Male Psyllids Differentially Learn in the Context of Copulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Colony
2.2. Experiment 1
2.3. Experiment 2
2.4. Experiment 3
2.5. Statistical Analysis
3. Results
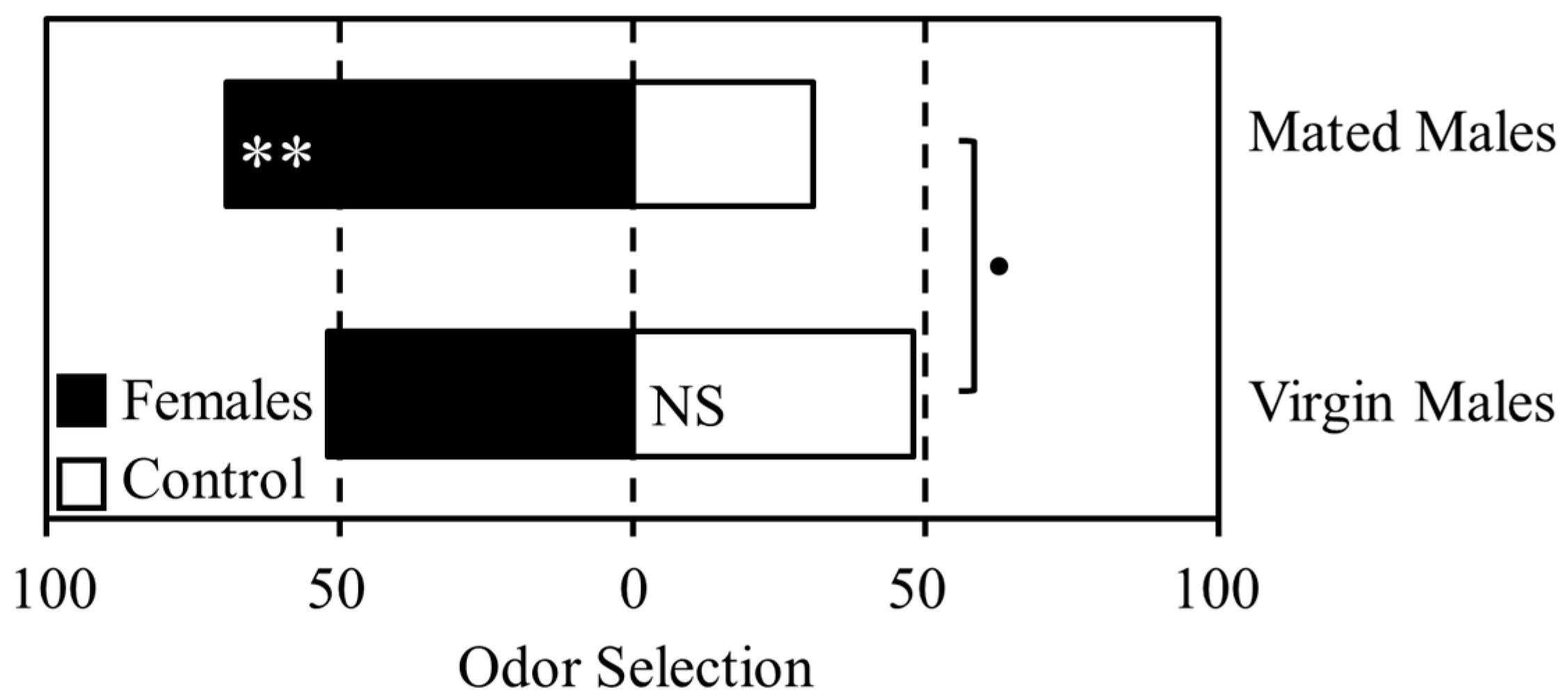
3.1. Experiment 1
3.2. Experiment 2
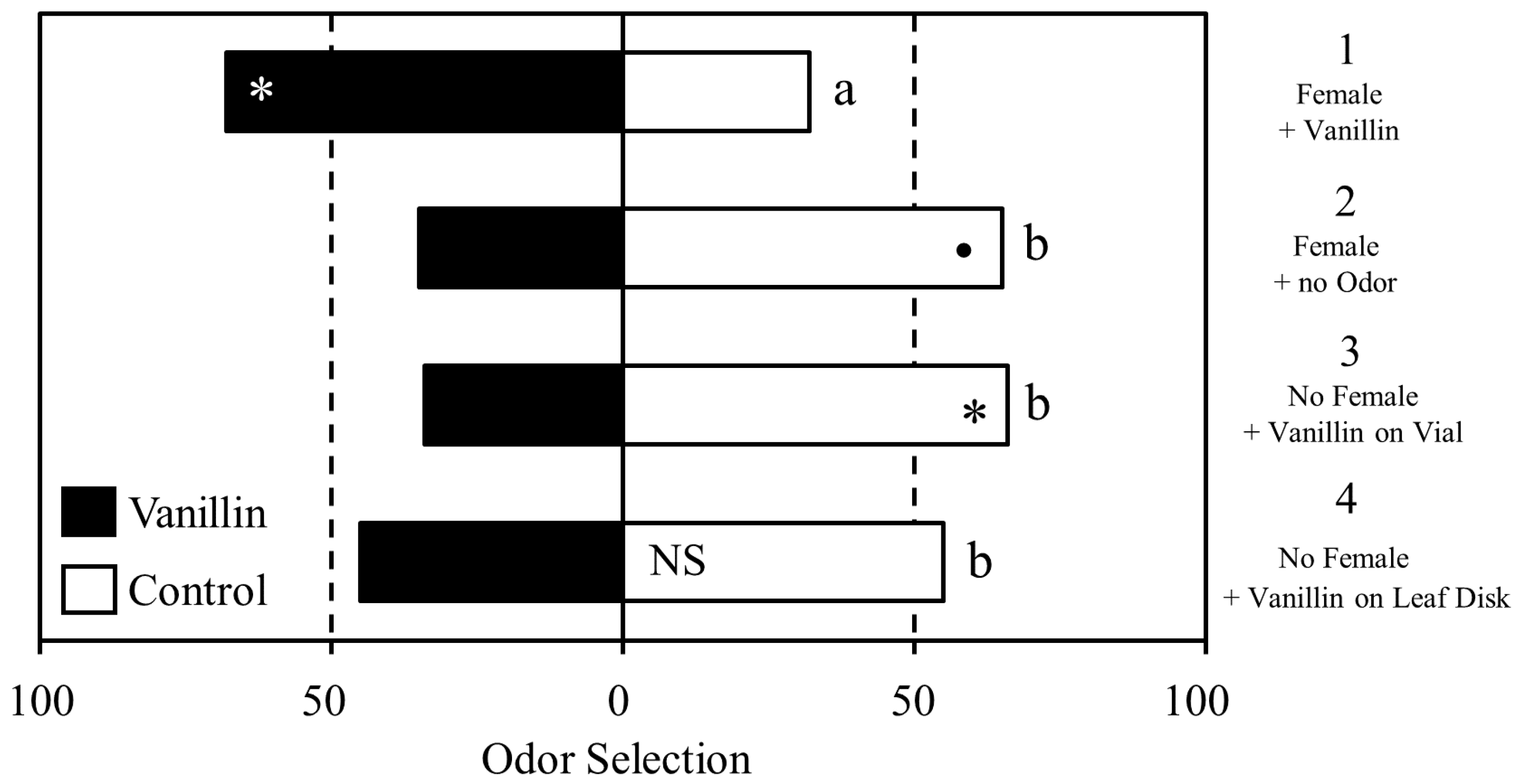
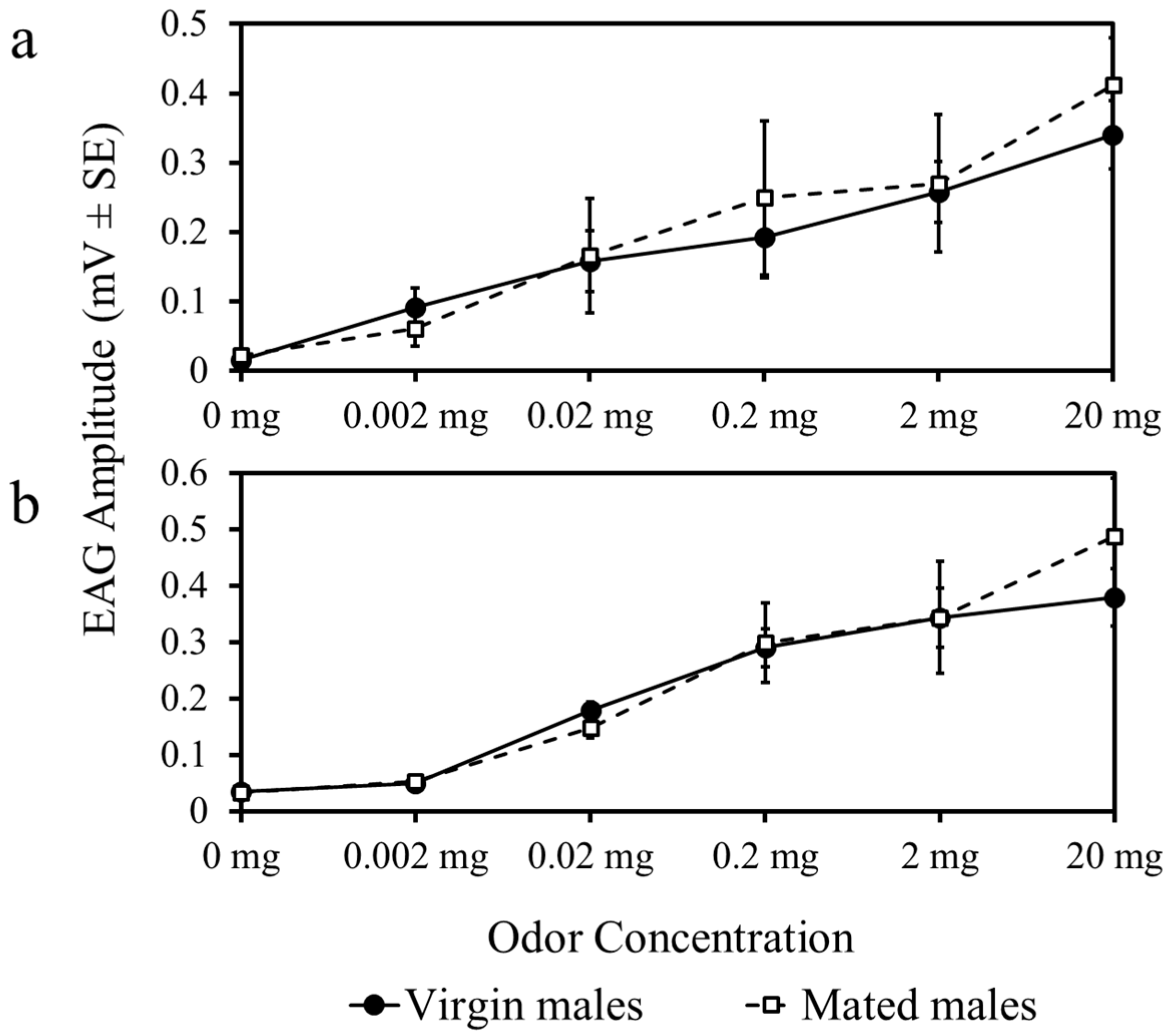
3.3. Experiment 3
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Halbert, S.E.; Manjunath, K.L. Asian citrus psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 2004, 87, 330–353. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Roles of olfactory cues, visual cues, and mating status in orientation of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) to four different host plants. Environ. Entomol. 2009, 38, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Lubanga, U.K.; Guedot, C.; Percy, D.M.; Steinbauer, M.J. Semiochemical and vibrational cues and signals mediating mate finding and courtship in Psylloidea (Hemiptera): A synthesis. Insects 2014, 5, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Tishechkin, D.Y. Vibratory communication in Psylloidea (Hemiptera). In Insect Sounds and Communication: Physiology, Behaviour, Ecology, and Evolution; Drosopoulos, S., Claridge, M.F., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2005. [Google Scholar]
- Tishechkin, D.Y. New data on vibratory communication in jumping plant lice of the families Aphalaridae and Triozidae (Homoptera, Psyllinea). Entomol. Rev. 2007, 87, 394–400. [Google Scholar] [CrossRef]
- Virant-Doberlet, M.; Cokl, A. Vibrational communication in insects. Neotrop. Entomol. 2004, 33, 121–134. [Google Scholar] [CrossRef]
- Borges, M.; Jepson, P.C.; Howse, P.E. Long-range mate location and close-range courtship behaviour of the Green Stink Bug, Nezara viridula and its mediation by sex pheromones. Entomol. Exp. Appl. 1987, 44, 205–212. [Google Scholar] [CrossRef]
- Todd, J.W. Ecology and behavior of Nezara viridula. Annu. Rev. Entomol. 1989, 34, 273–292. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Hall, D.G.; Mankin, R.W. Vibrational communication between the sexes in Diaphorina citri (Hemiptera: Psyllidae). Ann. Entomol. Soc. Am. 2009, 102, 547–555. [Google Scholar] [CrossRef]
