Biological Control beneath the Feet: A Review of Crop Protection against Insect Root Herbivores
Abstract
:1. Introduction
2. Viruses
3. Bacteria
4. Fungi
5. Nematodes
6. Macrofauna
7. Synergies between Different Bio-Control Agents
8. Interactions between Belowground Top-down and Bottom-up Forces
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Agriculture. In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Metz, D., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 497–540. [Google Scholar]
- Grassini, P.; Eskridge, K.M.; Cassman, K.G. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D. Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 1, pp. 89–111. [Google Scholar]
- Stehle, S.; Schulz, R. Pesticide authorization in the eu-environment unprotected? Environ. Sci. Pollut. Res. Int. 2015, 22, 19632–19647. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.A.; Nicholls, C.I.; Henao, A.; Lana, M.A. Agroecology and the design of climate change-resilient farming systems. Agron. Sustain. Dev. 2015, 35, 869–890. [Google Scholar] [CrossRef]
- Meehan, T.D.; Werling, B.P.; Landis, D.A.; Gratton, C. Agricultural landscape simplification and insecticide use in the midwestern united states. Proc. Natl. Acad. Sci. USA 2011, 108, 11500–11505. [Google Scholar] [CrossRef] [PubMed]
- Schoonhoven, L.M.; van Loon, J.J.A.; Dicke, M. Insect-Plant Biology; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Hunter, M.D. Out of sight, out of mind: The impacts of root-feeding insects in natural and managed systems. Agric. For. Entomol. 2001, 3, 3–9. [Google Scholar] [CrossRef]
- Koppenhofer, A.M.; Wilson, M.; Brown, I.; Kaya, H.K.; Gaugler, R. Biological control agents for white grubs (Coleoptera: Scarabaeidae) in anticipation of the establishment of the Japanese beetle in California. J. Econ. Entomol. 2000, 93, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gale, G. Saving the vine from Phylloxera. In Wine; CRC Press: Boca Raton, FL, USA, 2002; pp. 70–91. [Google Scholar]
- Johnson, S.N.; Benefer, C.M.; Frew, A.; Griffiths, B.S.; Hartley, S.E.; Karley, A.J.; Rasmann, S.; Schumann, M.; Sonnemann, I.; Robert, C.A. New frontiers in belowground ecology for plant protection from root-feeding insects. Appl. Soil Ecol. 2016, 108, 96–107. [Google Scholar] [CrossRef]
- Barsics, F.; Haubruge, E.; Verheggen, F.J. Wireworms’ management: An overview of the existing methods, with particular regards to Agriotes spp. (Coleoptera: Elateridae). Insects 2013, 4, 117–152. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.E.; Sappington, T.W.; Miller, N.J.; Moeser, J.; Bohn, M.O. Adaptation and invasiveness of western corn rootworm: Intensifying research on a worsening pest. Annu. Rev. Entomol. 2009, 54, 303–321. [Google Scholar] [CrossRef] [PubMed]
- Chandler, K. Strategies to Control Greyback Canegrub in Early Harvested Ratoon Crops; Bureau of Sugar Experiment Stations: Queensland, Australia, 2002; pp. 1–43. [Google Scholar]
- Van Geem, M.; Gols, R.; van Dam, N.M.; van der Putten, W.H.; Fortuna, T.; Harvey, J.A. The importance of aboveground-belowground interactions on the evolution and maintenance of variation in plant defense traits. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Hairston, N.G.; Smith, F.E.; Slobodkin, L.B. Community structure, population control, and competition. Am. Nat. 1960, 94, 421–425. [Google Scholar] [CrossRef]
- Price, P.W.; Bouton, C.E.; Gross, P.; McPheron, B.A.; Thompson, J.N.; Weis, A.E. Interactions among three trophic levels: Influence of plant on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 1980, 11, 41–65. [Google Scholar] [CrossRef]
- Kimura, M.; Jia, Z.-J.; Nakayama, N.; Asakawa, S. Ecology of viruses in soils: Past, present and future perspectives. Soil Sci. Plant Nutr. 2008, 54, 1–32. [Google Scholar] [CrossRef]
- Khachatourians, G.G. Production and use of biological pest control agents. Trends Biotechnol. 1986, 4, 120–124. [Google Scholar] [CrossRef]
- Erlandson, M. Insect pest control by viruses. In Encyclopedia of Virology; Mahy, B.W.J., van Regenmortel, M.H.V., Eds.; Elsevier: London, UK, 2008; Volume 3, pp. 125–133. [Google Scholar]
- Lapied, B.; Pennetier, C.; Apaire-Marchais, V.; Licznar, P.; Corbel, V. Innovative applications for insect viruses: Towards insecticide sensitization. Trends Biotechnol. 2009, 27, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moscardi, F.; de Souza, M.L.; de Castro, M.E.B.; Moscardi, M.L.; Szewczyk, B. Baculovirus pesticides: Present state and future perspectives. In Microbes and Microbial Technology: Agricultural and Environment Applications; Ahmad, I., Ahmad, F., Pitchel, J., Eds.; Springer: New York, NY, USA, 2011; pp. 415–445. [Google Scholar]
- Popham, H.J.; Nusawardani, T.; Bonning, B.C. Introduction to the use of baculoviruses as biological insecticides. In Baculovirus and Insect Cell Expression Protocols; Murhammer, D.W., Ed.; Humana Press: New York, NY, USA, 2016; Volume 388, pp. 383–392. [Google Scholar]
- Vega, F.E.; Kaya, H.K. Insect Pathology; Academic Press: London, UK, 2012. [Google Scholar]
- Beas-Catena, A.; Sánchez-Mirón, A.; García-Camacho, F.; Contreras-Gómez, A.; Molina-Grima, E. Baculovirus biopesticides: An overview. J. Anim. Plant Sci. 2014, 24, 362–373. [Google Scholar]
- Slack, J.; Arif, B.M. The baculoviruses occlusion-derived virus: Virion structure and function. Adv. Virus Res. 2007, 69, 99–165. [Google Scholar] [PubMed]
- Infante-Rodríguez, D.A.; Berber, J.J.; Mercado, G.; Valenzuela-González, J.; Muñoz, D.; Williams, T. Earthworm mediated dispersal of baculovirus occlusion bodies: Experimental evidence from a model system. Biol. Control 2016, 100, 18–24. [Google Scholar] [CrossRef]
- Nagamine, T.; Sako, Y. A role for the anti-viral host defense mechanism in the phylogenetic divergence in baculovirus evolution. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Kroschel, J.; Arthurs, S.P.; de La Rosa, F. Control microbiano de la palomilla de la papa phthorimaea operculella (lepidoptera: Gelechiidae). Rev. Colomb. Entomol. 2010, 36, 181–189. [Google Scholar]
- Rondon, S.I. The potato tuberworm: A literature review of its biology, ecology, and control. Am. J. Potato Res. 2010, 87, 149–166. [Google Scholar] [CrossRef]
- Ben Salah, H.; Aalbu, R. Field use of granulosis virus to reduce initial storage infestation of the potato tuber moth, Phthorimaea operculella (Zeller), in North Africa. Agric. Ecosyst. Environ. 1992, 38, 119–126. [Google Scholar] [CrossRef]
