Bee++: An Object-Oriented, Agent-Based Simulator for Honey Bee Colonies
Abstract
:1. Introduction
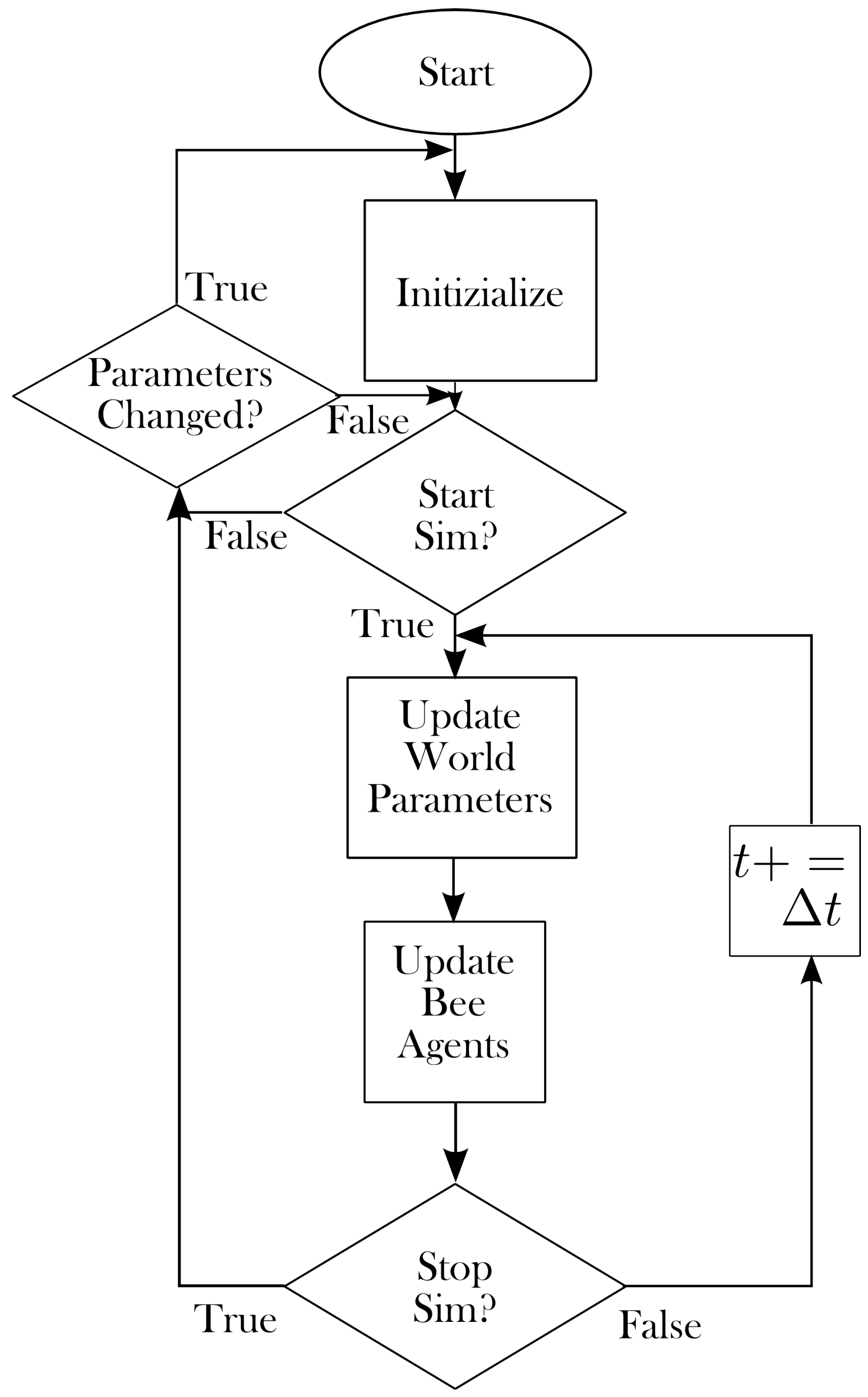
2. Model
2.1. Bees
2.2. Food Stores
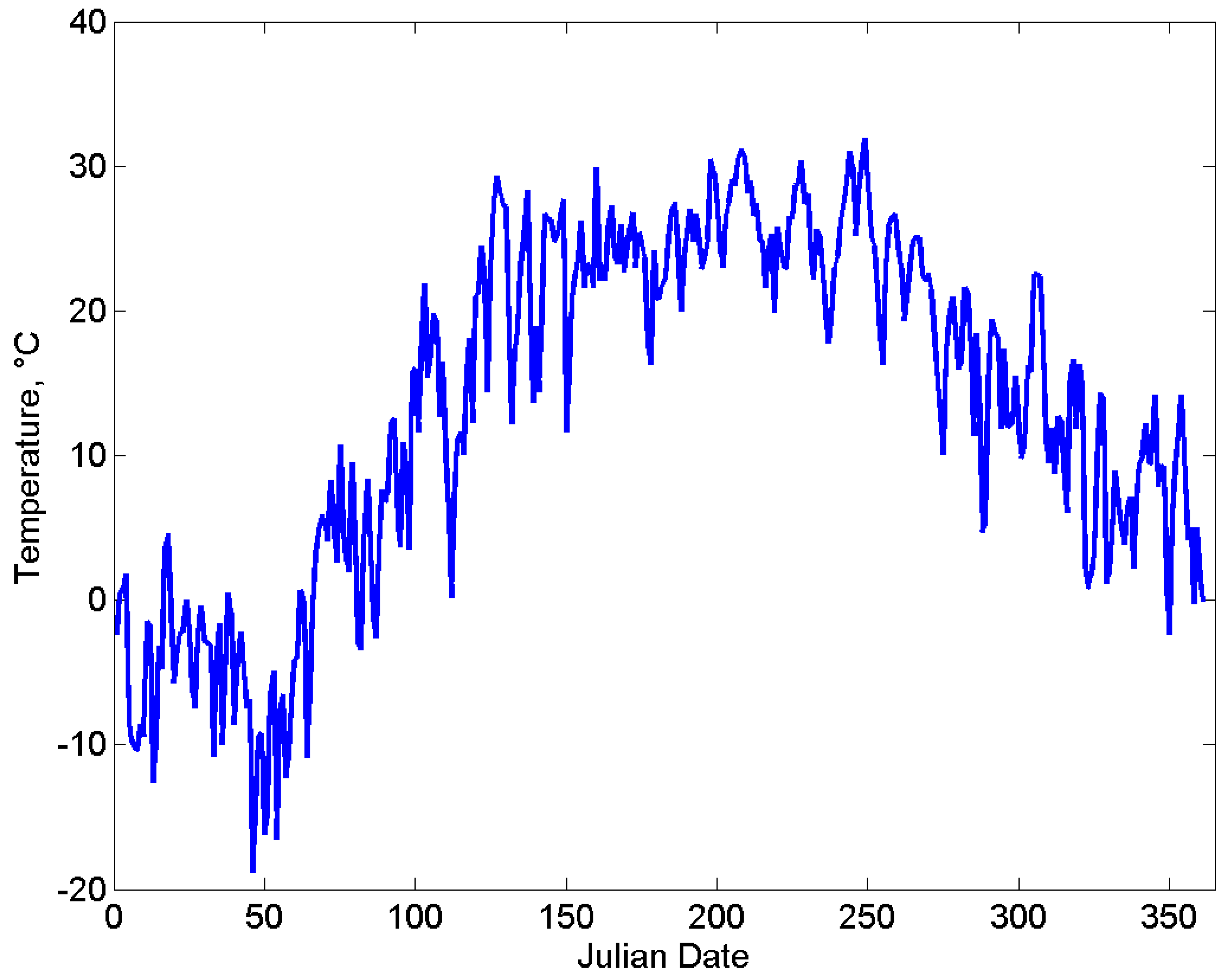
2.3. Environment
2.4. Pathogens
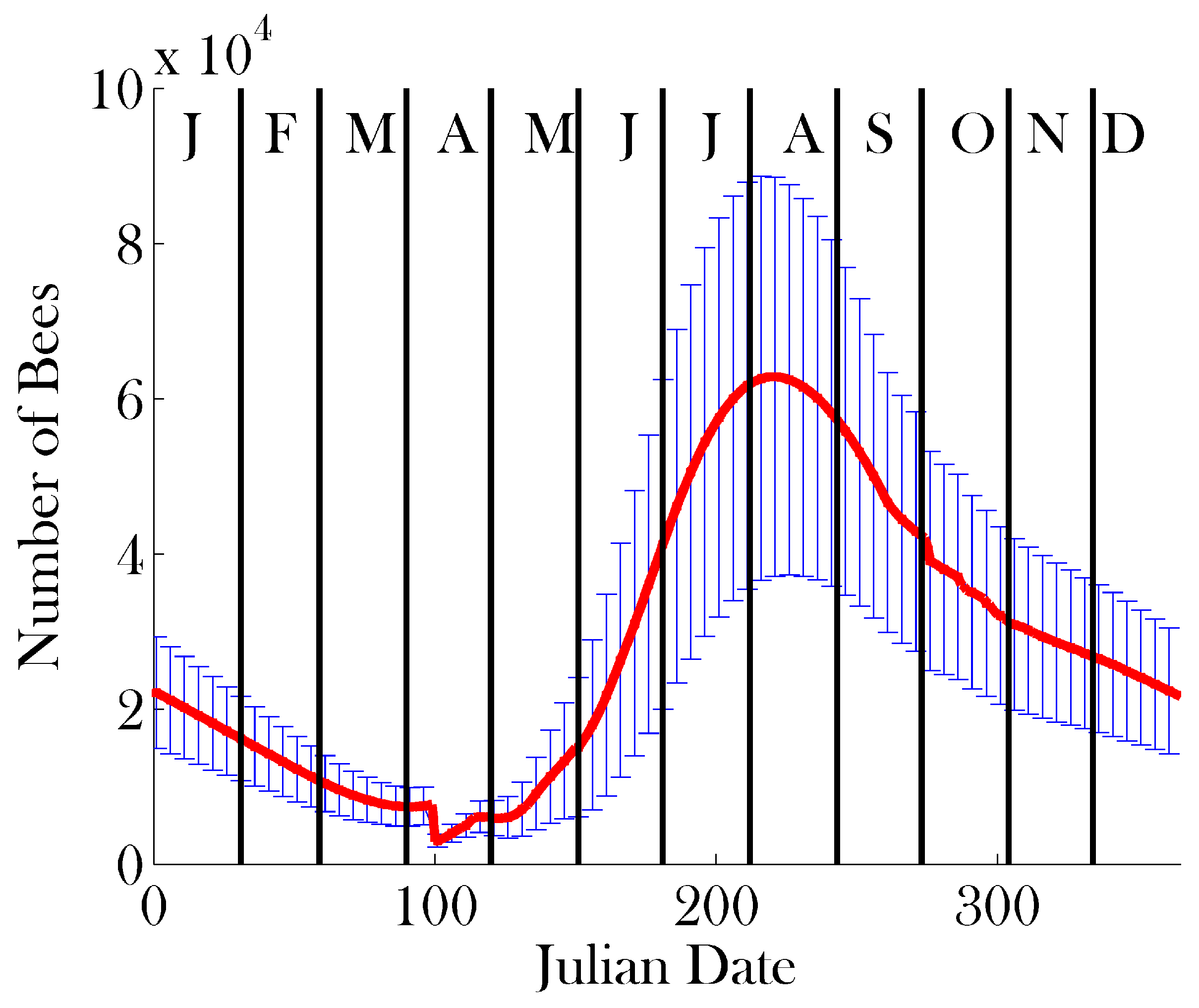
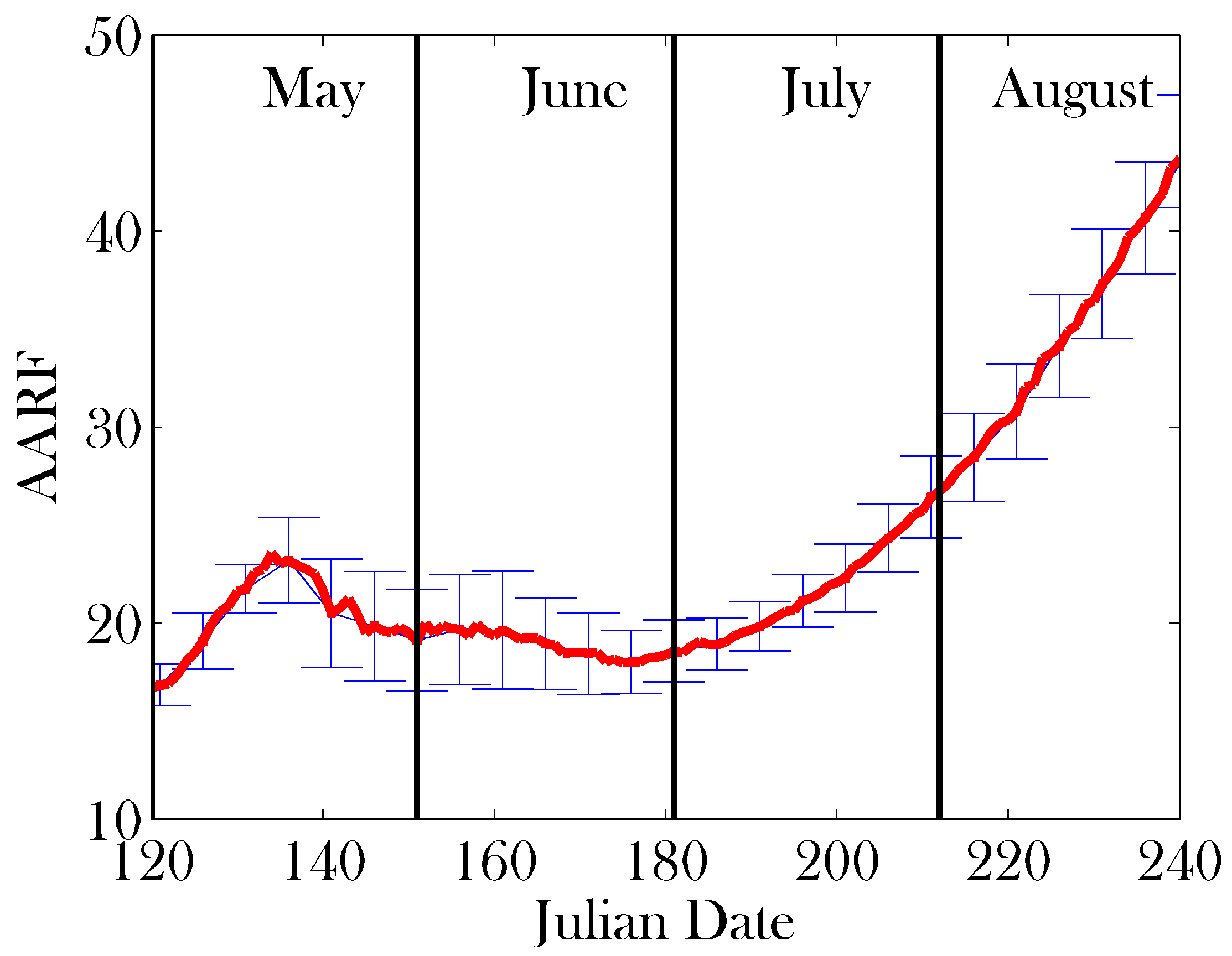
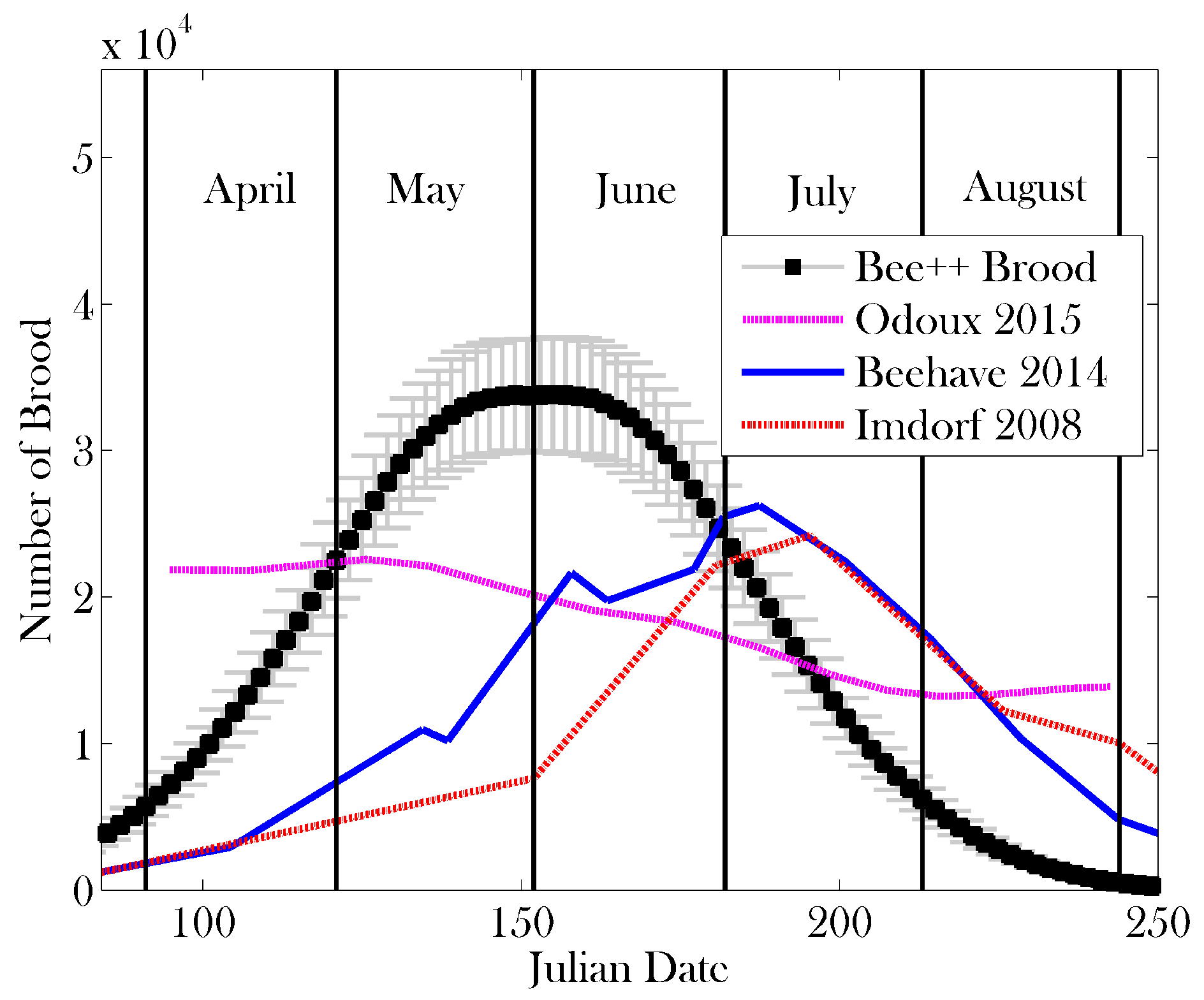
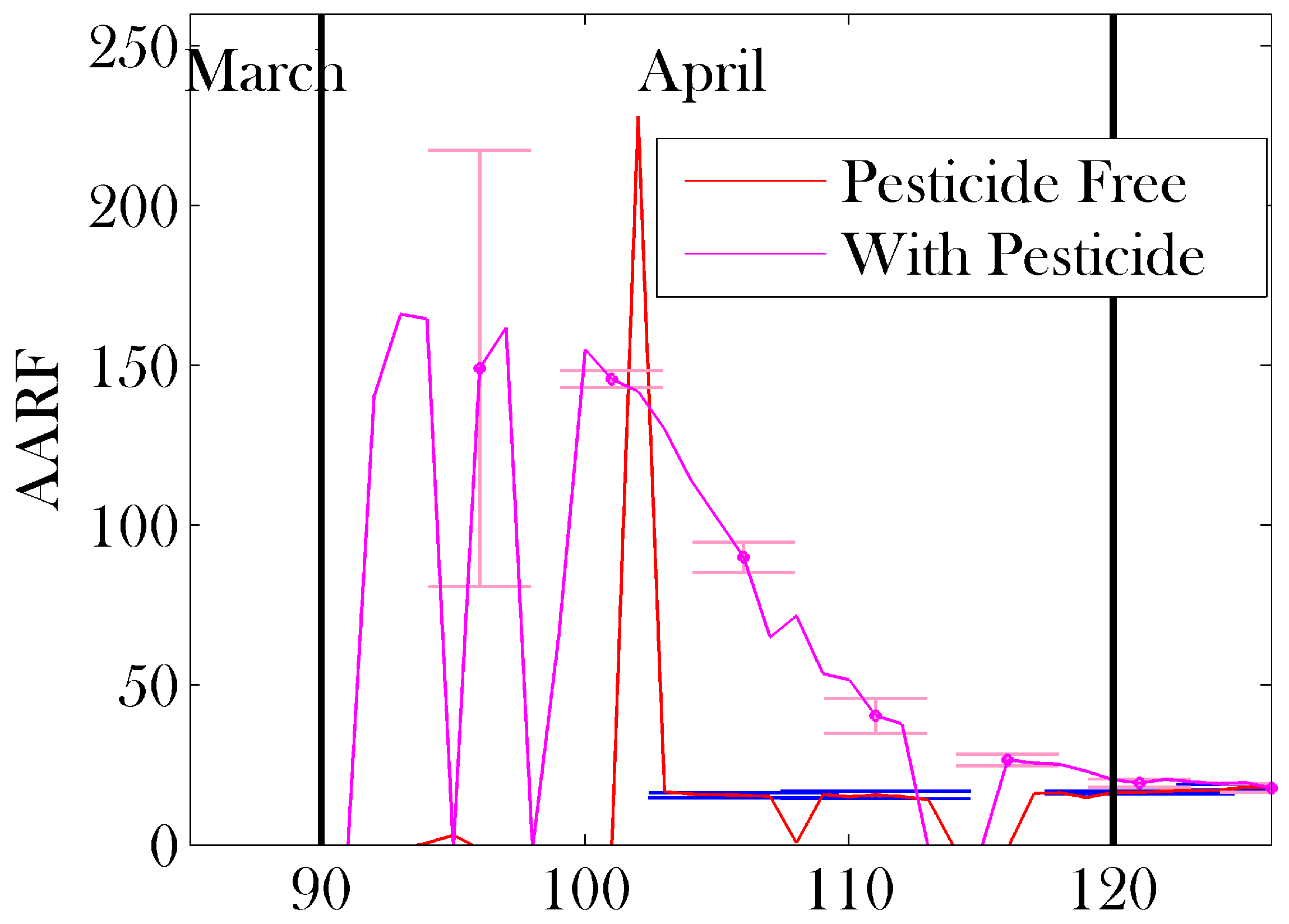
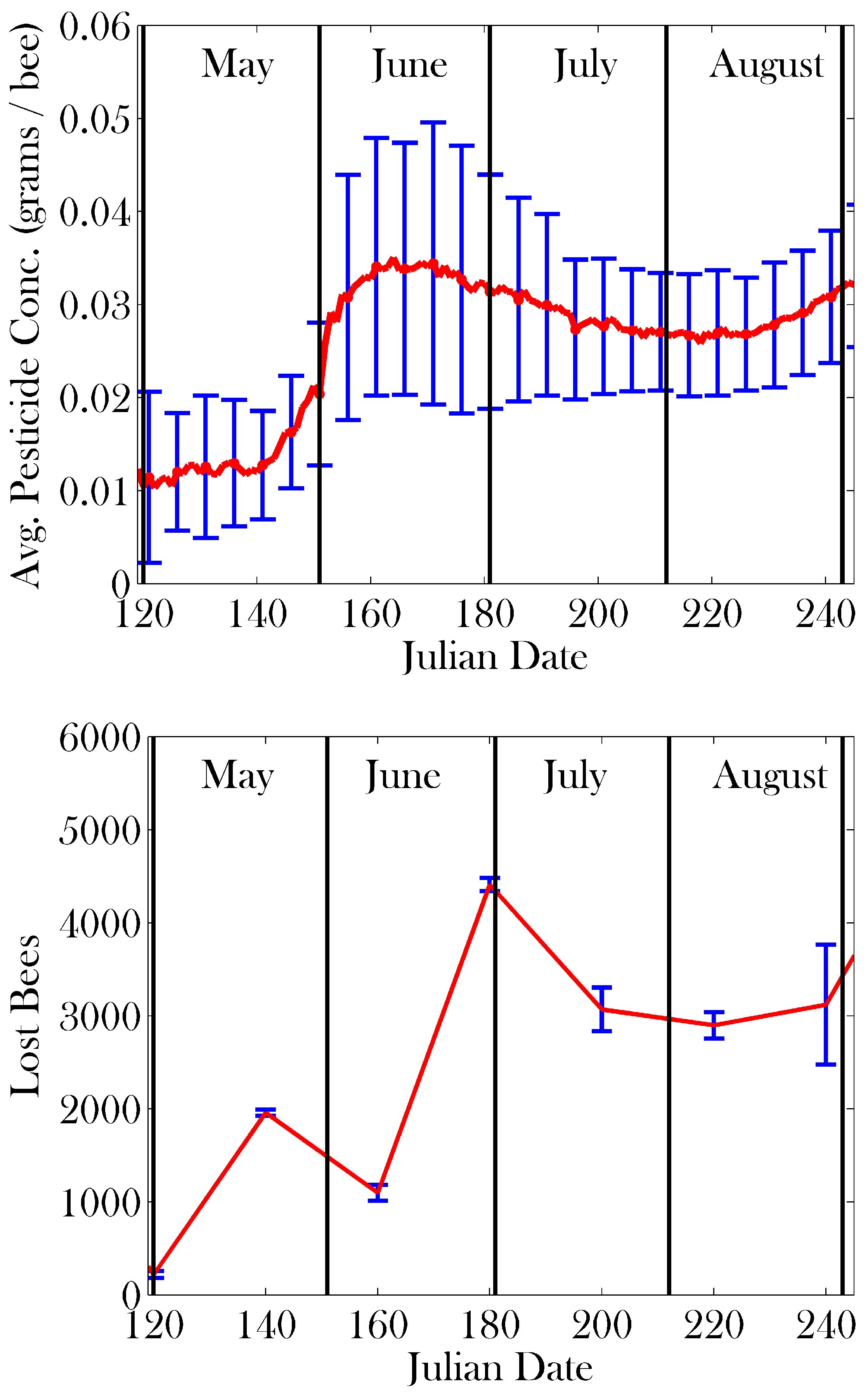
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
Appendix A
Appendix A.1. Drone Dynamics
Appendix A.2. Pathogen Dynamics
References
- Devillers, J. The ecological importance of honey bees and their relevance to ecotoxicology. In Honey Bees: Estimating the Environmental Impact of Chemicals; Devillers, J., Pham-Delègue, M., Eds.; Taylor and Francis: London, UK, 2002; pp. 1–11. [Google Scholar]
- Calderone, N.W. Insect Pollinated Crops, Insect Pollinators and US Agriculture: Trend Analysis of Aggregate Data for the Period 1992–2009. PLOS ONE 2012, 7, e37235. [Google Scholar] [CrossRef] [PubMed]
- Southwick, E.E.; Southwick, L., Jr. Estimating the economic value of honey bees (Hymenoptera: Apidae) as agricultural pollinators in the United States. J. Econ. Entomol. 1992, 85, 621–633. [Google Scholar] [CrossRef]
- Neumann, P.; Carreck, N.L. Honey bee colony losses. J. Apic. Res. 2010, 49, 1–6. [Google Scholar] [CrossRef]
- Evans, J.D.; Schwarz, R.S. Bees brought to their knees: Microbes affecting honey bee health. Trends Microbiol. 2011, 19, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.E. Colony Collapse Disorder: Many Suspects, No Smoking Gun. BioScience 2008, 58, 384–388. [Google Scholar] [CrossRef]
- Ho, M.W.; Cummins, J. Mystery of disappearing honeybees. Sci. Soc. 2007, 34, 35–36. [Google Scholar]
- Henry, M.; Beguin, M.; Requier, F.; Rollin, O.; Odoux, J.F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Favre, D. Mobile phone-induced honeybee worker piping. Apidologie 2011, 42, 270–279. [Google Scholar] [CrossRef]
- Khoury, D.S.; Myerscough, M.R.; Barron, A.B. A Quantitative Model of Honey Bee Colony Population Dynamics. PLoS ONE 2011, 6, e18491. [Google Scholar] [CrossRef] [PubMed]
