1. Introduction
Aquatic systems worldwide are being threatened by the expansion of human activities [
1], mainly by agriculture, cattle ranching, and urbanization [
2]. These activities affect not only the physical environment but also the diversity, structure, and function of aquatic communities that thrive in these environments. Tropical freshwater ecosystems are especially at risk of becoming increasingly imperiled by escalating anthropogenic impacts. Nonetheless, they are understudied and not well understood relative to temperate systems [
3].
With the exception of some remote Amazonian rivers, most aquatic environments have been altered by human activities in Brazil [
4], including wetland drainage, dams, road building, and deforestation for human settlements and intensive agriculture. All of these modifications have a negative impact on the hydrology, vegetation cover, and terrestrial-aquatic linkages of the affected systems [
5], but there is a shortage of studies focusing on the consequences of these impacts to ecosystem integrity in Cerrado and Amazonia watersheds.
The Brazilian Savannah (Cerrado) is considered a biodiversity hotspot for conservation priorities [
6]. The Cerrado biome is one of the most threatened ecosystems in Brazil. Especially in the Northern part, covered by the Tocantins and Araguaia hydrological region that supports the hydrological forces that link Amazonia production of waters and the Southeast Brazil [
7]. The Northern part of the Cerrado biome corresponds in great part to the State of Tocantins whose mosaic of ecosystems characterize the transition from Amazonia to Cerrado. Its functioning and diversity is almost unknown, although it faces the threat of fast occupation due to the creation of the state in the 1990s and the mechanized agriculture and huge damming projects in the Tocantins river mainly, but also in other tributaries.
Vast ecosystems of the Cerrado occupied by the newest Brazilian state have been suffering intense changes in land use [
8], mainly due to large-scale soybean agriculture and pasture establishment [
9]. In this newly occupied territory the degradation of the riparian stream zone, as well as loss of connectivity to downstream ecosystems, derives mainly from the damming of streams and rivers, often with the purpose of storing water for cattle, together with fast deforestation that threatens the biological integrity of river networks [
10]. However, the consequences on the structure of stream ecosystems have not been investigated in these fragile transition ecosystems between the Cerrado and Amazonia biomes. In the Tocantins and Araguaia river basins, large-scale deforestation has contributed to a 25% increase in river flow [
11]. In upper Xingu watersheds, covered by plantations in Brazilian Mato Grosso state, Hayhoe et al. [
9] reported a reduction in evapotranspiration as well as an increase in flow and seasonal variability compared to forested watersheds; this pattern could be mirrored in the agriculture-dominated landscapes of the Northern Brazilian Cerrado, causing important alterations in regional hydrology. In the neighboring biome, the Brazilian Amazonia, studies conducted in the Madeira River, Ji-Paraná, and upper Jamari basins showed that replacing riparian forest with pastures for grazing affects the hydrology, nutrient concentrations, and benthic habitats of streams. Pasture presence is a major factor affecting the chemical composition of waters [
12,
13], creating an increase in runoff and lowering habitat complexity from a channel composed of runs and pools and forest leaf detritus (50% cover) to a channel covered with grass (63%), mainly with low-moving waters [
14]. Given the steady increase in deforestation in the different ecosystems of the Cerrado in the Tocantins and Araguaia river basins and the fundamental role of its network of rivers in linking the Amazonia to the Southern Cerrado and Atlantic Rain Forest, there is an urgent need for monitoring indicators for the management of these ecosystems.
Among aquatic communities, macroinvertebrates stand out as biological indicators of freshwaters [
15,
16] since they respond to longer temporal and spatial impacts than the instant measures of physical and chemical variables due to their long-lasting life cycle in waters [
17,
18]. Also, they show less mobility than larger vertebrates such as fish, as they depend on drifting to move from the habitat if conditions are not suitable.