- Arthurs, S.P.; Lacey, L.A.; de la Rosa, F. Evaluation of a granulovirus (PoGV) and Bacillus thuringiensis subsp. kurstaki for control of the potato tuberworm (Lepidoptera: Gelechiidae) in stored tubers. J. Econ. Entomol. 2008, 101, 1540–1546. [Google Scholar] [PubMed]
- Espinel-Correal, C.; Lery, X.; Villamizar, L.; Gomez, J.; Zeddam, J.L.; Cotes, A.M.; Lopez-Ferber, M. Genetic and biological analysis of colombian Phthorimaea operculella granulovirus isolated from Tecia solanivora (Lepidoptera: Gelechiidae). Appl. Environ. Microbiol. 2010, 76, 7617–7625. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Correal, C.; Lopez-Ferber, M.; Zeddam, J.L.; Villamizar, L.; Gomez, J.; Cotes, A.M.; Lery, X. Experimental mixtures of Phthorimaea operculella granulovirus isolates provide high biological efficacy on both Phthorimaea operculella and Tecia solanivora (Lepidoptera: Gelechiidae). J. Invertebr. Pathol. 2012, 110, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Sporleder, M. The granulovirus of the potato tuber moth Phthorimaea operculella (Zeller): Characterisation and prospects for effective mass production and pest control. In Advances in Crop Research; Kroschel, J., Ed.; Margraf Verlag: Weikersheim, Germany, 2003; Volume 3, pp. 196–206. [Google Scholar]
- Gebhardt, M.M.; Eberle, K.E.; Radtke, P.; Jehle, J.A. Baculovirus resistance in codling moth is virus isolate-dependent and the consequence of a mutation in viral gene pe38. Proc. Natl. Acad. Sci. USA 2014, 111, 15711–15716. [Google Scholar] [CrossRef] [PubMed]
- Asser-Kaiser, S.; Fritsch, E.; Undorf-Spahn, K.; Kienzle, J.; Eberle, K.E.; Gund, N.A.; Reineke, A.; Zebitz, C.P.; Heckel, D.G.; Huber, J.; et al. Rapid emergence of baculovirus resistance in codling moth due to dominant, sex-linked inheritance. Science 2007, 317, 1916–1918. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, A.B.; Khushiramani, R.; Athawale, S.S.; Mishra, A.C.; Mourya, D.T. Characterization of a newly established potato tuber moth (Phthorimaea operculella Zeller) cell line. Indian J. Med. Res. 2005, 121, 159–163. [Google Scholar] [PubMed]
- Bravo, A.; Cristina del Rincon-Castro, M.; Ibarra, J.E.; Soberon, M. Chapter 8 towards a healthy control of insect pests: Potential use of microbial insecticides. In Green Trends in Insect Control; Royal Society of Chemistry: London, UK, 2011; pp. 266–299. [Google Scholar]
- Blackburn, D.; Shapiro-Ilan, D.I.; Adams, B.J. Biological control and nutrition: Food for thought. Biol. Control 2016, 97, 131–138. [Google Scholar] [CrossRef]
- Davidson, E.W. History of insect pathology. In Insect Pathology, 2nd ed.; Vega, F.E., Kaya, H.K., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 13–28. [Google Scholar]
- Oestergaard, J.; Belau, C.; Strauch, O.; Ester, A.; van Rozen, K.; Ehlers, R.-U. Biological control of Tipula paludosa (Diptera: Nematocera) using entomopathogenic nematodes (Steinernema spp.) and Bacillus thuringiensis subsp. israelensis. Biol. Control 2006, 39, 525–531. [Google Scholar] [CrossRef]
- Asano, S.-I.; Yamashita, C.; Iizuka, T.; Takeuchi, K.; Yamanaka, S.; Cerf, D.; Yamamoto, T. A strain of Bacillus thuringiensis subsp. galleriae containing a novel cry8 gene highly toxic to Anomala cuprea (Coleoptera: Scarabaeidae). Biol. Control 2003, 28, 191–196. [Google Scholar] [CrossRef]
- Bixby, A.; Alm, S.R.; Power, K.; Grewal, P.; Swier, S.R. Susceptibility of four species of turfgrass-infesting scarabs (Coleoptera: Scarabaeidae) to Bacillus thuringiensis serovar japonensis strain buibui. J. Econ. Entomol. 2007, 100, 1604–1610. [Google Scholar] [CrossRef]
- Osborne, L.S.; Boucias, D.G.; Lindquist, R.K. Activity of Bacillus thuringiensis var. israelensis on Bradysia coprophila (Diptera: Sciaridae). J. Econ. Entomol. 1985, 78, 922–925. [Google Scholar] [CrossRef]
- Taylor, M.D.; Willey, R.D.; Noblet, R. A 24-h potato-based toxicity test for evaluating Bacillus thuringiensis var. israelensis (h-14) against darkwinged fungus gnat Bradysia impatiens johannsen (Diptera: Sciaridae) larvae. Int. J. Pest Manag. 2007, 53, 77–81. [Google Scholar] [CrossRef]
- Chu, J.; Syrovy, L.; Meberg, H. The Effects of Novodor® (Bacillus thuringiensis subsp. tenebrionis) and Entrust® (Spinosad) on Reproduction and Feeding Activity of Epitrix tuberis (Coleoptera: Chrysomelidae) in Potato; Certified Organic Association of BC: Vernon, BC, Canada, 2006. [Google Scholar]
- Weathersbee, A.A.; Tang, Y.Q.; Doostdar, H.; Mayer, R.T. Susceptibility of Diaprepes abbreviatus (Coleoptera: Curculionidae) to a commercial preparation of Bacillus thuringiensis subsp. tenebrionis. Fla. Entomol. Soc. 2002, 85, 330–335. [Google Scholar] [CrossRef]
- Kirchmair, M.; Hoffmann, M.; Neuhauser, S.; Strasser, H.; Huber, L. Persistence of Granmet®, a Metarhizium anisopliae-Based Product, in Grape Phylloxera-Infested Vineyards. IOBC WPRS Bull. 2007, 30, 137–142. [Google Scholar]
- Ansari, M.A.; Butt, T.M. Influence of the application methods and doses on the susceptibility of black vine weevil larvae Otiorhynchus sulcatus to Metarhizium anisopliae in field-grown strawberries. BioControl 2013, 58, 257–267. [Google Scholar] [CrossRef]
- Bruck, D.J. Impact of fungicides on Metarhizium anisopliae in the rhizosphere, bulk soil and in vitro. BioControl 2009, 54, 597–606. [Google Scholar] [CrossRef]
- Berón, C.M.; Diaz, B.M. Pathogenicity of hyphomycetous fungi against Cyclocephala signaticollis. BioControl 2005, 50, 143–150. [Google Scholar] [CrossRef]
- Chandler, D.; Davidson, G. Evaluation of entomopathogenic fungus Metarhizium anisopliae against soil-dwelling stages of cabbage maggot (Diptera: Anthomyiidae) in glasshouse and field experiments and effect of fungicides on fungal activity. J. Econ. Entomol. 2005, 98, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Gold, C.S.; Pena, J.E.; Karamura, E.B. Biology and integrated pest management for the banana weevil Cosmopolites sordidus (Germar) (Coleoptera: Curculionidae). Integr. Pest Manag. Rev. 2001, 6, 79–155. [Google Scholar] [CrossRef]
- Avery, P.; Hunter, W.B.; Hall, D.G.; Jackson, M.A.; Powell, C.; Rogers, M. Potential of topic applications, leaf residues and soil drenches of the fungus Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) for management of the Diaprepes root weevil: Laboratory and greenhouse investigations. In Proceedings of the 41st Annual Society Meeting for Invertebrate Pathology, Warwick, UK, 3–7August 2008.