- Martini, X.; Kuhns, E.H.; Hoyte, A.; Stelinski, L.L. Plant volatiles and density-dependent conspecific female odors are used by Asian citrus psyllid to evaluate host suitability on a spatial scale. Arthropod-Plant Interact. 2014, 8, 453–460. [Google Scholar] [CrossRef]
- Rohde, B.; Paris, T.M.; Heatherington, E.M.; Hall, D.G.; Mankin, R.W. Responses of Diaphorina citri (Hemiptera: Psyllidae) to conspecific vibrational signals and synthetic mimics. Ann. Entomol. Soc. Am. 2013, 106, 392–399. [Google Scholar] [CrossRef]
- Percy, D.M.; Taylor, G.S.; Kennedy, M. Psyllid communication: Acoustic diversity, mate recognition and phylogenetic signal. Invertebr. Syst. 2006, 20, 431–445. [Google Scholar] [CrossRef]
- Mankin, R.W.; Rohde, B.; McNeill, S. Vibrational duetting mimics to trap and disrupt mating of the devastating Asian citrus psyllid insect pest. Proc. Meet. Acoust. 2016. [Google Scholar] [CrossRef]
- Mankin, R.; Rohde, B. Use of vibrational duetting mimics to trap and disrupt mating of the Asian citrus psyllid, a devastating pest in Florida groves. J. Acoust. Soc. Am. 2015. [Google Scholar] [CrossRef]
- Mankin, R.W.; Rohde, B.B.; Mcneill, S.A.; Paris, T.M.; Zagvazdina, N.I.; Greenfeder, S. Diaphorina citri (Hemiptera: Liviidae) responses to microcontroller-buzzer communication signals of potential use in vibration traps. Fla. Entomol. 2013, 96, 1546–1555. [Google Scholar] [CrossRef]
- Moghbeli, G.A.; Ziaaddini, M.; Jalali, M.A.; Michaud, J.P. Sex-specific responses of Asian citrus psyllid to volatiles of conspecific and host-plant origin. J. Appl. Entomol. 2014, 138, 500–509. [Google Scholar] [CrossRef]
- Mann, R.S.; Rouseff, R.L.; Smoot, J.; Rao, N.; Meyer, W.L.; Lapointe, S.L.; Robbins, P.S.; Cha, D.; Linn, C.E.; Webster, F.X.; et al. Chemical and behavioral analysis of the cuticular hydrocarbons from Asian citrus psyllid, Diaphorina citri. Insect Sci. 2013, 20, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Guédot, C.; Horton, D.R.; Landolt, P.J. Attraction of male winterform pear psylla to female-produced volatiles and to female extracts and evidence of male–male repellency. Entomol. Exp. Appl. 2009, 130, 191–197. [Google Scholar] [CrossRef]
