A Nutritional Profile of the Trap-Nesting Wasp Trypoxylon lactitarse (Hymenoptera: Crabronidae): Comparison of Sexes and Overwintering and Non-Overwintering Generations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trap-Nests
2.2. Study Sites
2.3. Monitoring and Field Collections
2.4. Opening Tubes and Rearing
2.5. Measurements
2.6. Micronutrient Analysis
2.7. Macronutrient Analyses
2.8. Data Analysis
2.8.1. Life Stage Comparisons
2.8.2. Changes during Development
2.8.3. Morphology and Nutrient Comparisons
3. Results
3.1. Eggs and Food
3.2. Immatures
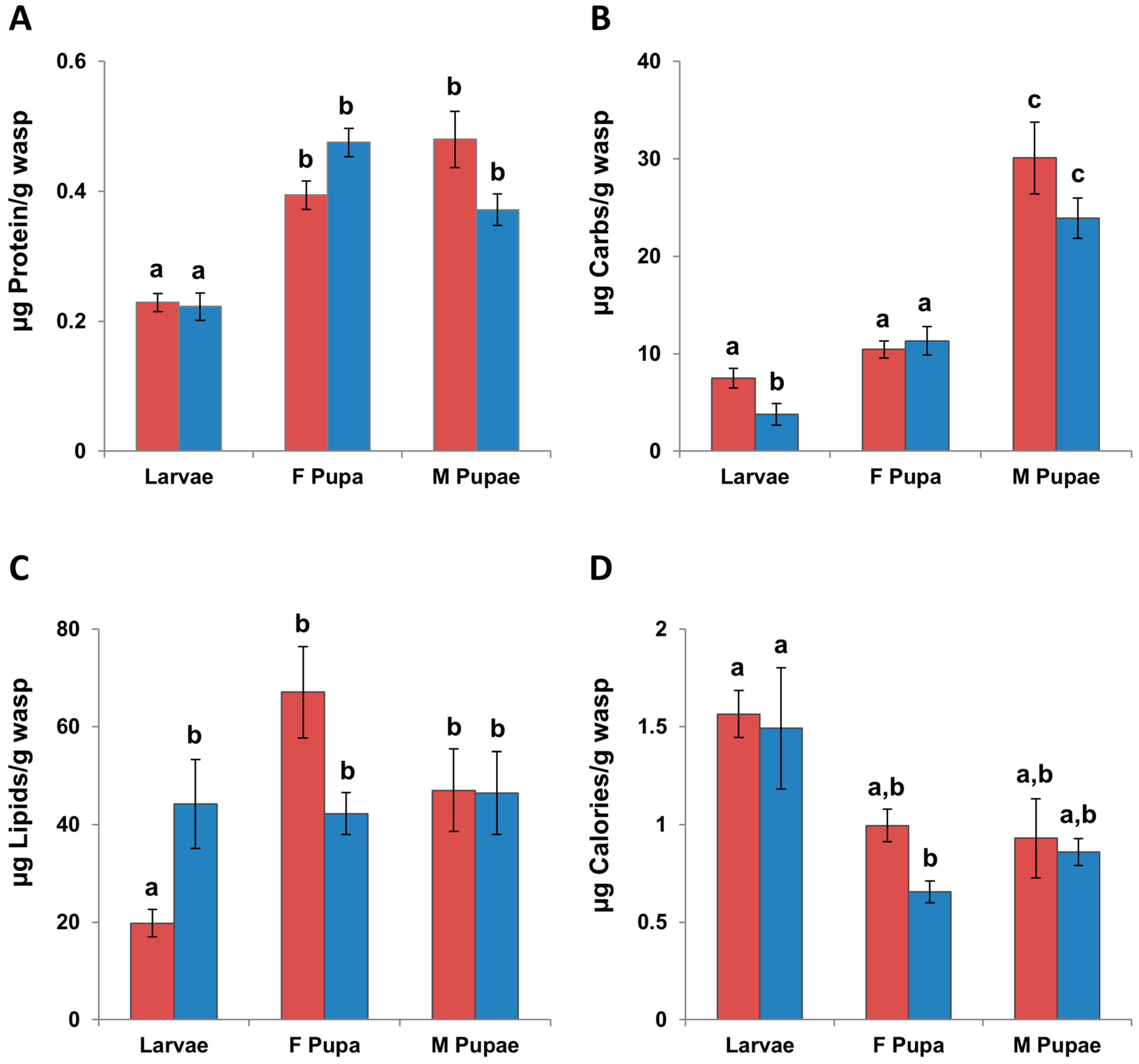
3.2.1. Macronutrients
3.2.2. Calories
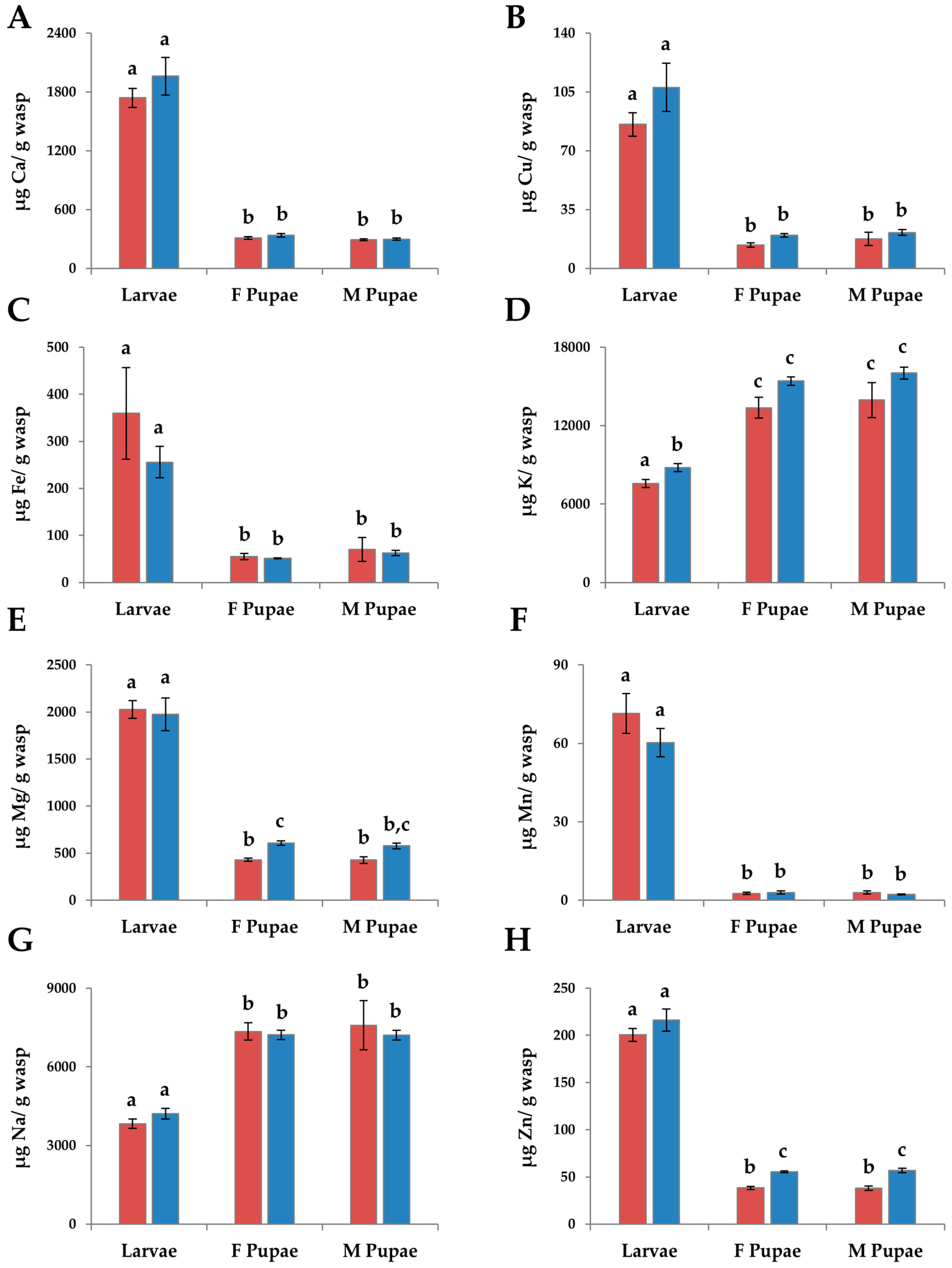
3.2.3. Micronutrients
3.3. Changes in Individuals from Initial Nutrients
3.4. Adults
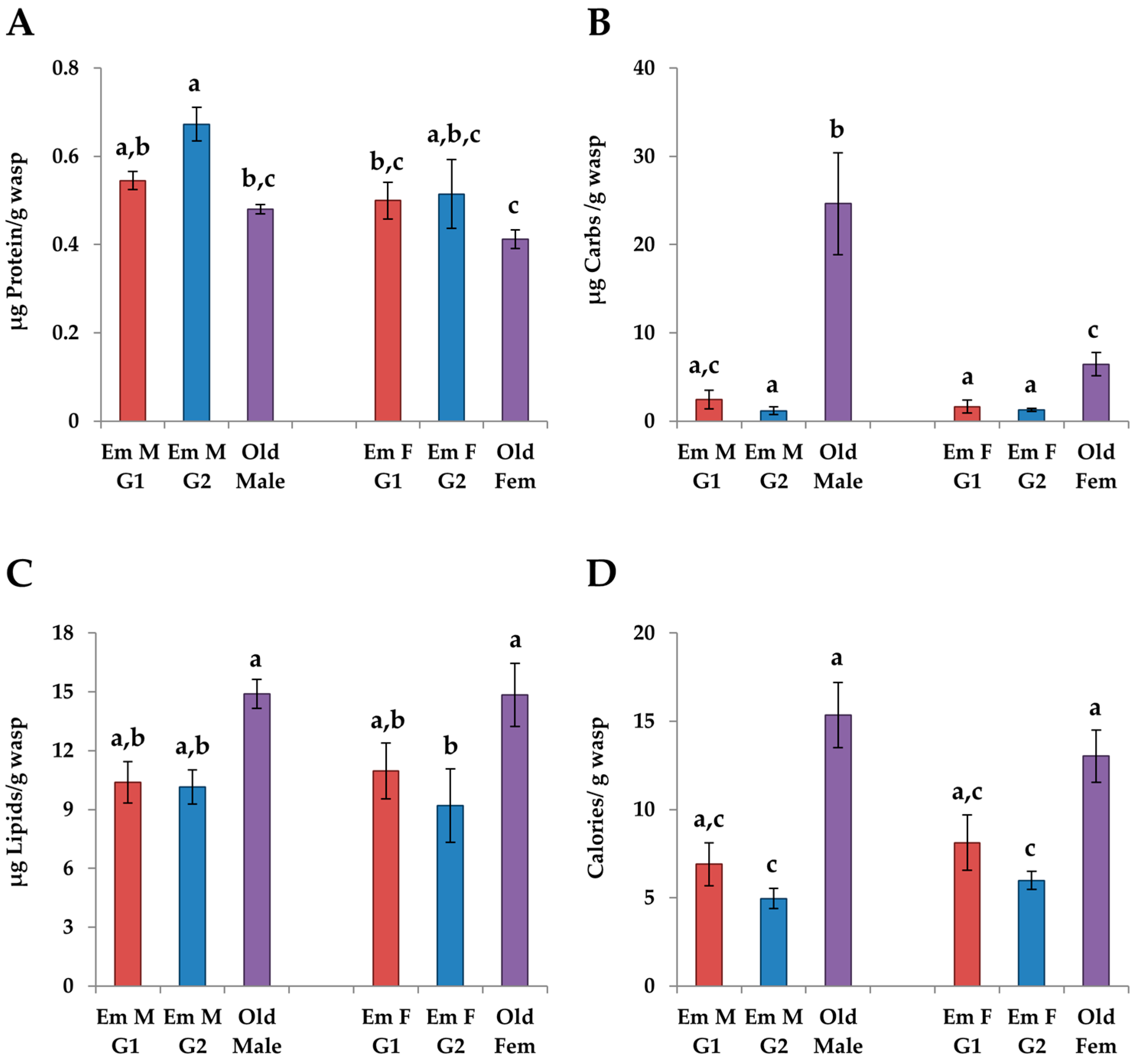
3.4.1. Changes in Age
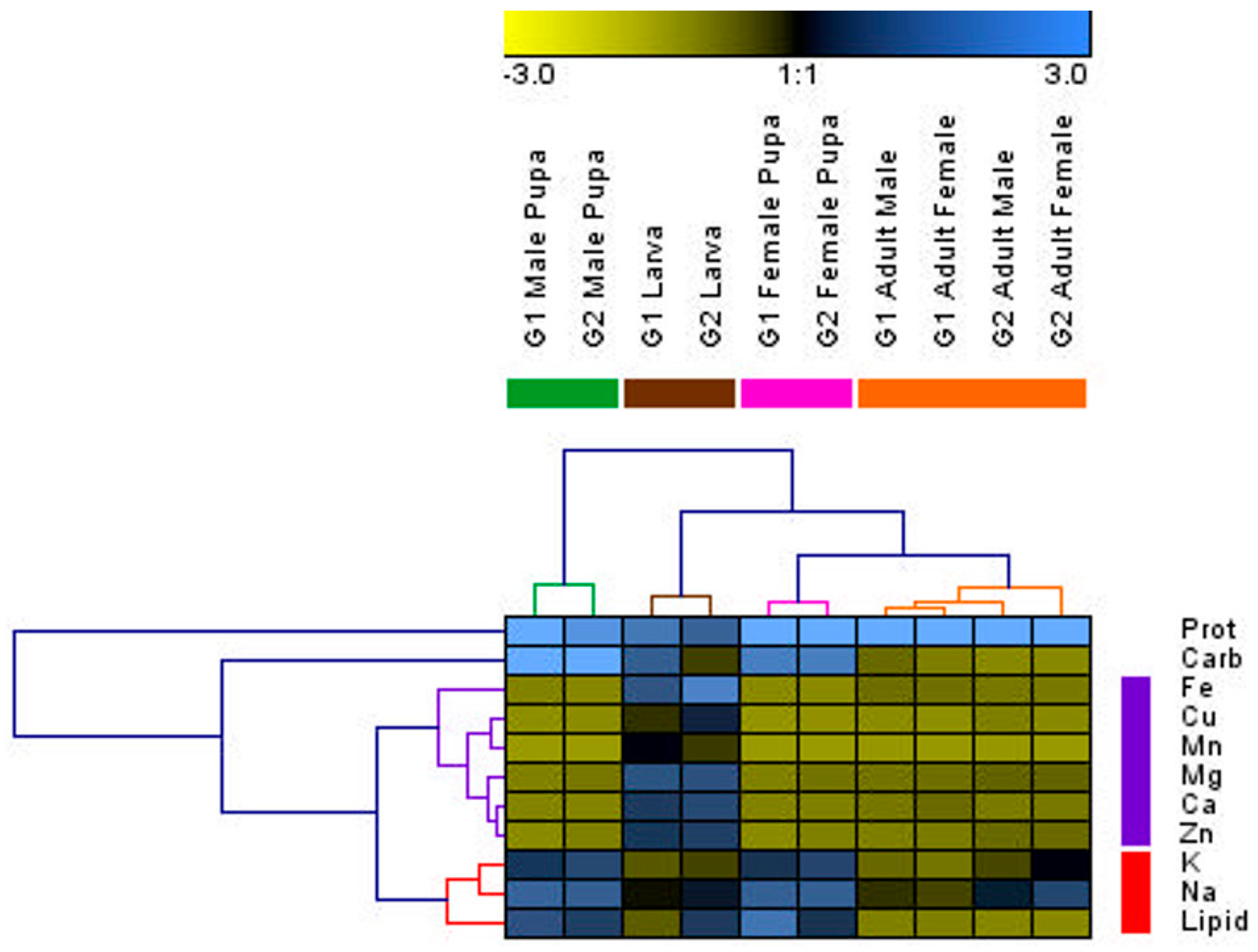
3.4.2. G1 vs. G2 Emerging Adults
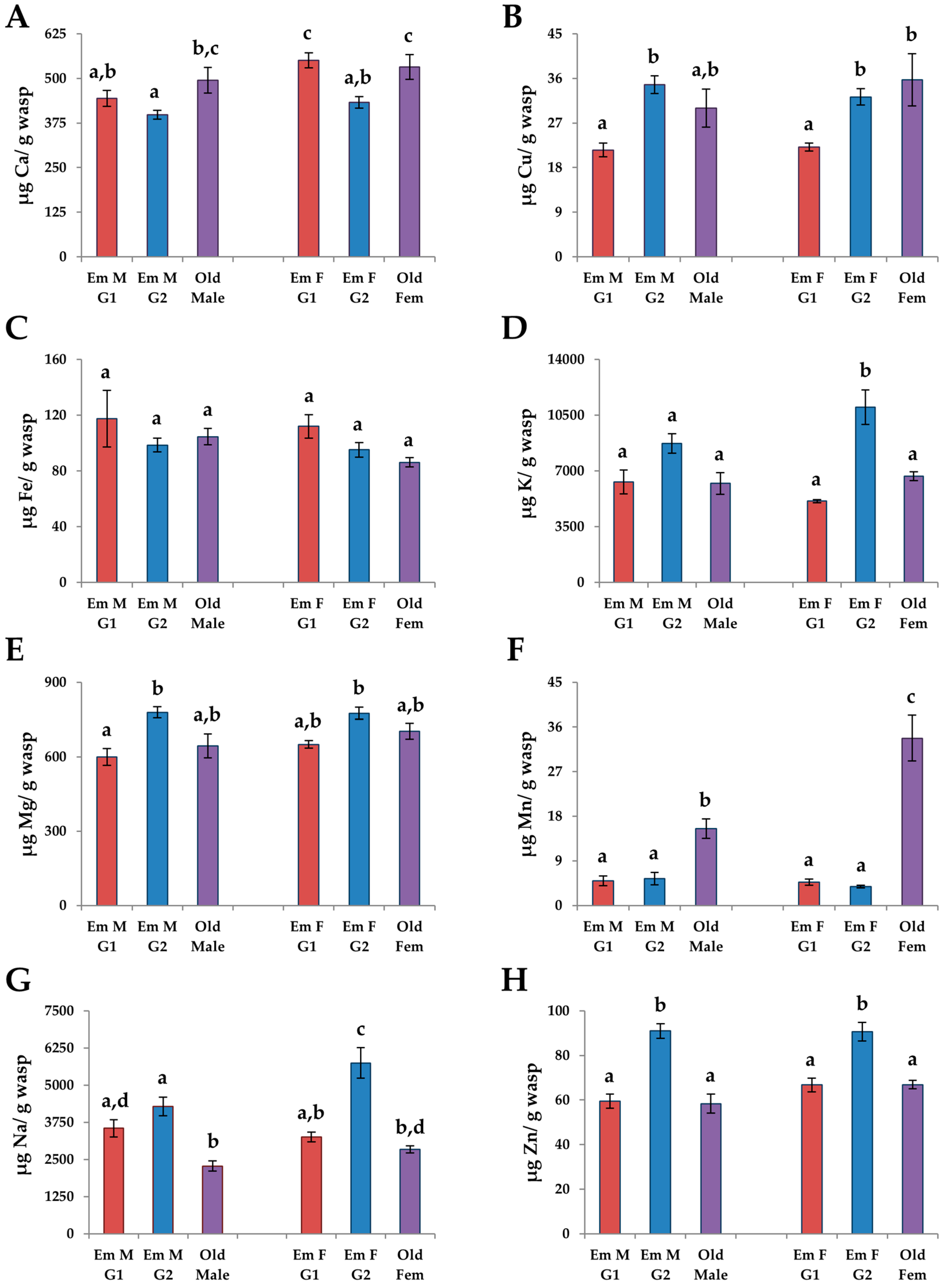
3.4.3. Males vs. Females
3.5. Size and Nutrient Relationships
3.5.1. Larvae
3.5.2. Pupae
3.5.3. Adults
4. Discussion
4.1. Changes During Development
4.2. Changes in Adults
4.3. Size and Nutrient Levels
4.4. G1 and G2 Generations and Social Insect Castes