- Beavers, J.B.; Selhime, A.G.; Denmark, H.A. Predation by Blattisocius keegani acarina-blattisocidae on egg masses of Diaprepes abbreviatus coleoptera-curculionidae in laboratory. J. Econ. Entomol. 1972, 65, 1483–1484. [Google Scholar] [CrossRef] [PubMed]
- Gabarty, A.; Salem, H.M.; Fouda, M.A.; Abas, A.A.; Ibrahim, A.A. Pathogencity induced by the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in Agrotis ipsilon (hufn.). J. Radiat. Res. Appl. Sci. 2014, 7, 95–100. [Google Scholar] [CrossRef]
- Samson, P.R.; Staier, T.N.; Bull, J.I. Evaluation of an application procedure for Metarhizium anisopliae in sugarcane ratoons for control of the white grub Dermolepida albohirtum. Crop Prot. 2006, 25, 741–747. [Google Scholar] [CrossRef]
- Eckard, S.; Ansari, M.A.; Bacher, S.; Butt, T.M.; Enkerli, J.; Grabenweger, G. Virulence of in vivo and in vitro produced conidia of Metarhizium brunneum strains for control of wireworms. Crop Prot. 2014, 64, 137–142. [Google Scholar] [CrossRef]
- Pilz, C.; Wegensteiner, R.; Keller, S. Natural occurrence of insect pathogenic fungi and insect parasitic nematodes in Diabrotica virgifera virgifera populations. BioControl 2008, 53, 353–359. [Google Scholar] [CrossRef]
- Rudeen, M.L.; Jaronski, S.T.; Petzold-Maxwell, J.L.; Gassmann, A.J. Entomopathogenic fungi in cornfields and their potential to manage larval western corn rootworm Diabrotica virgifera virgifera. J. Invertebr. Pathol. 2013, 114, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, E.; Sevim, A.; Demirbağ, Z.; Demir, İ. Isolation, characterization and virulence of entomopathogenic fungi from Gryllotalpa gryllotalpa (Orthoptera: Gryllotalpidae). Appl. Entomol. Zool. 2016, 51, 213–223. [Google Scholar] [CrossRef]
- Hirsch, J.; Reineke, A. Efficiency of commercial entomopathogenic fungal species against different members of the genus Otiorhynchus (Coleoptera: Curculionidae) under laboratory and semi-field conditions. J. Plant Dis. Prot. 2014, 121, 211–218. [Google Scholar] [CrossRef]
- Ansari, M.A.; Evans, M.; Butt, T.M. Identification of pathogenic strains of entomopathogenic nematodes and fungi for wireworm control. Crop Prot. 2009, 28, 269–272. [Google Scholar] [CrossRef]
- Lee, J.C.; Edwards, D.L. Impact of predatory carabids on below- and above-ground pests and yield in strawberry. BioControl 2012, 57, 515–522. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Fergen, J.K. Enhancing predation of a subterranean insect pest: A conservation benefit of winter vegetation in agroecosystems. Appl. Soil Ecol. 2011, 51, 9–16. [Google Scholar] [CrossRef]
- Prischmann-Voldseth, D.A.; Dashiell, K.E. Effects of releasing a generalist predator (Acari: Gaeolaelaps aculeifer) on a subterranean insect herbivore (Coleoptera: Diabrotica virgifera virgifera). Biol. Control 2013, 65, 190–199. [Google Scholar] [CrossRef]
- Shanchez-Contreras, M.; Vlisidou, I. The diversity of insect-bacteria interactions and its applications for disease control. Biotechnol. Genet. Eng. Rev. 2008, 25, 203–243. [Google Scholar] [CrossRef] [PubMed]
- Henkel, J.S.; Baldwin, M.R.; Barbieri, J.T. Toxins from bacteria. EXS 2010, 100, 1–29. [Google Scholar] [PubMed]
- Schünemann, R.; Knaak, N.; Fiuza, L.M. Mode of action and specificity of Bacillus thuringiensis toxins in the control of caterpillars and stink bugs in soybean culture. ISRN Microbiol. 2014, 2014, 135675. [Google Scholar] [CrossRef] [PubMed]
- Höfte, H.; Whiteley, H.R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 1989, 53, 242–255. [Google Scholar] [PubMed]
- Frankenhuyzen, K.V. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 2009, 101, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberon, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Walters, F.S.; deFontes, C.M.; Hart, H.; Warren, G.W.; Chen, J.S. Lepidopteran-active variable-region sequence imparts coleopteran activity in ecry3.1ab, an engineered Bacillus thuringiensis hybrid insecticidal protein. Appl. Environ. Microbiol. 2010, 76, 3082–3088. [Google Scholar] [CrossRef] [PubMed]
- Jakka, S.R.K.; Shrestha, R.B.; Gassmann, A.J. Broad-spectrum resistance to Bacillus thuringiensis toxins by western corn rootworm (Diabrotica virgifera virgifera). Sci. Rep. 2016, 6, 27860. [Google Scholar] [CrossRef] [PubMed]
- Saxena, D.; Stewart, C.N.; Altosaar, I.; Shu, Q.; Stotzky, G. Larvicidal cry proteins from Bacillus thuringiensis are released in root exudates of transgenic B. Thuringiensis corn, potato, and rice but not of B. thuringiensis canola, cotton, and tobacco. Plant Physiol. Biochem. 2004, 42, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Stotzky, G. Persistence and biological activity in soil of the insecticidal proteins from Bacillus thuringiensis, especially from transgenic plants. Plant Soil 2005, 266, 77–89. [Google Scholar] [CrossRef]
- Rosi-Marshall, E.J.; Tank, J.L.; Royer, T.V.; Whiles, M.R.; Evans-White, M.; Chambers, C.; Griffiths, N.A.; Pokelsek, J.; Stephen, M.L. Toxins in transgenic crop byproducts may affect headwater stream ecosystems. Proc. Natl. Acad. Sci. USA 2007, 104, 16204–16208. [Google Scholar] [CrossRef] [PubMed]