- Ratti, V.; Kevan, P.G.; Eberl, H.J. A mathematical model for population dynamics in honeybee colonies infested with Varroa destructor and the Acute Bee Paralysis Virus. Can. Appl. Math. Q. 2013, 21, 63–93. [Google Scholar]
- Sumpter, D.J.T.; Martin, S.J. The dynamics of virus epidemics in Varroa-infested honey bee colonies. J. Anim. Ecol. 2004, 73, 51–63. [Google Scholar] [CrossRef]
- Betti, M.I.; Wahl, L.M.; Zamir, M. Effects of Infection on Honey Bee Population Dynamics: A Model. PLoS ONE 2014, 9, e110237. [Google Scholar] [CrossRef] [PubMed]
- Roubik, D.W.; Wolda, H. Do competing honey bees matter? Dynamics and abundance of native bees before and after honey bee invasion. Popul. Ecol. 2001, 43, 53–62. [Google Scholar] [CrossRef]
- Eberl, H.J.; Frederick, M.R.; Kevan, P.G. Importance of brood maintenance terms in simple models of the honeybee—Varroa destructor—Acute Bee Paralysis Virus complex. Electron. J. Differ. Equ. 2010, 19, 85–98. [Google Scholar]
- Paxton, R.J.; Klee, J.; Korpela, S.; Fries, I. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 2007, 38, 558–565. [Google Scholar] [CrossRef]
- Higes, M.; Martin-Hernandez, R.; Garrido-Bailon, E.; Gonzalez-Porto, A.V.; Garcia-Palencia, P.; Meana, A. Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ. Microbiol. Rep. 2009, 1, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Barron, A.B.; Myerscough, M.R. Modelling Food and Population Dynamics in Honey Bee Colonies. PLoS ONE 2013, 8, e59084. [Google Scholar] [CrossRef] [PubMed]
- Vidau, C.; Diogon, M.; Aufauvre, J.; Fontbonne, R.; Viguès, B.; Brunet, J.L.; Texier, C.; Biron, D.G.; Blot, N.; El Alaoui, H.; et al. Exposure to sublethal doses of fipronil and thiacloprid highly increases mortality of honeybees previously infected by Nosema ceranae. PLoS ONE 2011, 6, e21550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratti, V.; Kevan, P.G.; Eberl, H.J. A Mathematical Model of the Honeybee-Varroa destructor-Acute Bee Paralysis Virus Complex with Seasonal Effects. Bull. Math. Biol. 2015, 77, 1493–1520. [Google Scholar] [CrossRef] [PubMed]