- Guédot, C.; Horton, D.R.; Landolt, P.J. Sex attraction in Bactericera cockerelli (Hemiptera: Triozidae). Environ. Entomol. 2010, 39, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Behavioral evidence for a female-produced sex attractant in Diaphorina citri. Entomol. Exp. Appl. 2008, 128, 450–459. [Google Scholar] [CrossRef]
- Vinauger, C.; Lahondère, C.; Cohuet, A.; Lazzari, C.R.; Riffell, J.A. Learning and memory in disease vector insects. Trends Parasitol. 2016, 32, 761–771. [Google Scholar] [CrossRef] [PubMed]
- McCall, P.J.; Kelly, D.W. Learning and memory in disease vectors. Trends Parasitol. 2002, 18, 429–433. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Lewis, W.J. Application of learning to pest management. In Insect Learning: Ecology and Evolutionary Perspectives; Papaj, D.R., Lewis, A.C., Eds.; Chapman and Hall: New York, NY, USA, 1993; pp. 308–342. [Google Scholar]
- Vinauger, C.; Lutz, E.K.; Riffell, J.A. Olfactory learning and memory in the disease vector mosquito Aedes aegypti. J. Exp. Biol. 2014, 217, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Menda, G.; Uhr, J.H.; Wyttenbach, R.A.; Vermeylen, F.M.; Smith, D.M.; Harrington, L.C.; Hoy, R.R. Associative learning in the dengue vector mosquito, Aedes aegypti: Avoidance of a previously attractive odor or surface color that is paired with an aversive stimulus. J. Exp. Biol. 2013, 216, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, N.M.; Brookfield, J.F.; Field, L.M.; Logan, J.G. Aedes aegypti mosquitoes exhibit decreased repellency by DEET following previous exposure. PLoS ONE 2013, 8, e54438. [Google Scholar] [CrossRef] [PubMed]
- Tomberlin, J.K.; Rains, G.C.; Allan, S.A.; Sanford, M.R.; Lewis, W.J. Associative learning of odor with food-or blood-meal by Culex quinquefasciatus Say (Diptera: Culicidae). Naturwissenschaften 2006, 93, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.S.; Lai, Y.L.; Giger, A.D. Learning and memory in the mosquito Aedes aegypti shown by conditioning against oviposition deterrence. Med. Vet. Entomol. 