The use of aquatic macroinvertebrates to assess water quality includes a variety of biotic indexes [
19]. The main biotic indices include the presence of sensitive species such as the EPT index (Ephemeroptera, Plecoptera, and Trichoptera) [
20] or the adoption of different values of tolerance to organic pollution for each family [
21]. Generally, these indices include biological responses in a numeric expression that can be easily understood [
22]. They also combine low cost and effort with high efficiency and a fast identification of organisms, even when considering the huge gap of knowledge about biodiversity distribution patterns in tropical areas.
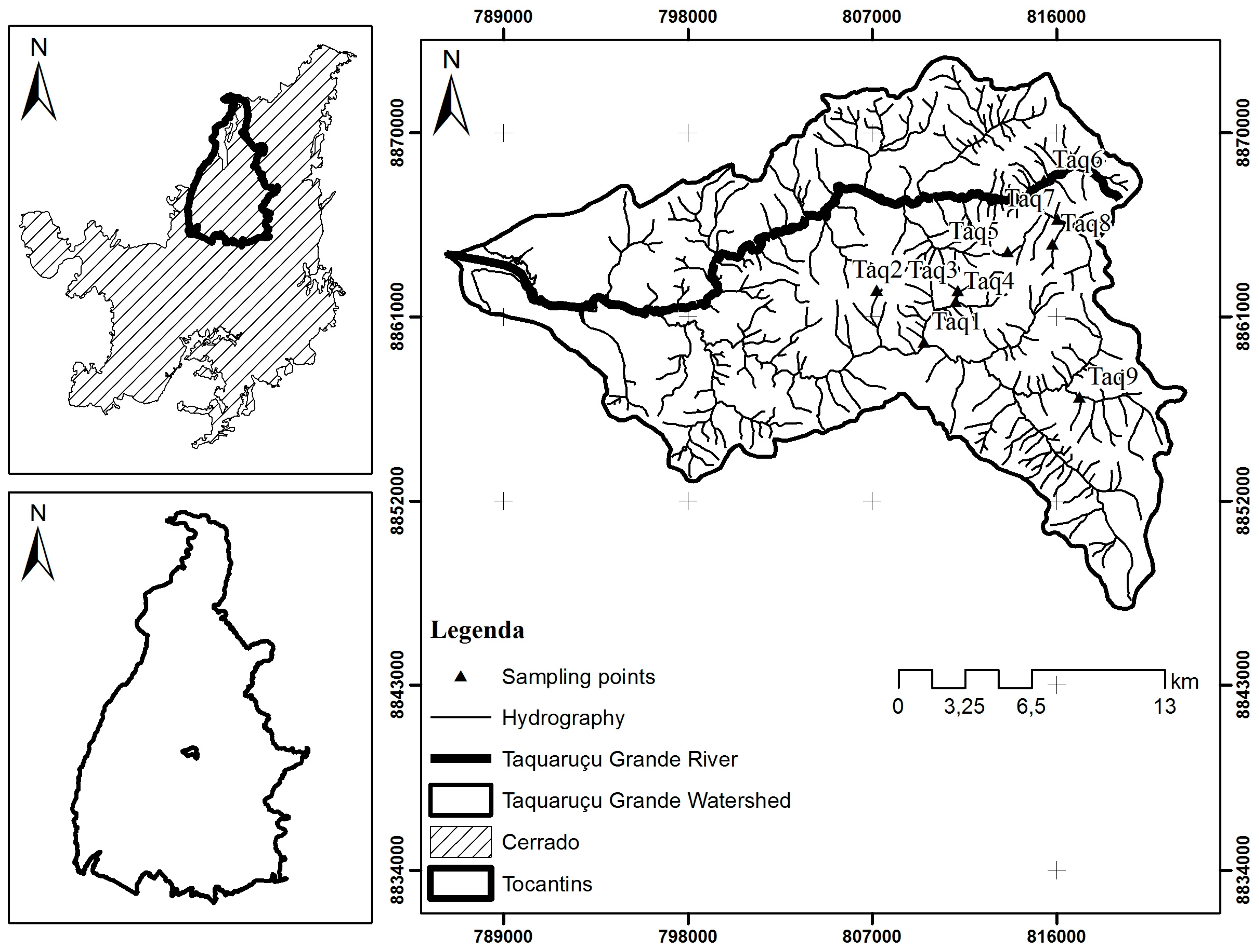
Therefore, this study aimed to evaluate the environmental quality of streams in a partially preserved watershed, the Taquaruçu Grande river basin, using biological metrics based on the macroinvertebrate fauna and abiotic variables such as Habitat Integrity Index. The Taquaruçu Grande river basin can be exemplary of the ecosystems of the mosaic Northern Cerrado territory. It is also facing threats of anthropization, but pristine aquatic environments are still found. The greater threats to this watershed are fast anthropic occupation and touristic activities in rural landscapes, but not organic or chemical pollution derived from urban settlements and agriculture. The effects of these impacts on macroinvertebrate communities and ecosystem health are practically unknown for this territory.
