Allogrooming, Self-Grooming, and Touching Behavior: Contamination Routes of Leaf-Cutting Ant Workers Using a Fat-Soluble Tracer Dye
Abstract
:1. Introduction
2. Material and Methods
- (1)
- Group 1-1: 1 worker + 1 worker with tracer;
- (2)
- Group 4-1: 4 workers + 1 worker with tracer;
- (3)
- Group 9-1: 9 workers + 1 worker with tracer;
- (4)
- Group 19-1: 19 workers + 1 worker with tracer.
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Britto, J.S.; Forti, L.C.; Caldato, N.; Zanuncio, J.C.; Oliveira, M.A.; Bonetti Filho, R.Z.; Loeck, A.E.; Lemes, P.G.; Nagamoto, N.S.; Camargo, R.S. Use of alternatives to PFOS, its salts and PFOSF for the control of leaf-cutting ants Atta and Acromyrmex. Int. J. Res. Environ. Stud. 2016, 3, 11–92. [Google Scholar]
- Forti, L.C.; Martins, F.S.D.; Yassu, W.K.; Pinhão, M.A.S. Trofalaxia entre operárias-larvas de Atta sexdens rubropilosa Forel, 1908 (Hymenoptera, Formicidae). In Proceedings of the Anais do 14° Congresso Brasileiro de Entomologia, Piracicaba, Brazil, 5–10 April 1993. [Google Scholar]
- Forti, L.C.; Pretto, D.R.; Nagamoto, N.S.; Padovani, C.R.; Camargo, R.S.; Andrade, A.P.P. Dispersal of the delayed action insecticide sulfluramid in colonies of the leaf-cutting ant Atta sexdens rubropilosa (Hymenoptera: Formicidae). Sociobiology 2007, 50, 1149–1163. [Google Scholar]
- Silva, A.C.; da Navas, C.A.; Ribeiro, P.L. Trophallaxis in dehydrated leaf cutting colonies of Atta sexdens rubropilosa (Hymenoptera: Formicidae). Sociobiology 2009, 54, 109–114. [Google Scholar]
- Moreira, D.D.O.; Dáttilo, W.; Morais, V.; Erthal, M.; Silva, C.P.; Samuels, R.I. Diet type modifies ingestion rates and trophallactic exchanges in leaf-cutting ants. Entomol. Exp. Appl. 2015, 154, 45–52. [Google Scholar] [CrossRef]
- Wilson, E.O. The Insect Societies; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1971. [Google Scholar]
- Samuels, R.I.; Erthal, M.; Moreira, D.D.O. Ocorrência de trofalaxia oral entre operárias de Acromyrmex subterraneus subterraneus (Hymenoptera: Formicidae). In Proceedings of the XX Congresso Brasileiro de Entomologia, Gramado, Rio Grande do Sul, Brazil, 5–10 September 2004. [Google Scholar]
- Paul, J.; Roces, F. Fluid intake rates in ants correlate with their feeding habits. J. Insect Physiol. 2003, 49, 347–357. [Google Scholar] [CrossRef]
- Hölldobler, B.; Wilson, E.O. The Ants; Harvard University Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Littledyke, M.; Cherrett, J.M. Direct ingestion of plant sap from cut leaves by the leaf-cutting ants Atta cephalotes (L.) and Acromyrmex octospinosus (Reich) (Formicidae, Attini). Bull. Entomol. Res. 1976, 66, 205–217. [Google Scholar] [CrossRef]
- Andrade, A.P.P.; Forti, L.C.; Moreira, A.A.; Boaretto, M.A.C.; Ramos, V.; Matos, C.A.O. Behavior of Atta sexdens rubropilosa (Hymenoptera: Formicidae) workers during the preparation of the leaf substrate for symbiont fungus culture. Sociobiology 2002, 40, 293–306. [Google Scholar]
- Camargo, R.S.; Forti, L.C.; Lopes, J.F.; Andrade, A.P.P. Age polyethism in the leaf-cutting ant Acromyrmex subterraneus brunneus Forel, 1911 (Hymenoptera: Formicidae). J. Appl. Entomol. 2007, 131, 139–145. [Google Scholar] [CrossRef]
- Bretz, F.; Hothorn, T.; Westfall, P. Multiple Comparisons Using R; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Cordeiro, G.M.; Demétrio, C.G. Modelos Lineares Generalizados e Extensões; Departamento de Ciências Exatas, ESALQ, USP: São Paulo, Brasil, 2008. [Google Scholar]
- Ayre, G.L. The relationships between food and digestive enzymes in five species of ants (Hymenoptera: Formicidae). Can. Entomol. 1967, 99, 408–411. [Google Scholar] [CrossRef]