2003, 17, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Vinauger, C.; Pereira, M.H.; Lazzari, C.R. Learned host preference in a Chagas disease vector, Rhodnius prolixus. Acta tropica 2012, 122, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Abramson, C.I.; Aldana, E.; Sulbaran-Romero, E.; Lizano, E. Fifth instar experience reduces aversiveness of the plant extract ruda (Ruta graveolens) in the adult triatomine Rhodnius prolixus Stal 1859. J. Vector Ecol. 2006, 31, 196–197. [Google Scholar] [CrossRef]
- Bouyer, J.; Pruvot, M.; Bengaly, Z.; Guerin, P.M.; Lancelot, R. Learning influences host choice in tsetse. Biol. Lett. 2007, 3, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, J.; Cuisance, D.; Messad, S.; Guerin, P.M. Learning affects host preference in tsetse flies. Revue d’Élevage et de Médecine Vétérinaire des Pays Tropicaux 2005, 58, 27–29. [Google Scholar]
- Guerra-Silva, N.M.M.; De Melo, S.C.C.S.; Massafera, R.; Rossi, R.M.; Silveira, T.G.V.; Teodoro, U. Dispersal and memory of sand flies in an endemic area of cutaneous leishmaniasis, southern Brazil. J. Med. Entomol. 2013, 50, 986–993. [Google Scholar] [CrossRef]
- Stockton, D.G.; Martini, X.; Patt, J.M.; Stelinski, L.L. The influence of learning on host plant preference in a significant phytopathogen vector, Diaphorina citri. PLoS ONE 2016, 11, e0149815. [Google Scholar] [CrossRef] [PubMed]
- Vreysen, M.J.; Seck, M.T.; Sall, B.; Bouyer, J. Tsetse flies: Their biology and control using area-wide integrated pest management approaches. J. Invertebr. Pathol. 2013, 112, S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Sadek, M.M.; Hansson, B.S. Pre-exposure modulates attraction to sex pheromone in a moth. Chem. Sens. 2003, 28, 285–291. [Google Scholar] [CrossRef]
- Anderson, P.; Hansson, B.S.; Nilsson, U.; Han, Q.; Sjöholm, M.; Skals, N.; Anton, S. Increased behavioral and neuronal sensitivity to sex pheromone after brief odor experience in a moth. Chem. Sens. 2007, 32, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Dukas, R. Male fruit flies learn to avoid interspecific courtship. Behav. Ecol. 2004, 15, 695–698. [Google Scholar] [CrossRef]
- Dukas, R. Dynamics of learning in the context of courtship in Drosophila persimilis and D. pseudoobscura. Anim. Behav. 2009, 77, 253–259. [Google Scholar] [CrossRef]