3. Results
The values of the abiotic variables and of the Habitat Integrity Index are shown in
Table 2. Site Taq9 was classified as an impacted environment with a HII of 0.56, due to a surrounding matrix of open pasture and narrow riparian cover together with proximity to a road and suburban area. Site Taq8 was classified as an altered environment with a HII of 0.77, and environmental changes measured in this site refer mainly to changes due to partial removal of marginal vegetation, resulting in a pioneer herbaceous and shrub cover with frequent breaks due to cattle activity. Furthermore, in both sites there were Cerrado patches among pasture areas, riparian vegetation varied between 1 and 5 m in width, there were retention mechanisms in the water course, such as stones and trunks, stable banks with some cutting, riverbeds with silt, gravel, and sand, and in some sites, leaf detritus and woody material with sediments. The other seven streams were classified as preserved environments with HII varying from 0.81 to 0.96. At these points, the predominant characteristics were: more than 50 m in width of gallery forest, which was continuous with the adjacent forest, retention mechanisms strongly fixed, lack of banks, little or no accumulation of sediments in the riverbed, with stones grouped together, mosses and algae patches, and leaf detritus and woody material without sediment.
In general, the water streams of the Taquaruçu Grande watershed were well oxygenated, with low electrical conductivity, acid pH, and low concentration of Total Dissolved Solids (STD), except for streams Taq8 and Taq9 that showed high values of electrical conductivity and STD (
Table 2).
A total of 615 individuals were collected that belong to nine orders and 30 families (
Table 3). The order Trichoptera was present in high abundance with 187 individuals, followed by Coleptera with 145 individuals, and they occurred in all streams. Perlidae, the only taxa belonging to Plecoptera in the samples, was the most abundant family in the streams, and it was present in seven streams, but not in Taq9, with the lowest HII score. The family Elmidae from the order Coleoptera was the second most abundant in the streams, but it was absent from Taq9. Also, the family Hydropsychidae, an abundant trichopteran in the streams (62 individuals) presented a low abundance in Taq9, where only two individuals were captured. The order Lepidoptera was represented by one individual of the family Pyralidae, and it was the rarer group represented in the samplings and occurred only in Taq7 stream. Also rare, the order Megaloptera was represented only by 56 individuals of the family Corylidae, although it occurred in five among the nine sampled streams.
The Trichoptera occurred in all streams. The Ephemeroptera was absent from Taq5, but it occurred in all other streams. The Plecoptera occurred in all but two streams, Taq4 and Taq9. Also, Coleptera, Diptera, Lepidoptera, and Megaloptera were absent from Taq9. The anisopterans (Odonata) were present in Taq9 where they represented 29% of the Odonata or 2.76 of the abundance of macroinvertebrates in all samples.
Lepidoptera, Plecoptera, and Megaloptera were represented by one taxon each, whereas Coleoptera and Odonata were represented by seven and six taxa each.
