholar] [CrossRef]
- Lapointe, S.L.; Shapiro, J.P. Effect of soil moisture on development of Diaprepes abbreviatus (Coleoptera: Curculionidae). Fla. Entomol. 1999, 82, 291–299. [Google Scholar] [CrossRef]
- Puterka, G.J. Fungal pathogens for arthropod pest control in orchard systems: Mycoinsecticidal approach for pear psylla control. Biocontrol 1999, 44, 183–210. [Google Scholar] [CrossRef]
- Subandiyah, S.; Nikoh, N.; Sato, H.; Wagiman, F.; Tsuyumu, S.; Fukatsu, T. Isolation and characterization of two entomopathogenic fungi attacking Diaphorina citri (Homoptera, Psylloidea) in Indonesia. Mycoscience 2000, 41, 509–513. [Google Scholar] [CrossRef]
- Slininger, P.; Behle, R.W.; Jackson, M.A.; Schisler, D.A. Discovery and development of biological agents to control crop pests. Neotrop. Entomol. 2003, 32, 183–195. [Google Scholar] [CrossRef]
- Dolinski, C.; Lacey, L. Microbial control of arthropod pests of tropical tree fruits. Neotrop. Entomol. 2007, 36, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Shapiro-Ilan, D.I. Microbial control of insect pests in temperate orchard systems: Potential for incorporation into IPM. Annu. Rev. Entomol. 2008, 53, 121–144. [Google Scholar] [CrossRef] [PubMed]
- Guerri-Agullo, B.; Gomez-Vidal, S.; Asensio, L.; Barranco, P.; Lopez-Llorca, L. Infection of the red palm weevil (Rhynchophorus ferrugineus) by entomopathogenic fungus Beauveria bassiana: A SEM study. Microsc. Res. Technol. 2010, 73, 714–725. [Google Scholar]
- Vallet-Gely, I.; Lemaitre, B.; Boccard, F. Bacterial strategies to overcome insect defenses. Nat. Rev. Microbiol. 2008, 6, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.; Leger, R. Interactions between fungal pathogens and insect hosts. Annu. Rev. Entomol. 1994, 39, 293–322. [Google Scholar] [CrossRef]
- Beavers, J.B.; McCoy, C.W.; Kanavel, R.F.; Sutton, R.A.; Selhime, A.G. Two muscardine fungi pathogenic to Diaprepes abbreviatus. Fla. Entomol. 1972, 55, 117–120. [Google Scholar] [CrossRef]
- Beavers, J.B.; Kaplan, D.T.; McCoy, C.W. Natural enemies of Diaprepes abbreviatus larvae in Florida. Proc. Fla. State Hortic. Soc. 1982, 95, 63–65. [Google Scholar]
- Peña, J.; Giblin-Davis, R.; Duncan, R. Impact of indigenous Beauveria bassiana (Balsamo) Vuillemin on banana weevil and rotten sugarcane weevil (Coleoptera: Curculionidae) populations in banana in Florida. J. Agric. Entomol. 1995, 12, 163–167. [Google Scholar]
- Hussain, A.; Rizwan-ul-Haq, M.; Al-Ayedh, H.; Ahmed, S.; Al-Jabr, A.M. Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. BioControl 2015, 60, 849–859. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.M.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Montemayor, C.; Avery, P.B.; Cave, R.D. Infection and mortality of Microtheca ochroloma (Coleoptera: Chrysomelidae) by Isaria fumosorosea (Hypocreales: Cordycipitaceae) under laboratory conditions. Biocontrol Sci. Technol. 2016, 26, 605–616. [Google Scholar] [CrossRef]
- Luangsa-Ard, J.; Houbraken, J.; van Doorn, T; Hong, S.B.; Borman, A.M.; Hywel-Jones, N.L.; Samson, R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011, 321, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Hoy, M.A.; Boucias, D.G. Isolation and characterization of an Isaria fumosorosea isolate infecting the Asian citrus psyllid in Florida. J. Invertebr. Pathol. 2008, 99, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Avery, P.B.; Hunter, W.B.; Hall, D.G.; Jackson, M.A.; Powell, C.A.; Rogers, M.E. Diaphorina citri (Hemiptera: Psyllidae) infection and dissemination of the entomopathogenic fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) under laboratory conditions. Fla. Entomol. 2009, 92, 608–618. [Google Scholar] [CrossRef]
- Hoy, M.; Singh, R.; Rogers, M.E. Evaluations of A novel isolate of Isaria fumosorosea for control of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Fla. Entomol. 2010, 93, 24–32. [Google Scholar] [CrossRef]
