1. Introduction
Both higher and lower termites interact with fungi [
1]. In particular, termites and wood-decay fungi both decompose woody substances; therefore, several forms of interactions likely formed between these two organisms. For example, non-entomopathogenic fungi can influence the palatability of wood [
2,
3], acting as feeding attractants, stimuli or preference inducers [
4,
5], or as deterrents [
6] towards termites. Thus, fungi-termite interactions are ubiquitous, although they are sometimes provisional in character and cannot be categorized as symbiosis [
7].
Factors such as fungal strain, degree of decay, as well as tree and termite species, should be considered in fungi-termite interactions, especially with respect to termite feeding preferences for fungus-decayed wood [
1,
2]. Wood-decay fungi facilitate termite consumption of lignocellulose [
8] through the degradation or modification of wood components. Wood-decay fungi trigger various responses in termite feeding behavior under field and laboratory conditions [
1,
4,
8]. For instance, fungal chelate accumulation during wood decay initiates lignin oxidation, cellulose depolymerization, and lignin degradation reactions, which may act as chemosensory signals to termites that the wood is heavily degraded and therefore nutritionally inadequate [
6]. However, wood-decay fungi also appear to detoxify wood extractives, rendering soft wood more suitable for termite consumption [
5].
Although interactions between termites and decay fungi are frequently observed on wood as a fungal substrate, it remains unclear whether these interactions are actually mediated by the presence of wood components or solely between the termite and fungus themselves. Therefore, it is important to clarify whether termite feeding is related to fungal secretion or products released during wood degradation. In our previous study, we reported a feeding deterrent effect from stakes decayed by brown rot basidiomycete
Fibroporia radiculosa [
9]. We determined that the compounds linked to this deterrent effect are also present in
n-hexane wood extracts; both field-decayed wood stakes and laboratory-decayed wood extract yielded the same outcome. This study aimed to further verify the identity and origin of the compound from
Fibroporia radiculosa (Peck) Parmasto that is responsible for the feeding deterrent effect on
Reticulitermes speratus (Kolbe).
2. Materials and Methods
2.1. Termites
Five R. speratus colonies were used in this study: three colonies (Oarai 1, 2, and 3) were obtained from Oarai, Ibaraki Prefecture, Japan, during October 2016. Two colonies (Kagoshima 1 and 2) were obtained from a field maintained by the Research Institute for Sustainable Humanosphere (RISH) in Kagoshima Prefecture, Japan, during December 2014. All termite colonies were housed in plastic containers in a temperature- and moisture-controlled laboratory until use. Different colonies were used for each test; no colony was used more than once.
2.2. Pure Cultures of Fibroporia radiculosa
Fibroporia radiculosa cultures were previously isolated, identified, and purified from decayed stakes obtained from a RISH field in 2009 [
9]. The fungus was maintained in a potato dextrose agar (PDA) slant containing 100 ppm benlate and 50 ppm tetracycline to prevent mold growth, and kept under refrigerated conditions (2 to 3 °C) until use.
2.3. Preparation of Decayed Wood and PDA Media
Laboratory decayed woods were prepared by drying block samples from sapwood of Pinus densiflora (20 mm × 20 mm × 5 mm) at 60 °C for 48 h, followed by sterilization by ethylene oxide gas. Three sterilized woods were aseptically placed on a F. radiculosa mycelium grown in a diluted potato dextrose agar medium and kept inside an incubator at 27 °C for eight weeks. To prepare diluted Potato Dextrose Agar (PDA) medium for laboratory decayed wood, 1.3 g of PDA powder (Nissui Pharmaceuticals, Tokyo, Japan) and 2.6 g of agar (Nacalai Tesque, Kyoto, Japan) were diluted in 100 mL tap water. The medium was poured into a 500 mL screw-capped bottle and sterilized by autoclaving at 121 °C for 15 min. Sterilized medium was inoculated with F. radiculosa pure cultures and incubated at 27 °C for seven days or until the mycelium has covered the diluted PDA surface.
Meanwhile, decayed PDA medium were prepared via mixing 3.9 g of PDA powder (Nissui Pharmaceuticals, Tokyo, Japan) with 100 mL of tap water, then poured into a 500-mL screw-capped bottle for autoclaving at 121 °C and 15 min. Pure culture of F. radiculosa were transferred aseptically to the media surface. Cultured medium were cultivated in a 27 °C incubator for eight weeks.