- Ruiu, L. Insect pathogenic bacteria in integrated pest management. Insects 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Butt, T.M.; Coates, C.J.; Dubovskiy, I.M.; Ratcliffe, N.A. Entomopathogenic fungi: New insights into host-pathogen interactions. Adv. Genet. 2016, 94, 307–364. [Google Scholar] [PubMed]
- Goettel, M.S.; Eilenberg, J.; Glare, T.R. Entomopathogenic fungi and their role in regulation of insect populations. In Comprehensive Molecular Insect Science; Gilbert, L.I., Iatrou, K., Gill, S., Eds.; Elsevier: London, UK, 2004; Volume 6, pp. 361–406. [Google Scholar]
- Hasan, S. Entomopathogenic fungi as potent agents of biological control. IJETR 2014, 2, 234–237. [Google Scholar]
- Babendreier, D.; Jeanneret, P.; Pilz, C.; Toepfer, S. Non-target effects of insecticides, entomopathogenic fungi and nematodes applied against western corn rootworm larvae in maize. J. Appl. Entomol. 2015, 139, 457–467. [Google Scholar] [CrossRef]
- Dromph, K.M.; Vestergaard, S. Pathogenicity and attractiveness of entomopathogenic hyphomycete fungi to collembolans. Appl. Soil Ecol. 2002, 21, 197–210. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Klingen, I.; Westrum, K.; Meyling, N.V. Effect of norwegian entomopathogenic fungal isolates against Otiorhynchus sulcatus larvae at low temperatures and persistence in strawberry rhizospheres. Biol. Control 2015, 81, 1–7. [Google Scholar] [CrossRef]
- Esther, C.-P.; Erika, A.-S.; Rosa María, M.-C.; de la Torre, M. Performance of two isolates of Isaria fumosorosea from hot climate zones in solid and submerged cultures and thermotolerance of their propagules. World J. Microbiol. Biotechnol. 2013, 29, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Pilz, C.; Enkerli, J.; Wegensteiner, R.; Keller, S. Establishment and persistence of the entomopathogenic fungus Metarhizium anisopliae in maize fields. J. Appl. Entomol. 2011, 135, 393–403. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Butt, T.M. Natural and released inoculum levels of entomopathogenic fungal biocontrol agents in soil in relation to risk assessment and in accordance with eu regulations. Biocontrol Sci. Technol. 2010, 20, 503–552. [Google Scholar] [CrossRef]
- Milner, R.J.; Samson, P.; Morton, R. Persistence of conidia of Metarhizium anisopliae in sugarcane fields: Effect of isolate and formulation on persistence over 3.5 years. Biocontrol Sci. Technol. 2003, 13, 507–516. [Google Scholar] [CrossRef]
- Mayerhofer, J.; Enkerli, J.; Zelger, R.; Strasser, H. Biological control of the European cockchafer: Persistence of Beauveria brongniartii after long-term applications in the euroregion tyrol. BioControl 2015, 60, 617–629. [Google Scholar] [CrossRef]
- Greenfield, M.; Jimenez, M.I.G.; Ortiz, V.; Vega, F.E.; Kramer, M.; Parsa, S. Beauveria bassiana and Metarhizium anisopliae endophytically colonize cassava roots following soil drench inoculation. Biol. Control 2016, 95, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Moonjely, S.; Barelli, L.; Bidochka, M.J. Insect pathogenic fungi as endophytes. In Genetics and Molecular Biology of Entomopathogenic fungi; Lovett, B., Stleger, R.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 94, pp. 107–135. [Google Scholar]
- Barelli, L.; Moonjely, S.; Behie, S.W.; Bidochka, M.J. Fungi with multifunctional lifestyles: Endophytic insect pathogenic fungi. Plant Mol. Biol. 2016, 90, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Behie, S.W.; Bidochka, M.J. Potential agricultural benefits through biotechnological manipulation of plant fungal associations. Bioessays 2013, 35, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Sasan, R.K.; Bidochka, M.J. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 2012, 99, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.; Jaber, L.R. Entomopathogenic fungi as endophytes: Plant-endophyte-herbivore interactions and prospects for use in biological control. Curr. Sci. 2015, 109, 46–54. [Google Scholar]
- Glaser, R.W.; Fox, H. A nematode parasite of the Japanese beetle (Popillia japonica Newm.). Science 1930, 71, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Ehlers, R.U. Entomopathogenic nematodes in biological plant protection. J. Nematol. 2014, 46, 157. [Google Scholar]
- Grewal, P.S.; Ehlers, R.U.; Shapiro-Ilan, D.I. Preface. In Nematodes as Biocontrol Agents; CABI: Wallingford, UK, 2005. [Google Scholar]
- Poinar, G.O. Taxonomy and biology of Steneirnematidae and Herorhabditidae. In Entomopathogenic nematodes in Biological Control; Gaugler, R., Kaya, H.K., Eds.; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Lacey, L.A.; Georgis, R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012, 44, 218–225. [Google Scholar] [PubMed]
- Rasmann, S.; Hiltpold, I.; Ali, J. The role of root-produced volatile secondary metabolites in mediating soil interactions. In Advances in Selected Plant Physiology Aspects; Montanaro, G., Ed.; InTech: Nappanee, IN, USA, 2012. [Google Scholar]
- Turlings, T.; Hiltpold, I.; Rasmann, S. The importance of root-produced volatiles as foraging cues for entomopathogenic nematodes. Plant Soil 2012, 358, 51–60. [Google Scholar] [CrossRef]