- Thorbek, P.; Campbell, P.J.; Sweeney, P.J.; Thompson, H.M. Using BEEHAVE to explore pesticide protection goals for European honeybee (Apis melifera L.) worker losses at different forage qualities. Environ. Toxicol. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Aufauvre, J.; Biron, D.G.; Vidau, C.; Fontbonne, R.; Roudel, M.; Diogon, M.; Viguès, B.; Belzunces, L.P.; Delbac, F.; Blot, N. Parasite-insecticide interactions: A case study of Nosema ceranae and fipronil synergy on honeybee. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Pettis, J.S.; Lichtenberg, E.M.; Andree, M.; Stitzinger, J.; Rose, R.; Vanengelsdorp, D. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLOS ONE 2013, 8, e70182. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E.; Botías, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.; Wahl, L.; Zamir, M. Age structure is critical to the population dynamics and survival of honeybee colonies. R. Soc. Open Sci. 2016, 3, 160444. [Google Scholar] [CrossRef] [PubMed]
- Becher, M.A.; Grimm, V.; Thorbek, P.; Horn, J.; Kennedy, P.J.; Osborne, J.L. BEEHAVE: A systems model of honeybee colony dynamics and foraging to explore multifactorial causes of colony failure. J. Appl. Ecol. 2014, 51, 470–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmickl, T.; Crailsheim, K. HoPoMo: A model of honeybee intracolonial population dynamics and resource management. Ecol. Model. 2007, 204, 219–245. [Google Scholar] [CrossRef]
- Stillman, R.A.; Railsback, S.F.; Giske, J.; Berger, U.; Grimm, V. Making predictions in a changing world: The benefits of individual-based ecology. BioScience 2015, 65, 140–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.M.; Wen, Z.; Schuler, M.A.; Berenbaum, M.R. Mediation of pyrethroid insecticide toxicity to honey bees (Hymenoptera: Apidae) by cytochrome P450 monooxygenases. J. Econ. Entomol. 2006, 99, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Dahlgren, L.; Siegfried, B.D.; Ellis, M.D. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS ONE 2013, 8, e54092. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.; Chen, H.; Graves, C.; Maisonnasse, A.; Le Conte, Y.; Plettner, E. Biosynthesis of ethyl oleate, a primer pheromone, in the honey bee (Apis mellifera L.). Insect Biochem. Mol. Biol. 2012, 42, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, I.; Le Conte, Y.; Costagliola, G.; Plettner, E.; Toth, A.L.; Wang, M. Regulation of behavioral maturation by a primer pheromone produced by adult worker honey bees. Proc. Natl. Acad. Sci. USA 2004, 101, 17559–17564. [Google Scholar] [CrossRef] [PubMed]