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Winfree, R.; Williams, N.M.; Dushoff, J.; Kremen, C. Native bees provide insurance against ongoing honey bee losses. Ecol. Lett. 2007, 10, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Van Driesch, R.; Hoddle, M.; Center, T. Control of Pests and Weeds by Natural Enemies: An Introduction to Biological Control; Blackwell Publishing: Malden, MA, USA, 2008. [Google Scholar]
- Amdam, G.V.; Norberg, K.; Fondrk, M.K.; Page, R.E. Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc. Natl. Acad. Sci. USA 2004, 101, 11350–11355. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.H. Evolution of castes in Polistes. Ann. Zool. Fennici 2006, 43, 407–422. [Google Scholar]
- West-Eberhard, M.J. Flexible strategy and social evolution. In Animal Societies: Theories and Facts; Itô, Y., Brown, J.L., Kikkawa, J., Eds.; Japanese Science Society Press: Tokyo, Japan, 1987; pp. 35–51. [Google Scholar]
- Judd, T.M.; Teal, P.E.A.; Hernandez, E.J.; Choudhury, T.; Hunt, J.H. Quantitative differences in nourishment affect caste-related physiology and development in the paper wasp Polistes metricus. PLoS ONE 2015, 10, e0116199. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.E. Developmental, and physiological determinants of caste in social hymenoptera: Evolutionary implications. Am. Nat. 1986, 128, 13–34. [Google Scholar] [CrossRef]
- Cross, E.A.; Mostafa, A.E.S.; Bauman, T.R.; Lancaster, I.J. Some aspects of energy transfer between the organ-pipe mud-dauber Trypoxylon politum and its araneid spider prey. Environ. Entomol. 1978, 7, 647–652. [Google Scholar] [CrossRef]