- Rasmann, S.; Turlings, T.C.J. Root signals that mediate mutualistic interactions in the rhizosphere. Curr. Opin. Plant Biol. 2016, 32, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; Kollner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Hiltpold, I.; Kollner, T.G.; Frey, M.; Gierl, A.; Gershenzon, J.; Hibbard, B.E.; Ellersieck, M.R.; Turlings, T.C.J. Restoring a maize root signal that attracts insect-killing nematodes to control a major pest. Proc. NatL. Acad. Sci. USA 2009, 106, 13213–13218. [Google Scholar] [CrossRef] [PubMed]
- Degenhardt, J.; Gershenzon, J.; Baldwin, I.T.; Kessler, A. Attracting friends to feast on foes: Engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr. Opin. Biotechnol. 2003, 14, 169–176. [Google Scholar] [CrossRef]
- Stenberg, J.A.; Heil, M.; Åhman, I.; Björkman, C. Optimizing crops for bocontrol of pests and disease. Trends Plant Sci. 2015, 20, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.A.M.; Erb, M.; Hiltpold, I.; Hibbard, B.E.; Gaillard, M.D.P.; Bilat, J.; Degenhardt, J.; Cambet-Petit-Jean, X.; Turlings, T.C.J.; Zwahlen, C. Genetically engineered maize plants reveal distinct costs and benefits of constitutive volatile emissions in the field. Plant Biotechnol. J. 2013, 11, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Hiltpold, I.; Baroni, M.; Toepfer, S.; Kuhlmann, U.; Turlings, T.C.J. Selection of entomopathogenic nematodes for enhanced responsiveness to a volatile root signal helps to control a major root pest. J. Exp. Biol. 2010, 213, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Tyagi, R.D.; Brar, S.K.; Surampalli, R.Y.; Prevost, D. Bio-encapsulation of microbial cells for targeted agricultural delivery. Crit. Rev. Biotechnol. 2011, 31, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Vemmer, M.; Patel, A.V. Review of encapsulation methods suitable for microbial biological control agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Kim, J.; Jaffuel, G.; Turlings, T.J. Enhanced alginate capsule properties as a formulation of entomopathogenic nematodes. BioControl 2015, 60, 527–535. [Google Scholar] [CrossRef]
- Hiltpold, I.; Hibbard, B.; French, B.W.; Turlings, T.J. Capsules containing entomopathogenic nematodes as a trojan horse approach to control the western corn rootworm. Plant Soil 2012, 358, 11–25. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Barbosa, P. Success in Conservation Biological Control of Arthropods; Springer: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Symondson, W.; Sunderland, K.; Greenstone, M. Can generalist predators be effective biocontrol agents? Annu. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [PubMed]
- Wyckhuys, K.A.G.; Lu, Y.; Morales, H.; Vazquez, L.L.; Legaspi, J.C.; Eliopoulos, P.A.; Hernandez, L.M. Current status and potential of conservation biological control for agriculture in the developing world. Biol. Control 2013, 65, 152–167. [Google Scholar] [CrossRef]
- Lang, A.; Filser, J.; Henschel, J.R. Predation by ground beetles and wolf spiders on herbivorous insects in a maize crop. Agric. Ecosyst. Environ. 1999, 72, 189–199. [Google Scholar] [CrossRef]
- Al Rehiayani, S.M.; Fouly, A.H. Cosmolaelaps simplex (Berlese), a polyphagous predatory mite feeding on root-knot. Pak. J. Biol. Sci. 2005, 8, 168–174. [Google Scholar]
- Putman, R.; Wratten, S.D. Principles of Ecology; Groom Helm: London, UK; Canberra, Australia, 1984. [Google Scholar]
- Ansari, M.A.; Shah, F.A.; Butt, T.M. Combined use of entomopathogenic nematodes and Metarhizium anisopliae as a new approach for black vine weevil, Otiorhynchus sulcatus, control. Entomol. Exp. Appl. 2008, 129, 340–347. [Google Scholar] [CrossRef]
- Jabbour, R.; Crowder, D.W.; Aultman, E.A.; Snyder, W.E. Entomopathogen biodiversity increases host mortality. Biol. Control 2011, 59, 277–283. [Google Scholar] [CrossRef]
- Tinzaara, W.; Gold, C.S.; Dicke, M.; Van Huis, A.; Nankinga, C.M.; Kagezi, G.H.; Ragama, P.E. The use of aggregation pheromone to enhance dissemination of Beauveria bassiana for the control of the banana weevil in Uganda. Biocontrol Sci. Technol. 2007, 17, 111–124. [Google Scholar] [CrossRef]
- Koppenhofer, A.M.; Kaya, H.K. Additive and synergistic interaction between entomopathogenic nematodes and Bacillus thuringiensis for scarab grub control. Biol. Control 1997, 8, 131–137. [Google Scholar] [CrossRef]
- Koppenhofer, A.M.; Kaya, H.K. Synergism of imidacloprid and an entomopathogenic nematode: A novel approach to white grub (Coleoptera: Scarabaeidae) control in turfgrass. J. Econ. Entomol. 1998, 91, 618–623. [Google Scholar] [CrossRef]
- Koppenhofer, A.M.; Brown, I.M.; Gaugler, R.; Grewal, P.S.; Kaya, H.K.; Klein, M.G. Synergism of entomopathogenic nematodes and imidacloprid against white grubs: Greenhouse and field evaluation. Biol. Control 2000, 19, 245–251. [Google Scholar] [CrossRef]
- Polavarapu, S.; Koppenhofer, A.M.; Barry, J.D.; Holdcraft, R.J.; Fuzy, E.M. Entomopathogenic nematodes and neonicotinoids for remedial control of oriental beetle, Anomala orientalis (Coleoptera : Scarabaeidae), in highbush blueberry. Crop Prot. 2007, 26, 1266–1271. [Google Scholar] [CrossRef]