- Slessor, K.N.; Winston, M.L.; Le Conte, Y. Pheromone communication in the honeybee (Apis mellifera L.). J. Chem. Ecol. 2005, 31, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Yang, S.; Wang, Z.W.; Radloff, S.E.; Oldroyd, B.P. Differences in foraging and broodnest temperature in the honey bees Apis cerana and A. mellifera. Apidologie 2012, 43, 618–623. [Google Scholar] [CrossRef]
- Joshi, N.C.; Joshi, P. Foraging behaviour of Apis spp. on apple flowers in a subtropical environment. N. Y. Sci. J. 2010, 3, 71–76. [Google Scholar]
- Blažytė-Čereškienė, L.; Vaitkevičienė, G.; Venskutonytė, S.; Būda, V. Honey bee foraging in spring oilseed rape crops under high ambient temperature conditions. Žemdirbystė Agric. 2010, 97, 61–70. [Google Scholar]
- De Vries, H.; Biesmeijer, J.C. Modelling collective foraging by means of individual behaviour rules in honey-bees. Behav. Ecol. Sociobiol. 1998, 44, 109–124. [Google Scholar] [CrossRef]
- Bukovac, Z.; Dorin, A.; Dyer, A. A-bees see: A simulation to assess social bee visual attention during complex search tasks. In Proceedings of the 12th European Conference on Artificial Life (ECAL 2013), Taormina, Italy, 2–6 September 2013; pp. 276–283.
- Reynolds, A.M.; Smith, A.D.; Menzel, R.; Greggers, U.; Reynolds, D.R.; Riley, J.R. Displaced honey bees perform optimal scale-free search flights. Ecology 2007, 88, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.M.; Smith, A.D.; Reynolds, D.R.; Carreck, N.L.; Osborne, J.L. Honeybees perform optimal scale-free searching flights when attempting to locate a food source. J. Exp. Biol. 2007, 210, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Biesmeijer, J.C.; de Vries, H. Exploration and exploitation of food sources by social insect colonies: A revision of the scout-recruit concept. Behav. Ecol. Sociobiol. 2001, 49, 89–99. [Google Scholar] [CrossRef]
- Abou-Shaara, H. The foraging behaviour of honey bees, Apis mellifera: A review. Vet. Med. 2014, 59, 1–10. [Google Scholar]
- Ratnieks, F.L.; Keller, L. Queen control of egg fertilization in the honey bee. Behav. Ecol. Sociobiol. 1998, 44, 57–61. [Google Scholar] [CrossRef]