- Kujtan, L.; Dukas, R. Learning magnifies individual variation in heterospecific mating propensity. Anim. Behav. 2009, 78, 549–554. [Google Scholar] [CrossRef]
- Dukas, R. Learning in the context of sexual behaviour in insects. Anim. Biol. 2006, 56, 125–141. [Google Scholar] [CrossRef]
- Dukas, R. Experience improves courtship in male fruit flies. Anim. Behav. 2005, 69, 1203–1209. [Google Scholar] [CrossRef]
- Wcislo, W.T. The role of learning in the mating biology of a sweat bee Lasioglossum zephyrum (Hymenoptera: Halictidae). Behav. Ecol. Sociobiol. 1987, 20, 179–185. [Google Scholar] [CrossRef]
- Smith, B.H. Recognition of female kin by male bees through olfactory signals. Proc. Natl. Acad. Sci. USA 1983, 80, 4551–4553. [Google Scholar] [CrossRef] [PubMed]
- Stelinski, L.L.; Miller, J.R.; Gut, L.J. Presence of long-lasting peripheral adaptation in oblique-banded leafroller, Choristoneura rosaceana and absence of such adaptation in redbanded leafroller, Argyrotaenia velutinana. J. Chem. Ecol. 2003, 29, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Wenninger, E.J.; Hall, D.G. Daily timing of mating and age at reproductive maturity in Diaphorina citri (Hemiptera: Psyllidae). Fla. Entomol. 2007, 90, 715–722. [Google Scholar] [CrossRef]
- Wenninger, E.J.; Stelinski, L.L.; Hall, D.G. Relationships between adult abdominal color and reproductive potential in Diaphorina citri (Hemiptera: Psyllidae). Ann. Entomol. Soc. Am. 2009, 102, 476–483. [Google Scholar] [CrossRef]
- Stelinski, L.L.; Tiwari, S. Vertical T-maze choice assay for arthropod response to odorants. J. Vis. Exp. 2013, 72, e50229. [Google Scholar] [CrossRef] [PubMed]
- Patt, J.M.; Stockton, D.; Meikle, W.G.; Sétamou, M.; Mafra-Neto, A.; Adamczyk, J.J. Innate and conditioned responses to chemosensory and visual cues in Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), vector of Huanglongbing pathogens. Insects 2014, 5, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Patt, J.M.; Setamou, M. Responses of the Asian citrus psyllid to volatiles emitted by the flushing shoots of its rutaceous host plants. Environ. Entomol. 