- Avery, P.B.; Wekesa, V.W.; Hunter, W.B.; Hall, D.G.; McKenzie, C.L.; Osborne, L.S.; Powell, C.A.; Rogers, M.E. Effects of the fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci. Technol. 2011, 21, 1065–1078. [Google Scholar] [CrossRef]
- Stauderman, K.; Avery, P.; Aristizabal, L.; Arthurs, S. Evaluation of Isaria fumosorosea (Hypocreales: Cordycipitaceae) for control of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci. Technol. 2012, 22, 747–761. [Google Scholar] [CrossRef]
- Avery, P.B.; Pick, D.A.; Aristizábal, L.F.; Kerrigan, J.; Powell, C.A.; Rogers, M.E.; Arthurs, S.P. Compatibility of Isaria fumosorosea (Hypocreales: Cordycipitaceae) blastospores with agricultural chemicals used for management of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae). Insects 2013, 4, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Zermeño, M.A.; Gallou, A.; Berlanga-Padilla, A.M.; Serna-Domínguez, M.G.; Arredondo-Bernal, H.C.; Montesinos-Matías, R. Characterisation of entomopathogenic fungi used in the biological control programme of Diaphorina citri in Mexico. Biocontrol Sci. Technol. 2015, 25, 1192–1207. [Google Scholar] [CrossRef]
- Casique-Valdés, R.; Sánchez-Lara, B.M.; Ek-Mass, J.; Hernández-Guerra, C.; Bidochka, M.; Guízar-Guzmán, L.; López-Arroyo, J.; Sánchez-Peña, S.R. Field trial of aqueous and emulsion preparations of entomopathogenic fungi against the Asian citrus psyllid (Hemiptera: Liviidae) in a lime orchard in Mexico. J. Entomol. Sci. 2015, 50, 79–87. [Google Scholar]
- Hunter, W.B.; Avery, P.B.; Pick, D.; Powell, C.A. Broad spectrum potential of Isaria fumosorosea on insect pests of citrus. Fla. Entomol. 2011, 94, 1051–1054. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Erhan, S.; Poprawski, T.J. Influence of formulation additives on the desiccation tolerance and storage stability of blastospores of the entomopathogenic fungus Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes). Biocontrol Sci. Technol. 2006, 16, 61–75. [Google Scholar] [CrossRef]
- McCoy, C.W.; Stuart, R.J.; Duncan, L.W.; Shapiro-Ilan, D.I. Application and evaluation of entomopathogens for citrus pest control. In Field Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Kaya, H.K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 567–581. [Google Scholar]
- Goettel, M.S.; Inglis, G.D. Fungi: Hyphomycetes. In Manual of Techniques in Insect Pathology; Lacey, L.A., Ed.; Academic Press: London, UK, 1997; pp. 213–249. [Google Scholar]
- Yaginuma, D.; Hiromori, H.; Hatsukade, M. Friction-associated conidial detachment of the entomopathogenic fungus Beauveria amorpha from the cuticle of a scarab grub, Anomala cuprea, in the soil. Appl. Entomol. Zool. 2006, 41, 613–620. [Google Scholar] [CrossRef]
- Quintela, E.D.; McCoy, C.W. Synergistic effect of imidacloprid and two entomopathogenic fungi on the behavior and survival of larvae of Diaprepes abbreviatus (Coleoptera: Curculionidae) in soil. J. Econ. Entomol. 1998, 91, 110–122. [Google Scholar] [CrossRef]
- Gillett-Kaufman, J.L.; Kimbrough, J.W. A modified method to visualize infection sites of spores of the entomopathogen Beauveria bassiana (Deuteromycotina: Hyphomycetes) on the exoskeleton of citrus root weevil Diaprepes abbreviatus (Coleoptera: Curculionidae) adults. Fla. Entomol. 2009, 92, 623–628. [Google Scholar] [CrossRef]
- Vega, F.E.; Meyling, N.V.; Luangsa-ard, J.J.; Blackwell, M. Fungal entomopathogens. In Insect Pathology, 2nd ed.; Kaya, H.K., Vega, F.E., Eds.; Academic Press: Waltham, MA, USA, 2012; pp. 171–220. [Google Scholar]
- Woods, S.P.; Grula, E.A. Utilizable surface nutrients on Heliothis zea available for growth of Beauveria bassiana. J. Invertebr. Pathol. 1984, 43, 259–269. [Google Scholar] [CrossRef]