- Strohm, E. Factors affecting body size and fat content in a digger wasp. Oecologia 2000, 123, 184–191. [Google Scholar] [CrossRef]
- Mitchell, P.S.; Hunt, J.H. Nutrient and energy assays of larval provisions and feces on the black and yellow mud dauber, Scelephron caementarium (Drury) (Hymenoptera: Sphecidae). J. Kansas Entomol. Soc. 1984, 57, 700–704. [Google Scholar]
- Krombein, K.V. Trap-Nesting Wasps and Bees; Smithsonian Press: Washington, DC, USA, 1967; p. 570. [Google Scholar]
- Budriene, A.; Budrys, E.; Nevronyte, Z. Sexual size dimorphism in the ontogeny of the solitary predatory wasp Symmorphus allobrogus (Hymenoptera: Vespidae). C. R. Biol. 2013, 336, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Hook, A.W.; Matthews, R.W. Nesting biology of Oxybelus sericeus with a discussion of nest guarding by male sphecid wasps (Hymenoptera). Psyche 1980, 87, 21–38. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Jones, S.A. Nutritional imbalance in an extreme generalist omnivore: Tolerance and recovery through complementary food selection. Anim. Behav. 2006, 71, 1253–1262. [Google Scholar] [CrossRef]
- Buschini, M.L.; Borba, N.A.; Brescovit, A.D. Patterns of prey selection of Trypoxylon (Trypargilum) lactitarse Saussure (Hymenoptera: CRABRONIDAE) in Southern Brazil. Braz. J. Biol. 2008, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Camillo, E.; Brescovit, A.D. Biological aspects of Trypoxylon (Trypargilum) lactitarse Saussure and Trypoxylon (Trypargilum) rogenhoferi Kohl (Hymenoptera: Sphecidae) in trap-nests, with special reference to their prey. An. Soc. Entomol. Bras. 1999, 28, 251–265. [Google Scholar] [CrossRef]
- Brockmann, H.J. Male behavior, courtship and nesting in Trypoxylon (Trypargilum) monteverdeae (Hymenoptera: Sphecidae). J. Kansas Entomol. Soc. 1992, 65, 66–84. [Google Scholar]
- Peckham, G.W.; Peckham, E.G. Notes on the habits of Trypoxylon rubrocinctum and Trypoxylon albopilosum. Psyche 1895, 7, 303–306. [Google Scholar] [CrossRef]
- Paetzel, M.M. Behavior of the male Trypoxylon Rubrocinctum. (Hymenoptera: Sphecidae). Pan-Pac. Entomol. 1973, 49, 26–30. [Google Scholar]
- Rau, P. Field studies in the behavior of the non-social wasps. Trans. Acad. Sci. St. Loius 1928, 25, 1–489. [Google Scholar]
- Buschini, M.L.T.; Fajardo, S. Biology of the solitary wasp Trypoxylon (Trypargilum) agamemnon Richards 1934 (Hymenoptera: Crabronidae) in trap-nests. Acta Zool. 2010, 91, 426–432. [Google Scholar] [CrossRef]
- Coville, R.E.; Griswold, C. Biology of Trypoxylon (Trypargilum) superbum (Hymenoptera: Sphecidae), a spider-hunting wasp with extended guarding of the brood by males. J. Kansas Entomol. Soc. 1984, 57, 365–376. [Google Scholar]