- Cappaert, D.L.; Koppenhofer, A.M. Steinernema scarabaei, an entomopathogenic nematode for control of the european chafer. Biol. Control 2003, 28, 379–386. [Google Scholar] [CrossRef]
- Saito, T.; Brownbridge, M. Compatibility of soil-dwelling predators and microbial agents and their efficacy in controlling soil-dwelling stages of western flower thrips Frankliniella occidentalis. Biol. Control 2016, 92, 92–100. [Google Scholar] [CrossRef]
- Van Groenigen, J.W.; Lubbers, I.M.; Vos, H.M.J.; Brown, G.G.; De Deyn, G.B.; van Groenigen, K.J. Earthworms increase plant production: A meta-analysis. Sci. Rep. 2014, 4, 6365. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; The University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- De Vos, M.; Van Oosten, V.R.; Van Poecke, R.M.P.; Van Pelt, J.A.; Pozo, M.J.; Mueller, M.J.; Buchala, A.J.; Metraux, J.P.; Van Loon, L.C.; Dicke, M.; et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant-Microbe Interact. 2005, 18, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.D.; Newton, A.C.; Walters, D.R. Induced resistance in crop protection: The future, drivers and barriers. In Induced Resistance for Plant Defense; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 316–325. [Google Scholar]
- Lu, J.; Robert, C.A.M.; Lou, Y.; Erb, M. A conserved pattern in plant-mediated interactions between herbivores. Ecol. Evol. 2016, 6, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.N.; Erb, M.; Hartley, S.E. Roots under attack: Contrasting plant responses to below- and aboveground insect herbivory. New Phytol. 2016, 210, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Smart, L.E.; Martin, J.L.; Limpalaër, M.; Bruce, T.J.A.; Pickett, J.A. Responses of herbivore and predatory mites to tomato plants exposed to jasmonic acid seed treatment. J. Chem. Ecol. 2013, 39, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.A.; Aradottír, G.I.; Birkett, M.A.; Bruce, T.J.A.; Hooper, A.M.; Midega, C.A.O.; Jones, H.D.; Matthes, M.C.; Napier, J.A.; Pittchar, J.O.; et al. Delivering sustainable crop protection systems via the seed: Exploiting natural constitutive and inducible defence pathways. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369. [Google Scholar] [CrossRef]
- Jisha, K.C.; Puthur, J.T. Seed priming with baba (β-amino butyric acid): A cost-effective method of abiotic stress tolerance in Vigna radiata (L.) wilczek. Protoplasma 2016, 253, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; De Vos, M.; Casteel, C.L.; Tian, D.; Halitschke, R.; Sun, J.Y.; Agrawal, A.A.; Felton, G.W.; Jander, G. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 2012, 158, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Burketova, L.; Trda, L.; Ott, P.G.; Valentova, O. Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Robert, C.A.M.; Riemann, M.; Cosme, M.; Mene-Saffrane, L.; Massana, J.; Stout, M.J.; Lou, Y.; Gershenzon, J.; Erb, M. Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol. 2015, 167, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Robert, C.A.M.; Marti, G.; Lu, J.; Doyen, G.R.; Villard, N.; Barriere, Y.; French, B.W.; Wolfender, J.-L.; Turlings, T.C.J.; et al. A physiological and behavioral mechanism for leaf herbivore-induced systemic root resistance. Plant Physiol. 2015, 169, 2884–2894. [Google Scholar] [CrossRef] [PubMed]
- Orrell, P.; Bennett, A.E. How can we exploit above–belowground interactions to assist in addressing the challenges of food security? Front. Plant Sci. 2013, 4, 432. [Google Scholar] [CrossRef] [PubMed]
- Spoel, S.H.; Dong, X.N. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Bakker, P.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting Rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Azcon-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Vannette, R.L.; Hunter, M.D. Mycorrhizal fungi as mediators of defence against insect pests in agricultural systems. Agric. For. Entomol. 2009, 11, 351–358. [Google Scholar] [CrossRef]
- Borowicz, V.A. Do arbuscular mycorrhizal fungi alter plant-pathogen relations? Ecology 2001, 82, 3057–3068. [Google Scholar]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Kissen, R.; Rossiter, J.T.; Bones, A.M. The “mustard oil bomb”: Not so easy to assemble?! Localization, expression and distribution of the components of the myrosinase enzyme system. Phytochem. Rev. 2009, 8, 69–86. [Google Scholar] [CrossRef]
- Potter, M.J.; Davies, K.; Rathjen, A.J. Suppressive impact of glucosinolates in brassica vegetative tissues on root lesion nematode pratylenchus neglectus. J. Chem. Ecol. 1998, 24, 67–80. [Google Scholar] [CrossRef]
- Gange, A.C.; Brown, V.K.; Aplin, D.M. Multitrophic links between arbuscular mycorrhizal fungi and insect parasitoids. Ecol. Lett. 2003, 6, 1051–1055. [Google Scholar] [CrossRef]
- Guerrieri, E.; Lingua, G.; Digilio, M.C.; Massa, N.; Berta, G. Do interactions between plant roots and the rhizosphere affect parasitoid behaviour? Ecol. Entomol. 2004, 29, 753–756. [Google Scholar] [CrossRef]