2010, 39, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.S.; Rouseff, R.L.; Smoot, J.M.; Castle, W.S.; Stelinski, L.L. Sulfur volatiles from Allium spp. affect Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), response to citrus volatiles. Bull. Entomol. Res. 2011, 101, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zagvazdina, N.Y.; Paris, T.M.; Udell, B.J.; Stanislauskas, M.; Mcneill, S.; Allan, S.A.; Mankin, R.W. Effects of atmospheric pressure trends on calling, mate-seeking, and phototaxis of Diaphorina citri (Hemiptera: Liviidae). Ann. Entomol. Soc. Am. 2015, 108, 762–770. [Google Scholar] [CrossRef]
- Magurran, A.E.; Ramnarine, I.W. Learned mate recognition and reproductive isolation in guppies. Anim. Behav. 2004, 67, 1077–1082. [Google Scholar] [CrossRef]
- Kozak, G.M.; Boughman, J.W. Learned conspecific mate preference in a species pair of sticklebacks. Behav. Ecol. 2009, 20, 1282–1288. [Google Scholar] [CrossRef]
- Takahashi, Y.; Watanabe, M. Male mate choice based on ontogenetic colour changes of females in the damselfly Ischnura senegalensis. J. Ethol. 2011, 29, 293–299. [Google Scholar] [CrossRef]
- Vet, L.E.; Groenewold, A.W. Semiochemicals and learning in parasitoids. J. Chem. Ecol. 1990, 16, 3119–3135. [Google Scholar] [CrossRef] [PubMed]
- Minoli, S.; Kauer, I.; Colson, V.; Party, V.; Renou, M.; Anderson, P.; Gadenne, C.; Marion-Poll, F.; Anton, S. Brief exposure to sensory cues elicits stimulus-nonspecific general sensitization in an insect. PLoS ONE 2012, 7, e34141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartell, R.J.; Roelofs, W.L. Inhibition of sexual response in males of the moth Argyrotaenia velutinana by brief exposures to synthetic pheromone and its geometric isomer. J. Insect Physiol. 1973, 19, 655–661. [Google Scholar] [CrossRef]
- Soroker, V.; Talebaev, S.; Harari, A.R.; Wesley, S.D. The role of chemical cues in host and mate location in the pear psylla Cacopsylla bidens (Homoptera: Psyllidae). J. Insect Behav. 2004, 17, 613–626. [Google Scholar] [CrossRef]
- Horton, D.R.; Landolt, P.J. Attraction of male pear psylla, Cacopsylla pyricola, to female-infested pear shoots. Entomol. Exp. Appl. 2007, 123, 177–183. [Google Scholar] [CrossRef]
- Guedot, C.; Horton, D.R.; Landolt, P.J.; Munyaneza, J.E. Effect of mating on sex attraction in Bactericera cockerelli with evidence of refractoriness. Entomol. Exp. Appl. 2013, 149, 27–35. [Google Scholar] [CrossRef]