The metrics based on macroinvertebrates fauna are presented in
Table 4 and
Table 5. According to the BMWP index from Monteiro et al. [
21], only Taq7 and Taq8 streams had satisfactory environmental quality, and Taq3 was classified as having very poor quality. This index was strongly related to the abundance of macroinvertebrates, total richness, and richness of EPT (
Table 6). Taq7 presented the greatest abundance and total richness of macroinvertebrates, which probably influenced its classification of being of “Good” environmental quality, whereas Taq3 presented the lowest abundance and EPT richness, which led to its “Very poor” classification (although it received a high HII score). Taq8 received a HII score equivalent to “Altered environment”, whereas it received a “Good quality” BMPW score. On the other hand, Taq1 presented a higher %EPT and EPT/total fauna ratio that was not correlated with the BMWP classification of “Poor quality” of this stream.
The metrics of the community (Shannon-Weiner index of diversity, Pielou index, and ratios of shredders and detritivores) did not correlate with the BMWP.
The BMWP index correlated positively to pH values, but not to other abiotic factors, including the Habitat Integrity Index (
Table 7). The values of electrical conductivity and STD do not correlate with the BMWP index, since Taq8 showed “Good” environmental quality but presented altered values of those two measures. The other metrics based on macroinvertebrates fauna were not correlated with any physical, chemical, or structural characteristics of the environment.
4. Discussion
Impacts of anthropic intervention in watersheds in Cerrado are usually linked to urbanization and agriculture, and the studies on biological monitoring of those streams focus on the effects of organic and agricultural pollution on aquatic communities [
11,
32]. The present study examines a set of streams impacted by rural settlements and touristic activities. The main human interventions in the area are river deviation for landscape building and damming of rivers for recreational (bathing) purposes or water extraction for cattle or small-scale agriculture, together with annual fire management of Cerrado vegetation. Touristic activities are concentrated in the dry season and include habitat alteration due to excessive use, deterioration of water quality, and deforestation of spots in stream margins (bathing areas).
These activities produced low physical alterations in the streams courses, such as few exposed riverbanks, rare fragmentation and siltation points, and pasture surrounding the riparian vegetation, and so contributed to a high value for the physical Habitat Integrity Index. Although most of the sampled streams exhibit good physical, chemical, and structural (HII) quality, when considering the sensitivity of taxa expressed as BMWP scores, the sampled streams have generally poor quality. This is probably because long-term fluctuating disturbances of surrounding environments, such as fire disturbances, affect the insect fauna both as adults and larvae [
33]. Nevertheless, fire management may produce long-term alteration in the diversity and abundance of macroinvertebrates that are probably reflected in the poor evaluation by BMWP. In a study of the responses of macroinvertebrate communities to fire via comparisons of streams in burned and unburned catchments in three fire-prone biomes that differ biogeographically and climatically (northwestern Mediterranean, southeastern Australia, and northwestern intermountain USA), Verkaik et al. [
34] found that the responses of macroinvertebrate communities in streams in burned catchments were similar in all biogeographic regions and corresponded to reduced measures of taxonomic richness and increased abundance, especially of
r-strategist taxa. Fire effects on streams generally result in long-term adverse changes in invertebrates leading to abundance and biomass decline and community composition shifts toward disturbance-adapted taxa [
35,
36]. It is possible that the vegetation in the Cerrado area studied recovers fast, but not the macroinvertebrate community, and the high HII scores do not account for the disturbance in the shifts of abundance and richness of sensitive families of macroinvertebrates as BMWP and EPT richness do.