- Wharton, K.E.; Dyer, F.C.; Huang, Z.Y.; Getty, T. The honeybee queen influences the regulation of colony drone production. Behav. Ecol. 2007, 18, 1092–1099. [Google Scholar] [CrossRef]
- McLellan, A. Growth and decline of honeybee colonies and inter-relationships of adult bees, brood, honey and pollen. J. Appl. Ecol. 1978, 155–161. [Google Scholar] [CrossRef]
- Harbo, J.R. Effect of population size on brood production, worker survival and honey gain in colonies of honeybees. J. Apic. Res. 1986, 25, 22–29. [Google Scholar] [CrossRef]
- Camazine, S. Self-organizing pattern formation on the combs of honey bee colonies. Behav. Ecol. Sociobiol. 1991, 28, 61–76. [Google Scholar] [CrossRef]
- Sakagami, S.; Fukuda, H. Life tables for worker honeybees. Res. Popul. Ecol. 1968, 10, 127–139. [Google Scholar] [CrossRef]
- Esther, E.; Smit, S.; Beukes, M.; Apostolides, Z.; Pirk, C.W.; Nicolson, S.W. Detoxification mechanisms of honey bees (Apis mellifera) resulting in tolerance of dietary nicotine. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Suchail, S.; De Sousa, G.; Rahmani, R.; Belzunces, L.P. In vivo distribution and metabolisation of 14C-imidacloprid in different compartments of Apis mellifera L. Pest Manag. Sci. 2004, 60, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Suchail, S.; Debrauwer, L.; Belzunces, L.P. Metabolism of imidacloprid in Apis mellifera. Pest Manag. Sci. 2004, 60, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Becher, M.A.; Osborne, J.L.; Thorbek, P.; Kennedy, P.J.; Grimm, V. REVIEW: Towards a systems approach for understanding honeybee decline: A stocktaking and synthesis of existing models. J. Appl. Ecol. 2013, 50, 868–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Botias, C.; Martin-Hernandez, R.; Barrios, L.; Meana, A.; Higes, M. Nosema spp. infection and its negative effects on honey bees (Apis mellifera iberiensis) at the colony level. Vet. Res. 2013, 44, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Seeley, T.D. Honeybee Ecology: A Study of Adaptation in Social Life; Princeton University Press: Princeton, NJ, USA, 2014. [Google Scholar]
- Kronenbergf, F. Colonial thermoregulation in honey bees. J. Comp. Physiol. B 1979, 148, 65–78. [Google Scholar] [CrossRef]