- Sosa-Gomez, D.R.; Boucias, D.G.; Nation, J.L. Attachment of Metarhizium anisopliae to the southern green stink bug Nezara viridula cuticle and fungistatic effect of cuticular lipids and aldehydes. J. Invertebr. Pathol. 1997, 69, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.W.A.; Borden, J.H.; Rahe, J.E.; Whitney, H.S. Nutrient-mediated germination of Beauveria bassiana conidia on the integument of the bark beetle Dendroctonus ponderosae (Coleoptera: Scolytidae). J. Invertebr. Pathol. 1984, 44, 304–314. [Google Scholar] [CrossRef]
- Oritz-Urquiza, A.; Keyhani, N.O. Action on the surface: Entomopathogenic fungi versus the insect cuticle. Insects 2013, 4, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.K.; Vega, F.E. Scope and basic principles of insect pathology. In Insect Pathology, 2nd ed.; Kaya, H.K., Vega, F.E., Eds.; Academic Press: Waltham, MA, USA, 2012; pp. 1–12. [Google Scholar]
- Fargues, J.; Delmas, J.C.; Lebrun, R.A. Leaf consumption by larvae of the Colorado potato beetle (Coleoptera: Chrysomelidae) infected with the entomopathogen, Beauveria bassiana. J. Econ. Entomol. 1994, 87, 67–71. [Google Scholar] [CrossRef]
- Ekesi, S. Pathogenicity and antifeedant activity of entomopathogenic hyphomycetes to the cowpea leaf beetle, Ootheca mutabilis Shalberg. Int. J. Trop. Insect Sci. 2001, 21, 55–60. [Google Scholar] [CrossRef]
- Gámez Herrera, C.; Niño, A.A.; Avery, P.B.; Cave, R.D. Efecto del hongo Isaria fumosorosea Wize sobre la supervivencia y el consumo de los adultos del escarabajo demargen amarillo, Microtheca ochroloma Stål (Coleoptera: Chrysomelidae). CEIBA 2016, in press. [Google Scholar]
- Duncan, L.W.; McCoy, C.W. Vertical distribution in soil, persistence, and efficacy against citrus root weevil (Coleoptera: Curculionidae) of two species of entomogenous nematodes (Rhabditida: Steinernematidae: Heterorhabditidae). Environ. Entomol. 1996, 25, 174–178. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Fuzy, E.M.; Crocker, R.L.; Gelernter, W.D.; Polavarapu, S. Pathogenicity of Steinernema scarabaei, Heterorhabditis bacteriophora and S. glaseri to twelve white grub species. Biocontrol Sci. Technol. 2004, 14, 87–92. [Google Scholar] [CrossRef]
- Koppenhöfer, A.M.; Grewal, P.S.; Fuzy, E.M. Virulence of the entomopathogenic nematodes Heterorhabditis bacteriophora, H. zealandica, and Steinernema scarabaei against five white grub species (Coleoptera: Scarabaeidae) of economic importance in turfgrass in North America. Biol. Control. 2006, 38, 397–404. [Google Scholar] [CrossRef]
- McCoy, C.W.; Duncan, L.W.; Quintela, E.D. A review of IPM strategies for citrus root weevils with emphasis on microbial control. Proc. Int. Soc. Citric. 1996, 1, 638–641. [Google Scholar]
- Georgis, R.; Gaugler, R. Predictability in biological control using entomopathogenic nematodes. J. Econ. Entomol. 1991, 84, 713–720. [Google Scholar] [CrossRef]
- Klein, M.G. Biological control of scarabs with entomopathogenic nematodes. In Nematodes and The Biological Control of Insect Pests; Bedding, R., Akhurst, R., Kaya, H.K., Eds.; CSIRO Publications: East Melbourne, Australia, 1993; pp. 49–57. [Google Scholar]
- Kaya, H.K. Natural enemies and other antagonists. In Entomopathogenic Nematology; Gaugler, R., Ed.; CABI Publishing: Wallingford, UK, 2002; pp. 189–204. [Google Scholar]
- Kaya, H.K.; Koppenhöfer, A.M. Effects of microbial and other antagonistic organisms and competition on entomopathogenic nematodes. Biocontrol Sci. Technol. 1996, 6, 357–371. [Google Scholar] [CrossRef]
- Kaya, H.K. Soil ecology. In Entomopathogenic Nematodes in Biological Control; Gaugler, R., Kaya, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 93–116. [Google Scholar]
- Smits, P.H. Post-application persistence of entomopathogenic nematodes. Biocontrol Sci. Technol. 1996, 6, 379–387. [Google Scholar] [CrossRef]