All streams presented riparian forests; this vegetation directly influences the community structure of aquatic insects, mainly by the input of nutrients and allochthonous energy [
37,
38,
39]. Sensitive taxa such as trichoperans and Odonata respond to variations in canopy cover [
30]. Monteiro-Junior et al. [
40] have shown that species richness of zygopterans is correlated positively with the integrity of riparian vegetation. In Taquaruçu Grande basin, zygopterans were collected in streams at Taq6 and Taq8, but not Taq9, showed the lowest HII score. On the other hand, anisopetrans were abundant and corresponded to 2.7% of the taxa present in Taq9. According to Monteiro-Junior et al. [
40], a negative relationship is expected between environmental integrity and the richness of anisopetrans. We have shown that the abundance of this subgroup of Odonata rises in streams with low HII. The order Trichoptera was the most abundant, although the most represented family Leptoceridae was absent from Taq8 and Taq9, the streams with altered HII score and high STD measures. This indicates that trichopterans may respond to the physical disturbances measured by HII. Our results agree with the findings of Mazeika et al. [
41] that EPT families are sensitive to stressors other than organic pollution, such as flow regimes and stream morphology. Also, Godoy et al. [
42] showed that changes in the physical structure of smaller streams led to an increase in the unpredictability of community dynamics in smaller impacted streams of Cerrado.
The gravelly substrate that is abundant in the streams studied is an important source of resources to several macroinvertebrates, including those of the order Trichoptera [
43,
44,
45], which may explain the higher abundance of this group in this basin.
Electrical conductivity and concentration of total dissolved solids, associated with anthropogenic impacts such as organic enrichment [
46] and soil erosion [
47], are two important variables in the structure of the aquatic community [
46,
48]. Measures of these two variables in two streams with high HII score (Taq8 and Taq9) were high, but there was no correlation with the biological metrics used. Taq8 was one of the two streams considered to have good environmental quality based on BMWP. These results could be a consequence of the predominant substrate in these streams, where the riverbed showed little accumulation of organic matter (considered a poor substrate), so that a small organic increment could contribute in favor of the fauna by acting as an intermediary disorder increasing taxa richness [
49] and consequently the score BMWP in Taq8, but a more effective increment, similar to what had happened in Taq9 (based on the values of electrical conductivity and STD) could negatively influence the community.
Melo [
50] showed that stream size and conductivity explained most of the variability in the macroinvertebrate community in a tropical stream. This is probably due to the fact that conductivity is as a general measure of disturbance, because it integrates the variables related to pollution, such as minerals and inorganic pollutants [
51]. Although electrical conductivity was not correlated with BMWP index in Taquaruçu Grande streams, it is possible that a continuous monitoring may reveal the influence of conductivity in the macroinvertebrate fauna through temporal variation related to pulses of organic increments in the water of streams.
Only the pH of the Taquaruçu Grande streams was correlated to biotic metrics, and it was associated with increased BMWP. Thus, it is not possible to infer that environmental impacts of anthropization are influencing the community of aquatic macroinvertebrates by measuring solely abiotic parameters. The BMWP was correlated to three other metrics considered important for water monitoring, especially the richness of EPT [
3] and richness of families [
52]. In addition, the EPT fauna and the richness of families have been considered to be highly congruent with the community; in other words, they can be representative of the community diversity [
32,
53]. Thus, its information cannot be ignored despite the high abiotic quality of most of the streams, and it should be considered in the evaluation of environmental quality of aquatic ecosystems of low order in Northern Brazil’s Cerrado. The catchment area to which Taquaruçu Grande basin belongs is known as the medium Tocantins basin area with 46,307.31 ha, it has about one hundred streams of first order that correspond to the main tributaries, which demand fast and low cost but effective monitoring protocols. HII may be recommended as part of those protocols, since it partly reveals effects of disturbance to the aquatic communities. HII remains an inexpensive measure of riparian zone structural variables such as canopy openness, litter bank volume, number of retention devices, proportion of benthic substrate components, and water temperature [
54], and should be considered for monitoring riparian deforestation in Northern Brazil’s Cerrado river ecosystems. The HII is directly related to the degree of environmental conservation and has been successfully used in other studies to evaluate the integrity in aquatic systems [
55,
56,
57,
58,
59]. The HII, which is applied rapidly and easily, provides environmental managers with an objective measure of the degree of alteration of aquatic habitats [
40].