2.4. Extract Preparation of Extract and PDA Media
Decayed P. densiflora blocks were removed from the incubation bottle. Blocks surface were carefully cleaned from fungal mycelium. The woods were freeze-dried overnight, cut into smaller pieces, and ground using a mortar and pestle to obtain decayed wood powder. Five grams of the decayed wood powder was inserted into a 200 mL conical flask and subjected to extraction with 100 mL of n-hexane at 18–25 °C overnight (10–12 h). The mixture was filtered using filter paper (No. 1, Advantec Toyo Roshi, Tokyo, Japan) and concentrated using a rotary evaporator into a 10 mL solution.
For extract preparation from fungal cultures cultivated on growth media, the mycelium-covered medium surface was carefully scraped using a spatula; the collected material was ground with a glass stirrer, and then added to a 300 mL conical flask. The ground medium (100 g) was extracted with 100 mL of n-hexane overnight or up to 24 h. The mixture was filtered using No. 1 filter paper (Advantec Toyo Roshi) and concentrated to 5 mL in a rotary evaporator at 10 °C, then refrigerated (2–3 °C) for a maximum of 14 days after extraction.
2.5. Purification of Extract
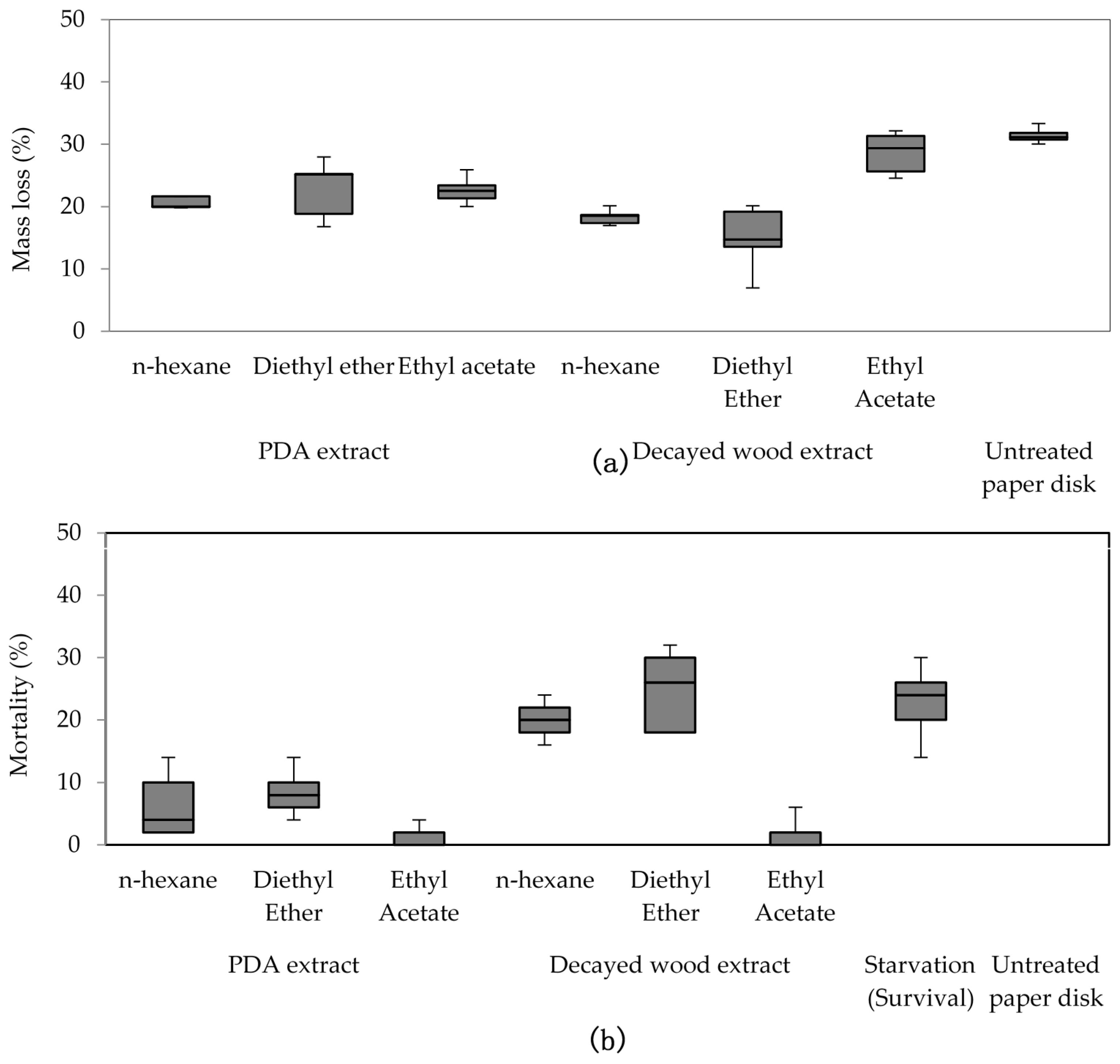
The decayed wood and PDA extracts were purified using small-scale column chromatography. Into a cotton wool plugged Pasteur pipette (146 mm, Corning, New York, NY, USA) silica gel (C200, Wako Pure Chemicals Industry, Osaka, Japan) was dry-packed (4 cm long). Then an aliquot of 4 mL of extract was added; n-hexane, diethyl ether, and ethyl acetate were used. Each portion was divided into two equal parts for bioassay and chemical analyses using gas chromatography/mass spectrometry (GC/MS).