- Glazer, I. Survival biology. In Entomopathogenic Nematology; Gaugler, R., Ed.; CAB International: Wallingford, UK, 2002; pp. 169–187. [Google Scholar]
- Glare, T.R.; Placet, C.; Nelson, T.L.; Reay, S.D. Potential of Beauveria and Metarhizium as control agents of pinhole borers (Platypus spp.). NZ Plant Protect. J. 2002, 55, 73–79. [Google Scholar]
- Mohammadyani, M.; Karimi, J.; Taheri, P.; Sadeghi, H.; Zare, R. Entomopathogenic fungi as promising biocontrol agents for the rosaceous longhorn beetle, Osphranteria coerulescens. BioControl 2016, 6, 579–590. [Google Scholar] [CrossRef]
- Wraight, S.P.; Inglis, G.D.; Goettel, M.S. Fungi. In Field Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Kaya, H.K., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 223–248. [Google Scholar]
- Hasan, S. Entomopathogenic fungi as potent agents of biological control. IJETR 2014, 2, 234–237. [Google Scholar]
- Jaques, R.; Morris, O.N. Compatibility of pathogens with other methods of pest control and with different crops. In Microbial Control of Pests and Plant Disease 1970–1980; Burges, H.D., Ed.; Academic Press: New York, NY, USA, 1981; pp. 283–302. [Google Scholar]
- Anbesse, S.A.; Adge, B.J.; Gebru, W.M. Laboratory screening for virulent entomopathogenic nematodes (Heterorhabditis bacteriophora and Steinernema yirgalemense) and fungi (Metarhizium anisopliae and Beauveria bassiana) and assessment of possible synergistic effects of combined use against grubs of the barley chafer Coptognathus curtipennis. Nematology 2008, 10, 701–709. [Google Scholar]
- Ansari, M.A.; Tirry, L.; Moens, M. Interaction between Metarhizium anisopliae CLO 53 and entomopathogenic nematodes for the control of Hoplia philanthus. Biol. Control 2004, 31, 172–180. [Google Scholar] [CrossRef]
- Ansari, M.A.; Shah, F.A.; Tirry, L.; Moens, M. Field trials against Hoplia philanthus (Coleoptera: Scarabaeidae) with a combination of an entomopathogenic nematode and the fungus Metarhizium anisopliae CLO 53. Biol. Control 2006, 39, 453–459. [Google Scholar] [CrossRef]
- Choo, H.Y.; Kaya, H.K.; Huh, J.; Lee, D.W.; Kim, H.H.; Lee, S.M.; Choo, Y.M. Entomopathogenic nematodes (Steinernema spp. and Heterorhabditis bacteriophora) and a fungus Beauveria brongniartii for biological control of the white grubs, Ectinohoplia rufipes and Exomala orientalis, in Korean golf courses. Biol. Control 2002, 47, 177–192. [Google Scholar]
- Hussein, H.M.; Skoková Habuštová, O.; Půža, V.; Zemek, R. Laboratory evaluation of Isaria fumosorosea CCM 8367 and Steinernema feltiae Ustinov against immature stages of the Colorado potato beetle. PLoS ONE 2016, 11, e0152399. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, R.; Crowder, D.W.; Aultman, E.A.; Snyder, W.E. Entomopathogen biodiversity increases host mortality. Biol. Control 2011, 59, 277–283. [Google Scholar] [CrossRef]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avery, P.B.; Hunter, W.B.; Hall, D.G.; Jackson, M.A.; Powell, C.A. Efficacy of Topical Application, Leaf Residue or Soil Drench of Blastospores of Isaria fumosorosea for Citrus Root Weevil Management: Laboratory and Greenhouse Investigations. Insects 2016, 7, 66. https://doi.org/10.3390/insects7040066
Avery PB, Hunter WB, Hall DG, Jackson MA, Powell CA. Efficacy of Topical Application, Leaf Residue or Soil Drench of Blastospores of Isaria fumosorosea for Citrus Root Weevil Management: Laboratory and Greenhouse Investigations. Insects. 2016; 7(4):66. https://doi.org/10.3390/insects7040066
Chicago/Turabian StyleAvery, Pasco B., Wayne B. Hunter, David G. Hall, Mark A. Jackson, and Charles A. Powell. 2016. "Efficacy of Topical Application, Leaf Residue or Soil Drench of Blastospores of Isaria fumosorosea for Citrus Root Weevil Management: Laboratory and Greenhouse Investigations" Insects 7, no. 4: 66. https://doi.org/10.3390/insects7040066