- Stephens, D.W. Learning and behavioral ecology: Incomplete information and environmental predictability. In Insect Learning: Ecology and Evolutionary Perspectives; Papaj, D.R., Lewis, A.C., Eds.; Chapman and Hall: New York, NY, USA, 1993; pp. 195–218. [Google Scholar]
- Dukas, R. Effects of learning on evolution: Robustness, innovation and speciation. Anim. Behav. 2013, 85, 1023–1030. [Google Scholar] [CrossRef]
- Papaj, D.R.; Prokopy, R.J. Ecological and evolutionary aspects of learning in phytophagous insects. Annu. Rev. Entomol. 1989, 34, 315–350. [Google Scholar] [CrossRef]
- Dukas, R.; Clark, C.W.; Abbott, K. Courtship strategies of male insects: When is learning advantageous? Anim. Behav. 2006, 72, 1395–1404. [Google Scholar] [CrossRef]
- Skals, N.; Anderson, P.; Kanneworff, M.; Löfstedt, C.; Surlykke, A. Her odours make him deaf: Crossmodal modulation of olfaction and hearing in a male moth. J. Exp. Biol. 2005, 208, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Bonduriansky, R. The evolution of male mate choice in insects: A synthesis of ideas and evidence. Biol. Rev. 2001, 76, 305–339. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.W.; Llorens, T.; Schinzig, M.; Hosken, D.; Craig, M. Sperm competition selects for male mate choice and protandry in the bushcricket, Requena verticalis (Orthoptera: Tettigoniidae). Anim. Behav. 1994, 47, 117–122. [Google Scholar] [CrossRef]
- Mazalov, V.; Perrin, N.; Dombrovsky, Y. Adaptive search and information updating in sequential mate choice. Am. Nat. 1996, 148, 123–137. [Google Scholar] [CrossRef]
- Long, C.E.; Markow, T.A.; Yaeger, P. Relative male age, fertility, and competitive mating success in Drosophila melanogaster. Behav. Genet. 1980, 10, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Moulin, B.; Rybak, F.; Aubin, T.; Jallon, J.M. Compared ontogenesis of courtship song components of males from the sibling species, D. melanogaster and D. simulans. Behav. Genet. 2001, 31, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, R.; Alcock, J. The Evolution of Insect Mating Systems; Harvard University Press: Cambridge, MA, USA, 1983. [Google Scholar]