- Coville, R.E.; Griswold, C.; Coville, P.L. Observations on the nesting biology and behavior of Trypoxylon (Trypargilum) vagulum (Hymenoptera: Sphecidae) in Costa Rica. Pan-Pac. Entomol. 2000, 76, 28–48. [Google Scholar]
- Cross, E.A.; Stith, M.G.; Bauman, T.R. Bionomics of the organ-pipe mud-dauber, Trypoxylon politum (Hymenoptera: Sphecoidea). Ann. Entomol. Soc. Am. 1975, 68, 901–916. [Google Scholar] [CrossRef]
- Musicante, M.L.; Salvo, A. Nesting biology of four species of Trypoxylon (Trypargilum) (Hymenoptera: Crabronidae) in Chaco Serrano woodland, Central Argentina. Rev. Biol. Trop. 2010, 58, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Judd, T.M.; Magnus, R.M.; Fasnacht, M.P. A nutritional profile of the social wasp Polistes metricus: Differences in nutrient levels between castes and changes within castes during the annual life cycle. J. Insect Physiol. 2010, 56, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.R. Analyzing tables of statistical tests. Evolution 1989, 43, 223–225. [Google Scholar] [CrossRef]
- Ashcraft, B.A.; Judd, T.M. Micronutrient changes in colonies of the ant Temnothorax curvispinosus (Hymenoptera: Formicidae) during the colony cycle. Ann. Entomol. Soc. Am. 2014, 107, 475–483. [Google Scholar] [CrossRef]