- Winston, M. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Ball, D.W. The chemical composition of honey. J. Chem. Educ. 2007, 84, 1643. [Google Scholar] [CrossRef]
- Wilson, W.T.; Moffett, J.O.; Harrington, H.D. Nectar and Pollen Plants of Colorado; Fort Collins: Fort Collins, CO, USA, 1958. [Google Scholar]
- Kralj, J.; Fuchs, S. Parasitic Varroa destructor mites influence flight duration and homing ability of infested Apis mellifera foragers. Apidologie 2006, 37, 577. [Google Scholar] [CrossRef]
- Smith, M.L. The Honey Bee Parasite Nosema ceranae: Transmissible via Food Exchange? PLoS ONE 2012, 7, e43319. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.D.; Beckman, R.J.; Conover, W.J. A comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics 2000, 42, 55–61. [Google Scholar] [CrossRef]
- Gary, N.E. Activities and behavior of honey bees. In The Hive and the Honey Bee; Langstroth, L.L., Ed.; Dadant & Sons: Sioux City, IA, USA, 1992; pp. 269–373. [Google Scholar]
- Fahrbach, S.; Robinson, G. Juvenile hormone, behavioral maturation and brain structure in the honey bee. Dev. Neurosci. 1996, 18, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Dukas, R. Mortality rates of honey bees in the wild. Insectes Sociaux 2008, 55, 252–255. [Google Scholar] [CrossRef]
- Russell, S.; Barron, A.B.; Harris, D. Dynamic modelling of honey bee (Apis mellifera) colony growth and failure. Ecol. Model. 2013, 265, 158–169. [Google Scholar] [CrossRef]
- Haydak, M.H. Honey bee nutrition. Annu. Rev. Entomol. 1970, 15, 143–156. [Google Scholar] [CrossRef]
- Rortais, A.; Arnold, G.; Halm, M.P.; Touffet-Briens, F. Modes of honeybees exposure to systemic insecticides: Estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie 2005, 36, 71–83. [Google Scholar] [CrossRef]
- Stanley, J.; Preetha, G. Pesticide Toxicity to Non-Target Organisms: Exposure, Toxicity and Risk Assessment Methodologies; Springer: Haarlem, The Netherlands, 2016. [Google Scholar]
- Stoner, K.A.; Eitzer, B.D. Movement of soil-applied imidacloprid and thiamethoxam into nectar and pollen of squash (Cucurbita pepo). PLoS ONE 2012, 7, e39114. [Google Scholar] [CrossRef] [PubMed]