Even though the Taquaruçu Grande is part of the Environmental Protection Area of Lajeado, it is suffering anthropic pressure by urbanization, agriculture, cattle farming, and tourism that negatively alter the integrity of the river ecosystems. Anthropic pressures bring significant loss of physical and chemical characteristics and therefore the reduction of aquatic biodiversity [
5].
This study did not focus on the seasonal fluctuations of water flow in Cerrado streams. Northern Cerrado rivers are similar to Amazonia rivers in that they vary throughout the rainfall and dry period cycle [
60]. Bleich et al. [
54] found severe alterations in habitat structure (especially water temperature, oxygen, suspended materials, and nitrite concentrations) and availability of substrates (litter, trunks, and retention devices) in altered streams (measured by HII) in comparison to pristine ones. According to Pearson [
61], current velocity is one of the main environmental correlates with species abundances and multivariate scores in tropical Australia. Faunal attributes cycled seasonally both in seasonal [
61,
62,
63] and aseasonal [
54,
64] tropics.
Also, a single biotic index proved confusing in the case of the Taquaruçu Grande basin. BMWP is a biotic index focused on an organism’s tolerance to organic pollution [
65,
66], with the tolerance determined relative to levels based on dissolved oxygen [
67,
68,
69]. However, Herman et al. [
70] point out that this approach does not take into account the combined impacts of multiple stressors within streams or the complex nature of stream ecosystems. In addition, BMWP was created in Britain and adapted for other regions. In Brazil, Junqueira et al. [
69] adapted BMWP for the Rio das Velhas basin, in the Southeast Brazil Cerrado area, and Monteiro et al. [
21] for the Meia Ponte River, also in the Southeast hydrogeographic region of Paraná River in Brazil. Monteiro et al. [
21] found different values for tolerance for the families: Elmidae, Hydrophilidae, Hydropsychidae, Leptoceridae, Odontoceridae, Leptophlebiidae, Baetidae, Perlidae, Gomphydae, Libellulidae, Calopterygidae, and Naucoridae, that are represented in the streams of Taquaruçu Grande. They may present a different value for tolerance in these streams when compared to the Meia Ponte basin streams, and further studies are needed to include local or regional values for tolerance in these families. It could also be recommended that other metrics should be included in a multi-metric index in order to better account for the complexity of stream ecosystems by way of a more comprehensive view of what is occurring within streams [
71,
72].
Also, according to Everaert et al. [
73], the associations between macroinvertebrates and abiotic factors appear to be river-specific and therefore not automatically transferable between river basins in the tropics. Low BMWP values that do not correlate with the physical and chemical variables may indicate that communities of macroinvertebrates in the Taquaruçu Grande river basin do not show the same correlation with the abiotic parameters as those in the study by Monteiro et al. [
21] in Meia Ponte River in the Cerrado of Goiás State, Brazil. This highlights the importance of establishing knowledge on the macroinvertebrate responses to alterations in different stream ecosystems in Cerrado. The Taquaruçu Grande basin is exemplary of the Northern Cerrado transition ecosystems that differ from the Cerrado of Goiás, which are more similar to the core of Southeast Brazil’s Cerrado landscapes. Although there is no detailed geographic classification of Brazilian ecoregions [
74], it is widely known that the Cerrado biome is a mosaic of different vegetation ecosystems and the Taquaruçu Grande basin is in the core of a vast transition zone of Cerrado with the biomes Caatinga and Amazonia. Ligeiro et al. [
74] point out that reference sites should be specific for a particular typology (e.g., altitude, stream size, and predominant substrate) and geographic domain (biome and ecoregion). As human modifications are widespread and occur across most landscapes on a worldwide basis, sites in least-disturbed condition may be used to represent reference conditions [
75,
76,
77]. This is the first study on the macroinvertebrate fauna in these ecosystems, and it raises the question as to whether studies in Cerrado ecosystems in the Southeast and West Brazil can model the parameters for biomonitoring of river ecosystems in the Northern part of this threatened biome.