- Sturn, A.; Quackenbush, J.; Trajanoski, Z. Genesis: Cluster analysis of microarray data. Bioinformatics 2002, 18, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Bergey, L.L.; Weis, J.S. Molting as a mechanism of depuration of metals in the fiddler crab, Uca pugnax. Mar. Environ. Res. 2007, 64, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Raessler, M.; Rothe, J.; Hilke, I. Accurate determination of Cd, Cr, Cu and Ni in woodlice and their skins—Is moulting a means of detoxification? Sci. Total Environ. 2005, 337, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Jungreis, A.M.; Tojo, S. Potassium and uric acid content in tissues of the silkmoth Hyalophora cecropia. Am. J. Physiol. 1973, 224, 21–26. [Google Scholar] [PubMed]
- Kristiansen, E.; Zachariassen, K.E. Effect of freezing on the transmembrane distribution of ions in freeze-tolerant larvae of the wood fly Xylophagus cinctus (Diptera, Xylophagidae). J. Insect Physiol. 2001, 47, 585–592. [Google Scholar] [CrossRef]
- Zachariassen, K.E.; Kristiansen, E. What determines the strategy of cold-hardiness? Acta Soc. Zool. Bohem. 2003, 67, 51–58. [Google Scholar]
- Zachariassen, K.E.; Kristiansen, E.; Pedersen, S.A. Inorganic ions in cold-hardiness. Cryobiology 2004, 48, 126–133. [Google Scholar] [PubMed]