- Odoux, J.F.; Aupinel, P.; Gateff, S.; Requier, F.; Henry, M.; Bretagnolle, V. ECOBEE: A tool for long-term honey bee colony monitoring at the landscape scale in West European intensive agroecosystems. J. Apic. Res. 2014, 53, 57–66. [Google Scholar] [CrossRef]
- Omholt, S.W. A model for intracolonial population dynamics of the honeybee in temperate zones. J. Apic. Research 1986, 25, 9–21. [Google Scholar] [CrossRef]
- Bühlmann, G. Assessing population dynamics in a honeybee colony. Mitteilungen der Deutschen Gesellschaft fuer Allgemeine und Angewandte Entomologie (Germany, FR) 1985, 4, 312–316. [Google Scholar]
- Imdorf, A.; Ruoff, K.; Fluri, P. Volksentwicklung bei der Honigbiene. Available online: http://alvearium3.kozo.ch/index.php?option=com_attachmentstask=downloadid=157 (accessed on 3 October 2016).
- Van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLOS ONE 2009, 4, e6481. [Google Scholar]
- Callahan, J.R. Honeybee Colony collapse disorder. Emerg. Biol. Threats 2009, 13, 141. [Google Scholar]
- Ratnieks, F.L. Egg-laying, egg-removal, and ovary development by workers in queenright honey bee colonies. Behav. Ecol. Sociobiol. 1993, 32, 191–198. [Google Scholar] [CrossRef]
- Capinera, J.L. Encyclopedia of Entomology; Springer: New York, NY, USA, 2008. [Google Scholar]
- Ginsberg, H.S. Foraging ecology of bees in an old field. Ecology 1983, 64, 165–175. [Google Scholar] [CrossRef]
- Grimm, V.; Revilla, E.; Berger, U.; Jeltsch, F.; Mooij, W.M.; Railsback, S.F.; Thulke, H.H.; Weiner, J.; Wiegand, T.; DeAngelis, D.L. Pattern-oriented modeling of agent-based complex systems: lessons from ecology. Science 2005, 310, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Ball, B.; Allen, M. The prevalence of pathogens in honey bee (Apis mellifera) colonies infested with the parasitic mite Varroa jacobsoni. Ann. Appl. Biol. 1988, 113, 237–244. [Google Scholar] [CrossRef]
- Perry, C.J.; Søvik, E.; Myerscough, M.R.; Barron, A.B. Rapid behavioral maturation accelerates failure of stressed honey bee colonies. Proc. Natl. Acad. Sci. USA 2015, 112, 3427–3432. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, L. Rusty Patched Bumble Bee Proposed for U.S. Endangered Species Status. Available online: http://www.reuters.com/article/us-usa-bumblebee-idUSKCN11R2TI,2016 (accessed on 3 October 2016).