2.6. Feeding Deterrent Bioassays (No-Choice and Two-Choice Feeding Tests)
2.6.1. No-Choice Feeding Test of Crude and Purified Extract from Wood and PDA Media Decayed by F. radiculosa
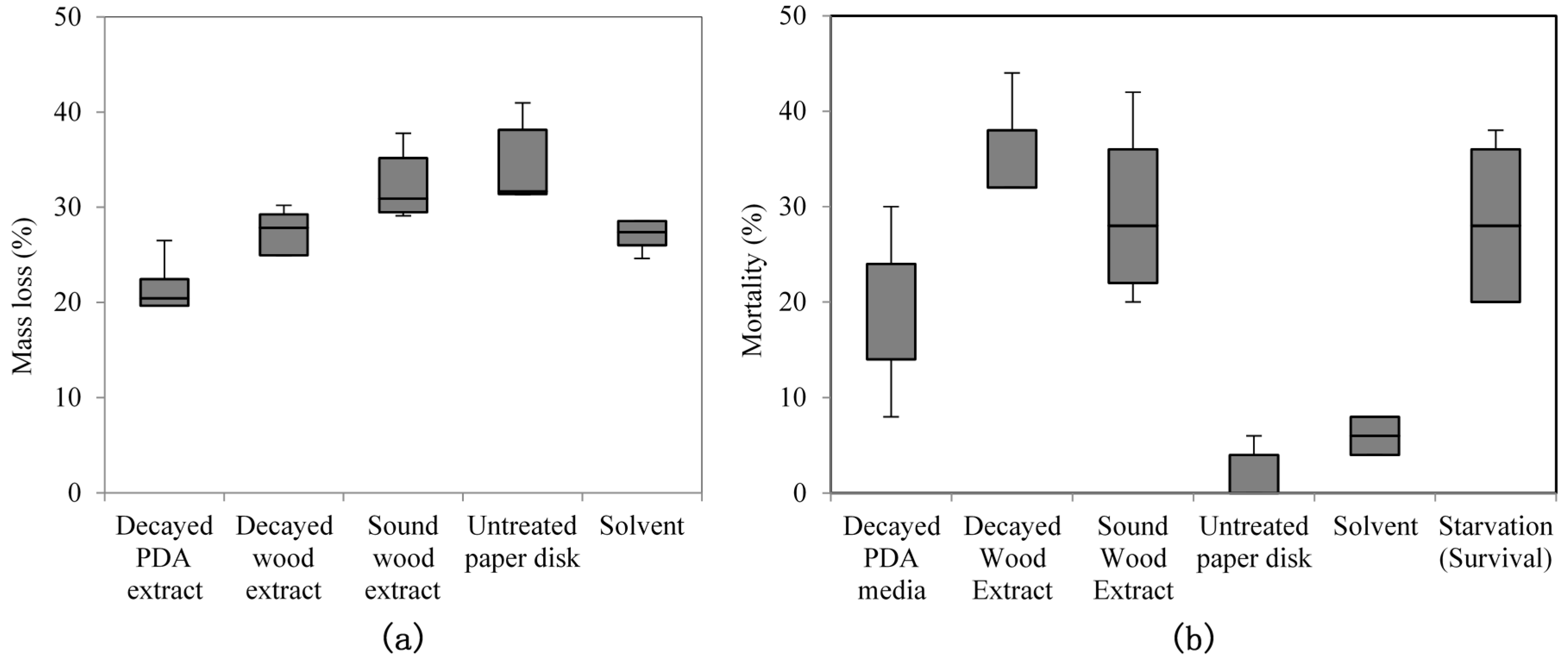
No-choice feeding tests were conducted as follows: paper disks (Ø 8 mm, thick type; Advantec Toyo Roshi) were soaked with 100 μL of decayed wood extract (containing 50 mgeq (milligrams equivalent) of decayed wood), 100 μL of decayed PDA extract (containing 500 mgeq of decayed PDA media), or 100 μL of sound wood extract (containing 5 mgeq of sound wood) used as control. The disks were then treated with 100 μL of n-hexane were used as a solvent treatment. Treated paper disks were placed inside a fume hood for 15 min to remove the solvent before being transferred into individual plastic saucer (Ø 1 cm) on top of 15-mm-thick hard plaster (New Plastone Dental Stone, GC Corp., Tokyo, Japan). The top of hard plaster was covered with a thin layer of wet sand on which a sample disk on the saucer was placed. The bottom of each cup was perforated (perforations 10 mm in diameter) to supply moisture through the plaster layer, which was in contact with a damp paper pad at the bottom of the test chamber. Fifty R. speratus workers of Kagoshima 1 colony were introduced to each cup. Test chambers were kept inside an incubator for five days at 25 °C and 70%–80% relative humidity (RH). Seven biological replicates were performed (seven cups each with 50 R. speratus) for each treatment. At the end of the test period, paper disks were weighed to assess mass loss from termite feeding and the remaining termites were counted to determine the mortality percentage.