- Rapparini, F.; Llusia, J.; Penuelas, J. Effect of arbuscular mycorrhizal (AM) colonization on terpene emission and content of Artemisia annua l. Plant Biol. 2008, 10, 108–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontana, A.; Reichelt, M.; Hempel, S.; Gershenzon, J.; Unsicker, S. The effects of arbuscular mycorrhizal fungi on direct and indirect defense metabolites of Plantago lanceolata. J. Chem. Ecol. 2009, 35, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, D.; Vierheilig, H.; Peneder, S.; Schausberger, P. Mycorrhiza modulates aboveground tri-trophic interactions to the fitness benefit of its host plant. Ecol. Entomol. 2011. [Google Scholar] [CrossRef]
- Wooley, S.C.; Paine, T.D. Infection by Mycorrhizal fungi increases natural enemy abundance on tobacco (Nicotiana rustica). Environ. Entomol. 2011, 40, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Schausberger, P.; Peneder, S.; Jurschik, S.; Hoffmann, D. Mycorrhiza changes plant volatiles to attract spider mite enemies. Funct. Ecol. 2012, 26, 441–449. [Google Scholar] [CrossRef]
- Erb, M.; Robert, C.A.M. Sequestration of plant secondary metabolites by insect herbivores: Molecular mechanisms and ecological consequences. Curr. Opin. Insect Sci. 2016, 14, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Opitz, S.; Müller, C. Plant chemistry and insect sequestration. Chemoecology 2009, 19, 117–154. [Google Scholar] [CrossRef]
- Douglas, W.T.; Daryl, P.W.; Ferdinand, D.; David, A.F.; Piotr, M.G.; Peter, W.G. Sequestered cucurbitacins and pathogenicity of Metarhizium anisopliae (Moniliales: Moniliaceae) on spotted cucumber beetle eggs and larvae (Coleoptera: Chrysomelidae). Environ. Entomol. 1998, 27, 366–372. [Google Scholar]
- Bale, J.S.; van Lenteren, J.C.; Bigler, F. Biological control and sustainable food production. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Holt, R.D.; Hochberg, M.E. When is biological control evolutionarily stable (or is it)? Ecology 1997, 78, 1673–1683. [Google Scholar] [CrossRef]
- Bardin, M.; Ajouz, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, R.-U. Current and future use of nematodes in biocontrol: Practice and commercial aspects with regard to regulatory policy issues. Biocontrol Sci. Technol. 1996, 6, 303–316. [Google Scholar] [CrossRef]
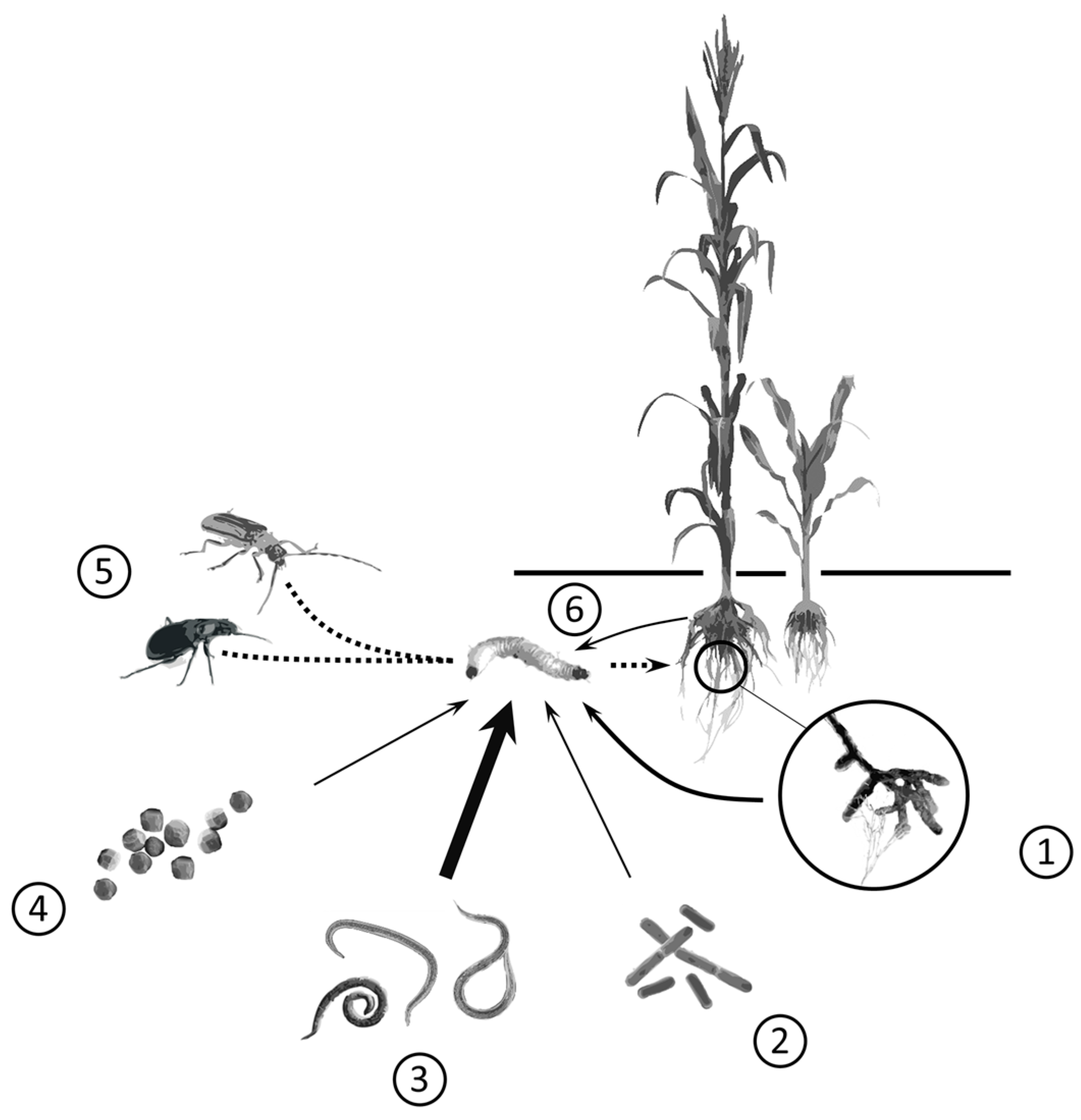
| Biocontrol Agents | Root-Pest Common Name | Root-Pest Scientific Name 1 | Key Crops Targeted | Entomopathogenic Species Used 2 | Biocontrol Method | Status | Potential Future Use | References |
|---|---|---|---|---|---|---|---|---|
| Virus | ||||||||
| Potato tuber moth | Phthorimaea operculella (1) | Potato | Granulovirus (PhopGV) | Inundative | Government agencies | Yes | [32] | |
| [29] | ||||||||
| [33] | ||||||||
| Potato tuber moth | Tecia solanivora (1) | Potato | Granulovirus (PhopGV) | Inundative | Government agencies | Yes | [33,34] | |
| Bacteria | ||||||||
| Japanese beetle | Popillia japonica (2) | Turf | Paenibacillus popilliae | Inundative | Registered | Yes | [41] | |
| Crane fly | Tipula paludos (3) | Pasture, turf | Bt subsp. israelensis | Inundative | Experimental | Yes | [42] | |
| Cupreous chafer | Anomala cuprea (2) | Peanut | Bt subsp. galleriae | Inundative | Experimental | Yes | [43] | |
| Oriental beetle | Anomala orientalis (2) | Turf | Bt subsp. japonensis | Inundative | Experimental | Yes | [44] | |
| Japanese beetle | Popillia japonica (2) | Turf | Bt subsp. japonensis | Inundative | Experimental | Yes | [44] | |
| Fungus gnat | Bradysia spp. (4) | Horticulture | Bt subsp. israelensis | Inundative | Registered | Yes | [45] | |
| [46] | ||||||||
| Tuber flea beetle | Epitrix tuberis (5) | Potato | Bt subsp. tenebrionis | Inundative | Registered | No | [47] | |
| Root weevil | Diaprepes abbreviatus (6) | Citrus | Bt subsp. tenebrionis | Inundative | Registered | No | [48] | |
| Fungi | ||||||||
| Grapevine phylloxera | Daktulosphaira vitifoliae (7) | Vineyard | Ma | Inundative | Registered | Yes | [49] | |
| Black vine weevil | Otiorhynchus sulcatus (6) | Berries | Ma, Bb | Inundative | Registered | Yes | [50] | |
| [51] | ||||||||
| White grub | Cyclocephala signaticollis (2) | Crops, fruit, ornamentals, turf and pasture | Bb | Inundative | Experimental | Yes | [52] | |
| Cabbage root fly | Delia radicum (8) | Cabbage | Ma | Inundative | Experimental | Yes | [53] | |
| Banana root borer | Cosmopolites sordidus (6) | Banana | Bb, Ma | Inundative | Experimental | No | [54] | |
| Diaprepes root weevil | Diaprepes abbreviatus (6) | Citrus, sugar cane | If, Bb | Inundative | Experimental | No, Yes | [55] | |
| [56] | ||||||||
| Black cutworm | Agrotis ipsilon (9) | Turf, vegetables | Ma, Bb | Inundative | Experimental | Yes | [57] | |
| Greyback cane beetle | Dermolepida albohirtum (2) | Sugar cane | Ma | Inundative | Registered | No | [58] | |
| Wireworms | Coleoptera: Elateriadae | Potatoes, vegetables | Mb | Inundative | Experimental | Yes | [59] | |
| Onion maggot | Delia antiqua (8) | Bulbous plants | Ma | Inundative | Experimental | Yes | [53] | |
| Crane fly | Tipula paludosa (3) | Diff. crops | Mr | Inundative | Experimental | Yes | [59] | |
| Rootworm | Diabrotica virgifera virgifera (5) | Corn | Ma, Bb | Inundative | Experimental | Yes | [60] | |
| [61] | ||||||||