- Linksvayer, T.A.; Wade, M.J. The evolutionary origin and elaboration of sociality in the aculeate Hymenoptera: Maternal effects, sib-social effects, and Heterochrony. Q. Rev. Biol. 2005, 80, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Orgad, S.; Nelson, H.; Segal, D.; Nelson, N. Metal ions suppress the abnormal taste behavior in the Drosophila mutant malvolio. J. Exp. Biol. 1998, 201, 115–120. [Google Scholar] [PubMed]
- Ben-Shahar, Y.; Dudek, N.L.; Robinson, G.E. Phenotypic deconstruction reveals involvement of manganese transporter malvolio in honey bee division of labor. J. Exp. Biol. 2004, 207, 3281–3288. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.L.; Bilof, K.B.J.; Henshaw, M.T.; Hunt, J.H.; Robinson, G.E. Lipid stores, ovary development, and brain gene expression in Polistes metricus females. Insect Soc. 2009, 56, 77–84. [Google Scholar] [CrossRef]
- Brockmann, H.J. Diversity in the nesting behavior of mud-daubers (Trypoxylon politum Say; Sphecidae). Fla. Entomol. 1980, 63, 53–64. [Google Scholar] [CrossRef]
- Brockmann, H.J. Mate conflict and male behavior in a solitary wasp, Trypoxylon (Trypargilum) politum (Hymenoptera: Sphecidae). Anim. Behav. 1989, 37, 232–255. [Google Scholar] [CrossRef]
- Coville, R.E.; Coville, P.L. Nesting biology and male behavior of Trypoxylon (Trypargilum) tenoctitlan in Costa Rica (Hymenoptera: Sphecidae). Ann. Entomol. Soc. Am. 1980, 73, 110–119. [Google Scholar] [CrossRef]
- Judd, T.M.; Southeast Missouri State University, Cape Girardeau, MO, USA. Personal observation, 2011.