Purified extracts bioassay was conducted in a similar manner to crude extracts, but with 300 μL (150 mgeq decayed and sound wood, 1500 mgeq of decayed PDA) of purified extracts. The tests were conducted for four days at 25 °C and 70%–80% RH, and replicated five times (five cups of 50 R. speratus for each treatment) using Oarai 3 colony. In no-choice tests, starvation treatment was used as a control towards termite mortality. Fifty termite workers (per cup) were not given any food during test period. The number of replication for starvation treatment is identical to other treatments (seven times for crude extracts and five times for purified extracts) in each feeding test. After the period finished, dead termite were counted to determine the mortality percentage.
2.6.2. Two-Choice Feeding Test Using Extract from F. radiculosa Decayed Wood and PDA Media
Two-choice feeding test were performed to assess termite preferences between paper disks treated with decayed wood and PDA media extract. Inside a 200 mL cup (prepared as described in
Section 2.6.1), paper disks treated with 100 μL of either decayed wood or PDA extract were placed, 4 cm apart, in a single plastic saucer. One hundred termite workers were introduced into each cup. Test chambers were kept inside an incubator for five days at 25 °C and 70%–80% RH. One
R. speratus colony (Kagoshima 2) from Kagoshima and two colonies from Oarai (Oarai 1 and 2) were used in this test. Eight biological replications (eight cups each with 100
R. speratus) were performed for each colony. Paper disks were weighed at the end of the test period to assess the mass loss from termite feeding.
2.7. Gas Chromatography and Mass Spectrometer Analyses
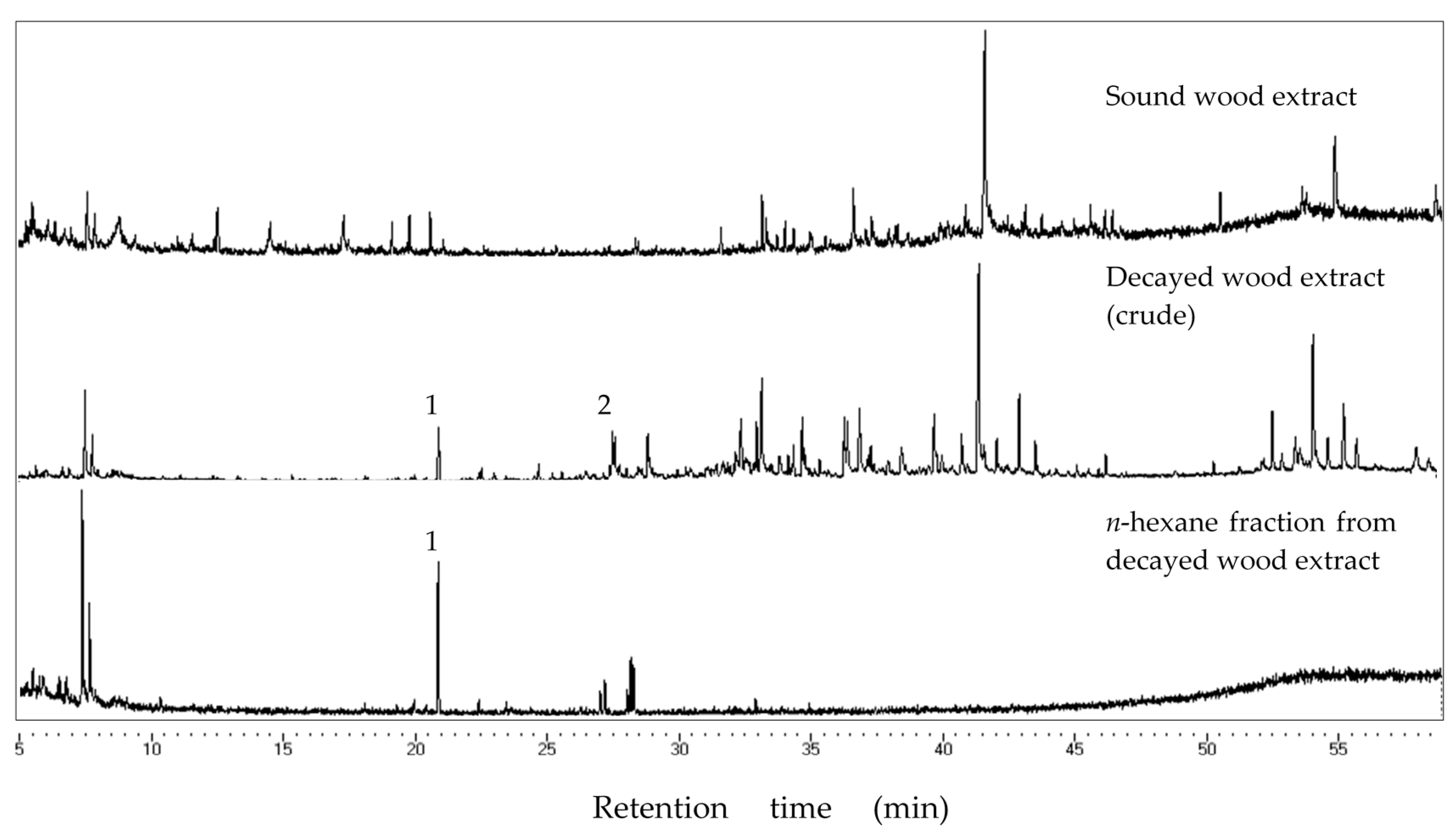
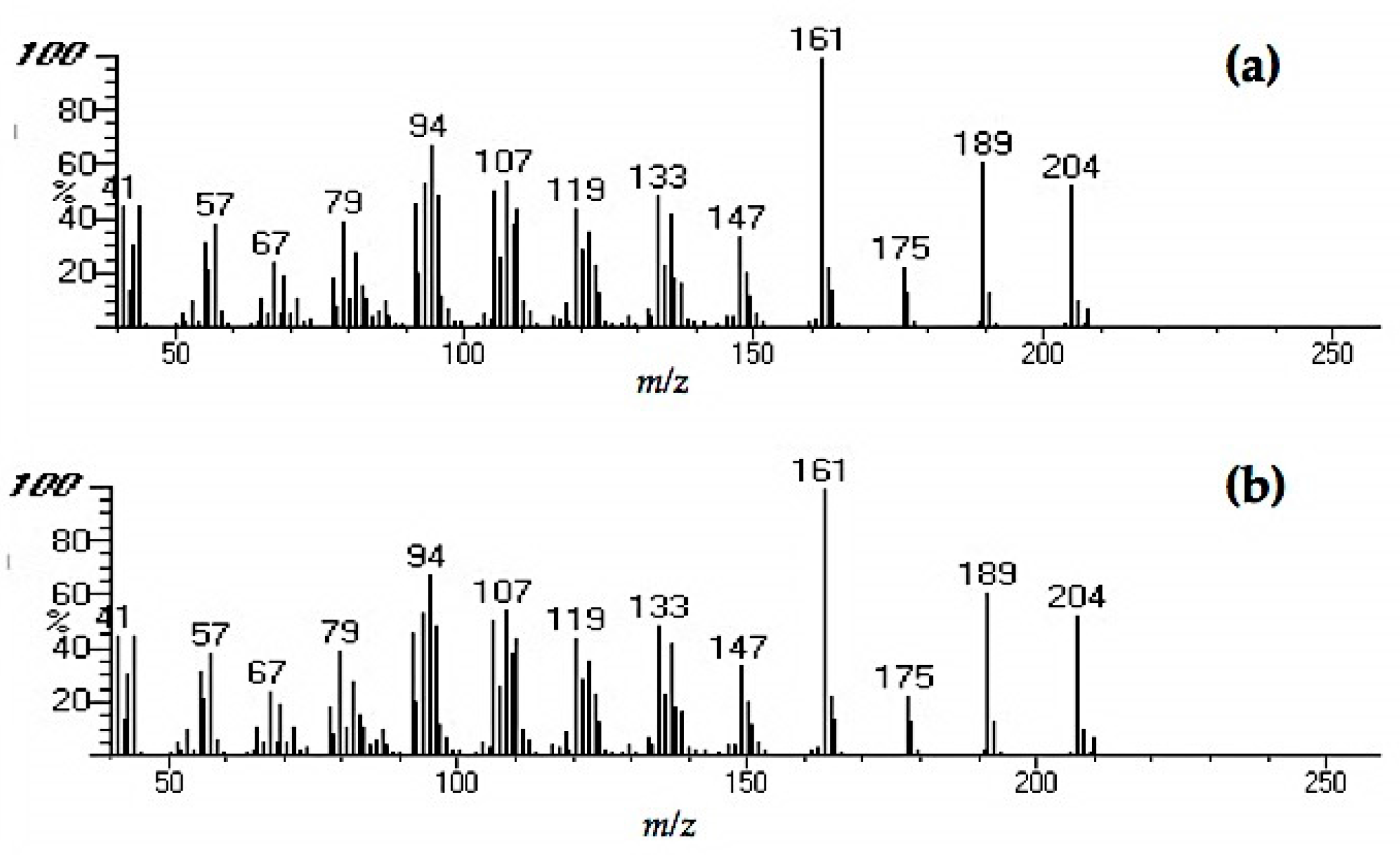
Chemical analyses of the bioactive fraction in decayed wood and PDA extract was carried out using GC-MS analysis. JEOL MS-600 (JEOL Ltd., Tokyo, Japan) mass spectrometer was coupled with Hewlett-Packard 6890N (Hewlett-Packard, Palo Alto, CA, USA) gas chromatograph was used for GC-MS and helium was used as the carrier gas at 1 mL/min in a constant flow mode. Samples were injected at 200 °C in the split-less mode (sampling time: 0.75 min), separated with a fused silica capillary column (DB-5MS, 25 m × 0.25 mm, 0.25 µm film thickness, Agilent Technology Inc., Santa Clara, CA, USA). Oven temperature was maintained at 50 °C for 3 min, increased to 300 °C at a rate of 5 °C/min, and kept steady for 6 min. The transfer line was kept at 280 °C. Mass spectra were obtained by electron ionization at 70 eV, at a scan speed of 0.29 s with a mass range of 40–600 amu. Major peaks in the chromatogram were compared to those of compounds in the National Institute of Science and Technology (USA) Mass Spectral (NIST MS) library for identification.
2.8. Statisitical Analysis
For feeding tests, treatment and control samples were compared using non-parametric test, Kruskal-Wallis H. The significance level was set at p = 0.05. All tests were performed using IBM SPSS Statistics version 22 software (IBM Corp., New York, NY, USA).
5. Conclusions
Extracts from fungi-decayed PDA media inhibited R. speratus feeding, indicating that the deterrent substance originated from F. radiculosa rather than the wood decay process. Despite the stronger feeding deterrent effect of the crude PDA extract, its effects differed from those of decayed wood extract. Conceivably, decayed wood extract may contain two bioactive compounds that deter feeding and are toxic to termites. Chromatography easily removed the decayed PDA media–derived bioactive compound, causing the purified extract to lose its deterrent characteristics and weakly suppress termite feeding. According to the NIST MS match, the bioactive compounds were indicated to be sesquiterpenes—the decayed wood extract contained two bioactive suspect compounds, longifolene (similarity index 88.9) and hinesol (similarity index 78.2), while the PDA extract contained only hinesol. The F. radiculosa growth medium appears to be primarily responsible for the differences in the bioactive compound produced. Future studies on termite-fungi interactions should aim to further investigate the effects of F. radiculosa growth media and variations in feeding deterrents produced.