- Seeley, T.D. The Wisdom of the Hive: The Social Physiology of Honey Bee Colonies; Harvard University Press: Boston, MA, USA, 2009. [Google Scholar]
- Fries, I. Nosema apis—A parasite in the honey bee colony. Bee World 1993, 74, 5–19. [Google Scholar] [CrossRef]
- Huang, W.F.; Solter, L.F.; Yau, P.M.; Imai, B.S. Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog. 2013, 9, e1003185. [Google Scholar] [CrossRef] [PubMed]
- Martin, S. Hygienic behaviour: An alternative view. Bee Improv. 2000, 7, 6–7. [Google Scholar]
- Martin, S. Biology and life-history of Varroa mites. In Mites of the Honey Bee; Dadant & Sons: Hamilton, IL, USA, 2001; pp. 131–148. [Google Scholar]
- Sammataro, D.; Gerson, U.; Needham, G. Parasitic mites of honey bees: Life history, implications, and impact. Annu. Rev. Entomol. 2000, 45, 519–548. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Pennacchio, F.; Caprio, E.; Boncristiani, H.F., Jr.; Evans, J.D.; Chen, Y. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J. Gen. Virol. 2011, 92, 151–155. [Google Scholar] [CrossRef] [PubMed]





| L | maximum rate of egg laying | 1500 eggs/day | [62] |
| number of hive bees for 50% egg survival | 1000 bees | ||
| mass of food stored for 50% egg survival | 500 g/day | [18] | |
| age at which hive bees begin brood care | 4 days | [48] | |
| age at which hive bees end brood care | 16 days | [48] | |
| minimum age of recruitment to foraging | 4 days | [63] | |
| age at which rate of recruitment is 50% of max. | 10 days | ||
| α | maximum rate of recruitment | 1 /day | |
| maximum fraction of colony that can be foraging | [10] | ||
| natural death rate of foragers outside the hive | equation (10) | [64] | |
| nectar gathered per day per forager | 0.03 g/day/bee | [65] | |
| pollen gathered per day per forager | 0.016 g/day/bee | [65] | |
| daily food requirement per hive bee | 0.08 g/bee | [66,67] | |
| daily food requirement per forager | 0.13 g/bee | [66,67] | |
| daily food requirement per drone | 0.1 g/bee | [66,67] | |
| daily pollen requirement per uncapped brood cell | 0.067 g/bee | [66,67] | |
| daily nectar requirement per uncapped brood cell | 0.018 g/bee | [66,67] | |
| Shape parameter for exit probability of forager | 30 min | ||
| Minimum time in hive | 1 h | ||
| Time forager spends searching for target | 15 min | ||
| Maximum flight time | 45 min | ||
| Temperature at which foraging begins | 10 C | [34,35] | |
| Ideal ambient temperature * | 25 C | [34,35,54] | |
| Minimum foraging temperature | 10 C | [34,35,54] | |
| Maximum foraging temperature | 40 C | [36] | |
| Rate at which pesticides are metabolised | /day | [50,51] | |
| Concentration of pesticide in nectar | ng/g | [68,69] | |
| Error in forager navigation | |||
| φ | Scaling of pesticide effect on navigation | 10 |
| % Change from Mean | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | 1.152 | −5.9516 | 13.53 | 11.145 | −10.583 | −1.5224 | −12.415 | 6.49 | −17.051 | 19.035 |
| w | −14.492 | −18.092 | 2.3472 | 15.129 | 16.566 | −11.444 | 6.8644 | −2.4616 | −7.118 | 8.1808 |
| b | 12.322 | −17.539 | 7.3496 | −15.187 | −4.4552 | −8.6352 | 10.577 | 16.609 | −2.3312 | 1.6552 |
| K | 16.297 | 13.7 | 7.96 | −11.238 | 9.5648 | −17.92 | −5.0736 | −13.032 | −1.924 | 3.0908 |
| 19.806 | −12.677 | −5.0336 | 7.2084 | 1.5772 | −11.295 | 8.7576 | 12.791 | −18.832 | −3.4376 | |
| −19.124 | 3.1848 | −3.9732 | 15.626 | −14.602 | 7.1392 | −4.9756 | 18.641 | 11.208 | −10.027 | |
| −3.8032 | −9.5784 | 17.662 | −6.426 | -14.858 | 2.394 | 12.565 | 8.318 | 4.9968 | −17.142 | |
| −7.1872 | 15.429 | −10.018 | 1.5572 | −14.815 | −17.533 | −2.9148 | 4.4508 | 19.777 | 11.447 | |
| −19.452 | −1.6868 | −9.6452 | −7.8364 | 16.999 | 0.076 | 7.066 | 11.615 | −13.538 | 13.999 | |
| −18.281 | 12.094 | 10.028 | 6.3964 | −15.98 | −9.044 | −6.6612 | −3.8572 | 17.315 | 2.8032 | |
| 13.876 | −0.006 | 16.465 | −17.618 | 2.7952 | −11.802 | −5.8424 | 10.85 | −12.338 | 5.6712 | |
| 7.3876 | 15.708 | −10.322 | −13.148 | 18.552 | −2.8992 | −7.4332 | 2.6084 | −19.847 | 8.1856 | |
| −4.824 | 12.927 | 9.538 | 0.3244 | −14.41 | -2.8084 | 17.025 | −9.5404 | −17.006 | 7.8528 | |
| −5.8884 | −10.58 | 6.884 | 13.928 | 11.017 | −17.19 | −14.602 | −3.5656 | 16.556 | 3.1604 | |
| 10.404 | 12.448 | −5.026 | −19.867 | 1.5232 | 7.3388 | −11.305 | −2.6228 | −14.186 | 18.995 | |
| −0.1608 | 3.0664 | −5.4444 | −14.534 | −19.944 | 15.171 | 8.9716 | −11.545 | 18.111 | 7.3644 | |
| L | 8.7564 | 18.094 | −16.465 | −7.5028 | −2.5408 | 7.6108 | −11.634 | 15.86 | −12.159 | 0.0456 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betti, M.; LeClair, J.; Wahl, L.M.; Zamir, M. Bee++: An Object-Oriented, Agent-Based Simulator for Honey Bee Colonies. Insects 2017, 8, 31. https://doi.org/10.3390/insects8010031
Betti M, LeClair J, Wahl LM, Zamir M. Bee++: An Object-Oriented, Agent-Based Simulator for Honey Bee Colonies. Insects. 2017; 8(1):31. https://doi.org/10.3390/insects8010031
Chicago/Turabian StyleBetti, Matthew, Josh LeClair, Lindi M. Wahl, and Mair Zamir. 2017. "Bee++: An Object-Oriented, Agent-Based Simulator for Honey Bee Colonies" Insects 8, no. 1: 31. https://doi.org/10.3390/insects8010031