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.N. Gluconeogenesis and effect of nutritional status on TCA cycle activity in the insect Manduca sexta. Biochim. Biophys. Acta 1995, 1245, 376–384. [Google Scholar] [CrossRef]
- Thompson, S.N.; Dahlman, D.L. Blood sugar formation due to abnormally elevated gluconeogenesis: Aberrant regulation in a parasitized insect, Manduca sexta Linnaeus. BBA-Mol. Basis Dis. 1999, 1454, 133–142. [Google Scholar] [CrossRef]
- Brothers, D.J. Phylogeny and evolution of wasps, ants and bees (Hymenoptera, Chrysidoidea, Vespoidea and Apoidea). Zool. Scr. 1999, 28, 233–250. [Google Scholar] [CrossRef]
- Berens, A.J.; Hunt, J.H.; Toth, A.L. Nourishment level affects caste-related gene expression in Polistes wasps. BMC Genet. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.K. Effects of environment and juvenile hormone on ovaries of the wasp, Polistes metricus. J. Insect Physiol. 1972, 18, 1875–1883. [Google Scholar] [CrossRef]
- Tibbetts, E.A.; Levy, S.; Donajkowski, K. Reproductive plasticity in Polistes paper wasp workers and the evolutionary origins of sociality. J. Insect Physiol. 2011, 57, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, E.A.; Sheehan, M.J. The effect of juvenile hormone on Polistes wasp fertility varies with cooperative behavior. Horm. Behav. 2012, 61, 559–564. [Google Scholar] [CrossRef] [PubMed]
| Nutrient | df | F | p |
|---|---|---|---|
| Macronutrient Analysis | |||
| Protein | 5 | 21.76 | p < 0.0001 |
| Carbohydrates | 5 | 31.29 | p < 0.0001 |
| Lipid | 5 | 7.92 | p < 0.0001 |
| Micronutrient Analysis | |||
| Ca | 5 | 183.43 | p < 0.0001 |
| Cu | 5 | 80.91 | p < 0.0001 |
| Fe | 5 | 32.79 | p < 0.0001 |
| K | 5 | 68.79 | p < 0.0001 |
| Mg | 5 | 120.71 | p < 0.0001 |
| Mn | 5 | 232.26 | p < 0.0001 |
| Na | 5 | 45.60 | p < 0.0001 |
| Zn | 5 | 344.00 | p < 0.0001 |
| Nutrient | df | F | p |
|---|---|---|---|
| Macronutrient Analysis | |||
| Protein | 5 | 6.27 | p = 0.0003 |
| Carbohydrates | 5 | 15.30 | p < 0.0001 |
| Lipid | 5 | 4.13 | p = 0.005 |
| Micronutrient Analysis | |||
| Ca | 5 | 7.16 | p < 0.0001 |
| Cu | 5 | 8.22 | p < 0.0001 |
| Fe | 5 | 0.93 | p = 0.47 |
| K | 5 | 8.57 | p < 0.0001 |
| Mg | 5 | 7.23 | p < 0.0001 |
| Mn | 5 | 36.44 | p < 0.0001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judd, T.M.; Fasnacht, M.P. A Nutritional Profile of the Trap-Nesting Wasp Trypoxylon lactitarse (Hymenoptera: Crabronidae): Comparison of Sexes and Overwintering and Non-Overwintering Generations. Insects 2017, 8, 3. https://doi.org/10.3390/insects8010003
Judd TM, Fasnacht MP. A Nutritional Profile of the Trap-Nesting Wasp Trypoxylon lactitarse (Hymenoptera: Crabronidae): Comparison of Sexes and Overwintering and Non-Overwintering Generations. Insects. 2017; 8(1):3. https://doi.org/10.3390/insects8010003
Chicago/Turabian StyleJudd, Timothy M., and Matthew P. Fasnacht. 2017. "A Nutritional Profile of the Trap-Nesting Wasp Trypoxylon lactitarse (Hymenoptera: Crabronidae): Comparison of Sexes and Overwintering and Non-Overwintering Generations" Insects 8, no. 1: 3. https://doi.org/10.3390/insects8010003