- White, T.C. An index to measure weather-induced stress of trees associated with outbreaks of psyllids in Australia. Ecology 1969, 50, 905–909. [Google Scholar] [CrossRef]
- Krysan, J.L. Laboratory study of mating behavior as related to diapause in overwintering Cacopsylla pyricola (Homoptera: Psyllidae). Environ. Entomol. 1990, 19, 551–557. [Google Scholar] [CrossRef]
- Kozlowski, M.W.; Aoxiang, S. Ritual behaviors associated with spermatophore transfer in Deuterosminthurus bicinctus (Collembola: Bourletiellidae). J. Ethol. 2006, 24, 103–109. [Google Scholar] [CrossRef]
- Farooqui, T. Review of octopamine in insect nervous systems. Open Access Insect Physiol. 2012, 4, 1–17. [Google Scholar] [CrossRef]
- Berry, M.D. Mammalian central nervous system trace amines: Pharmacologic amphetamines, physiologic neuromodulators. J. Neurochem. 2004, 90, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.; Guzmán, M.G.; Morales-Matos, C.; Díaz, R.A.D.V.; Abramson, C.I.; Giray, T. Dopamine and octopamine influence avoidance learning of honey bees in a place preference assay. PLoS ONE 2011, 6, e25371. [Google Scholar] [CrossRef] [PubMed]
- Mizunami, M.; Unoki, S.; Mori, Y.; Hirashima, D.; Hatano, A.; Matsumoto, Y. Roles of octopaminergic and dopaminergic neurons in appetitive and aversive memory recall in an insect. BMC Biol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Unoki, S.; Matsumoto, Y.; Mizunami, M. Roles of octopaminergic and dopaminergic neurons in mediating reward and punishment signals in insect visual learning. Euro. J. Neurosci. 2006, 24, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stockton, D.G.; Martini, X.; Stelinski, L.L. Male Psyllids Differentially Learn in the Context of Copulation. Insects 2017, 8, 16. https://doi.org/10.3390/insects8010016
Stockton DG, Martini X, Stelinski LL. Male Psyllids Differentially Learn in the Context of Copulation. Insects. 2017; 8(1):16. https://doi.org/10.3390/insects8010016
Chicago/Turabian StyleStockton, Dara G., Xavier Martini, and Lukasz L. Stelinski. 2017. "Male Psyllids Differentially Learn in the Context of Copulation" Insects 8, no. 1: 16. https://doi.org/10.3390/insects8010016