| Mole crickets | Orthoptera: Gryllotalpidae | Turf, vegetables, tree seedlings | Ma | Inundative | Experimental | Yes | [62] | |
| Root weevil | Otiorhynchus spp. (6) | Diff. crops | Bb | Inundative | Registered | Yes | [63] | |
| Nematodes | ||||||||
| Banana root borer | Cosmopolites sordidus (6) | Banana | Sc, Sf, Sg | Inundative | Registered | Yes | * | |
| Billbug | Sphenophorusspp. (6) | Turf | Hb, Sc | Inundative | Registered | Yes | * | |
| Black cutworm | Agrotis ipsilon (9) | Turf, vegetables | Sc | Inundative | Registered | Yes | * | |
| Black vine weevil | Otiorhynchus sulcatus (6) | Berries, ornamentals | Hb, Hd, Hm, Hmeg, Sc, Sg | Inundative | Registered | Yes | * | |
| Borers | Synanthedon spp. (10) | Fruit trees and ornamentals | Hb, Sc, Sf | Inundative | Registered | Yes | * | |
| Citrus root weevil | Pachnaeusspp. (6) | Citrus, ornamentals | Sr, Hb | Inundative | Registered | Yes | * | |
| Corn rootworm | Diabrotica spp. (6) | Vegetables | Hb, Sc | Inundative | Registered | Yes | * | |
| Cranberry girdler | Chrysoteuchia topiaria (11) | Cranberries | Sc | Inundative | Registered | Yes | * | |
| Crane fly | Diptera: Tipulidae | Turf | Sc | Inundative | Registered | Yes | * | |
| Diaprepes root weevil | Diaprepes abbreviatus (6) | Citrus, ornamentals | Hb, Sr | Inundative | Registered | Yes | * | |
| Fungus gnats | Diptera: Sciaridae | Mushrooms, greenhouse | Sf, Hb | Inundative | Registered | Yes | * | |
| Grape root borer | Vitacea polistiformis (10) | Grapes | Hz, Hb | Inundative | Registered | No | * | |
| Iris borer | Macronoctua onusta (9) | Iris | Hb, Sc | Inundative | Registered | Yes | * | |
| Mole crickets | Scapteriscus spp. (12) | Turf | Sc, Sr, Scap | Inundative | Registered | Yes | * | |
| Scarab grubs | Coleoptera: Scarabaeidae | Turf, ornamentals | Hb, Sc, Sg, Ss, Hz | Inundative | Registered | Yes | * | |
| Strawberry root weevil | Otiorhynchus ovatus (6) | Berries | Hm | Inundative | Registered | Yes | * | |
| Sugarbeet weevil | Temnorhinus mendicus (6) | Sugar beets | Hb, Sc | Inundative | Registered | No | * | |
| Sweetpotato weevil | Cylas formicarius (6) | Sweet potato | Hb, Sc, Sf | Inundative | Registered | Yes | * | |
| Wireworms | Coleoptera: Elateridae | Vegetables | Hb, Hm, Sc | Inundative | Registered | Yes | [64] | |
| Arthropods | ||||||||
| Carabid | Black vine weevil | Otiorhynchus sulcatus (6) | Strawberry | Carabus nemoralis | Conservation | Experimental | No | [65] |
| Black vine weevil | Otiorhynchus sulcatus (6) | Strawberry | Nebria brevicollis | Conservation | Experimental | No | [65] | |
| Black vine weevil | Otiorhynchus sulcatus (6) | Strawberry | Pterostichus algidu | Conservation | Experimental | No | [65] | |
| Black vine weevil | Otiorhynchus sulcatus (6) | Strawberry | Pterostichus melanarius | Conservation | Experimental | No | [65] | |
| Black vine weevil | Otiorhynchus sulcatus (6) | Strawberry | Scaphinotus marginatus | Conservation | Experimental | No | [65] | |
| Western corn rootworm | Diabrotica virgifera virgifera (5) | Maize | Pterostichus permundus | Conservation | Experimental | Yes | [66] | |
| Western corn rootworm | Diabrotica virgifera virgifera (5) | Maize | Poecilus chalcites | Conservation | Experimental | No | [66] | |
| Western corn rootworm | Diabrotica virgifera virgifera (5) | Maize | Cyclotrachelus alternans | conservation | experimental | No | [66] | |
| Western corn rootworm | Diabrotica virgifera virgifera (5) | Maize | Poecilus lucublandus | conservation | experimental | No | [66] | |
| Acari | Western corn rootworm | Diabrotica virgifera virgifera (5) | Maize | Gaeolaelaps aculeifer | conservation | experimental | No | [67] |
| Orthoptera | Western corn rootworm | Diabrotica virgifera virgifera (5) | Maize | Allonemobius spp. | conservation | experimental | No | [66] |
| Opiliones | Western corn rootworm | Diabrotica virgifera virgifera (5) | Maize | Phalangium opilio | conservation | experimental | No | [66] |
| Hymenoptera | Western corn rootworm | Diabrotica virgifera virgifera (5) | Maize | Hymenoptera: Formicidae | conservation | experimental | Yes | [66] |
| Hemiptera | Western corn rootworm | Diabrotica virgifera virgifera (5) | Maize | Geocoris sp. | conservation | experimental | No | [66] |
| Araneae | Western corn rootworm | Diabrotica virgifera virgifera (5) | Maize | Linyphiidae | conservation | experimental | No | [66] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kergunteuil, A.; Bakhtiari, M.; Formenti, L.; Xiao, Z.; Defossez, E.; Rasmann, S. Biological Control beneath the Feet: A Review of Crop Protection against Insect Root Herbivores. Insects 2016, 7, 70. https://doi.org/10.3390/insects7040070
Kergunteuil A, Bakhtiari M, Formenti L, Xiao Z, Defossez E, Rasmann S. Biological Control beneath the Feet: A Review of Crop Protection against Insect Root Herbivores. Insects. 2016; 7(4):70. https://doi.org/10.3390/insects7040070
Chicago/Turabian StyleKergunteuil, Alan, Moe Bakhtiari, Ludovico Formenti, Zhenggao Xiao, Emmanuel Defossez, and Sergio Rasmann. 2016. "Biological Control beneath the Feet: A Review of Crop Protection against Insect Root Herbivores" Insects 7, no. 4: 70. https://doi.org/10.3390/insects7040070