- Amaral, J.B.; Caetano, F.H. The intramandibular gland of leaf-cutting ants (Atta sexdens rubropilosa Forel 1908). Micron 2006, 37, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Janet, M.C.H. Sur les nématodes des glandes pharyngiennes des fourmis (Pelodora sp.). C.R.l’ Acad. Sci. Paris 1893, 117, 700–703. [Google Scholar]
- Caetano, F.H. Morphology of the digestive tract and associated excretory organs of ants. In Applied Myrmecology: A World Perspective. Boulder; Vander Meer, R.K., Jaffé, K., Cedeno, A., Eds.; Westview Press: Boulder, CO, USA, 1990; p. 741. [Google Scholar]
- Hefetz, A.; Errard, C.; Cojocaru, M. Heterospecific substances in the post-pharyngeal gland secretion of ants reared in mixed groups. Naturwissenschaften 1992, 79, 417–420. [Google Scholar] [CrossRef]
- Caetano, F.H. Anatomia, histologia e histoquímica do sistema digestivo e excretor de operárias de formigas (Hymenoptera: Formicidae). Naturalia 1988, 13, 129–174. (In Portuguese) [Google Scholar]
- Caetano, F.H.; Jaffé, K.; Zara, F.J. Formigas: Biologia e Anatomia; Editora Topázio: Rio Claro, Brazil, 2002; p. 131. [Google Scholar]
- Vieira, A.S.; Bueno, O.C. Mitochondrial and peroxisomal population in post-pharyngeal glands of leaf-cutting ants after lipid supplementation. Micron 2015. [CrossRef] [PubMed]
- Decio, P.; Vieira, A.S.; Dias, N.B.; Palma, M.S.; Bueno, O.C. The post-pharyngeal gland: Specialized organ for lipid nutrition in leaf-cutting ants. PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- Rosengaus, R.B.; Maxmen, A.; Coates, L.E.; Traniello, J.F.A. Disease resistance: A benefit of sociality in the dampwood termite Zootermopsis angusticollis (Isoptera: Termopsidae). Behav. Ecol. Sociobiol. 1998, 44, 125–134. [Google Scholar] [CrossRef]
- Hughes, W.O.H.; Eilenberg, J.; Boomsma, J.J. Trade-offs in group living: Transmission and disease resistance in leaf-cutting ants. Proc. R. Soc. Lond. B 2002, 269, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, A.; Yokohari, F.; Shimizu, S. Defense mechanism of the termite, Coptotermes formosanus Shiraki, to entomopathogenic fungi. J. Invertebr. Pathol. 2008, 97, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.N.; Hughes, W.H.O. Adaptive social immunity in leaf-cutting ants. Biol. Lett. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Morelos-Juárez, C.; Walker, T.N.; Lopes, J.F.S.; Hughes, W.H.O. Ant farmers practice proactive personal hygiene to protect their fungus crop. Curr. Biol. 2010, 20, R553–R554. [Google Scholar] [CrossRef] [PubMed]
- Crozier, R.H.; Dix, M.W. Analysis of two genetic models for the innate components of colony odor in social Hymenoptera. Behav. Ecol. Sociobiol. 1979, 4, 217–224. [Google Scholar] [CrossRef]
- Fletcher, D.J.C.; Michener, C.D. Kin Recognition in Animals; John Wiley Press: New York, NY, USA, 1987. [Google Scholar]
- Soroker, V.; Vienne, C.; Hefetz, A.; Nowbahari, E. Thepost-pharyngeal gland as a “Gestalt” organ for nestmate recognition in the ant Cataglyphisniger. Naturwissenschaften 1994, 81, 510–513. [Google Scholar]
- Nielsen, J.; Boomsma, J.J.; Oldham, N.J.; Petersen, H.C.; Morgan, E.D. Colony-level and season-specific variation in cuticular hydrocarbon profiles of individual workers in the ant Formica truncorum. Insectes Sociaux 1999, 46, 58–65. [Google Scholar] [CrossRef]
- Silverman, J.; Liang, D. Colony disassociation following diet partitioning in a unicolonial ant. Naturwissenschaften 2001, 88, 73–77. [Google Scholar] [PubMed]
- Richard, F.J.; Poulsen, M.; Hefetz, A.; Errard, C.; Nash, D.R.; Boomsma, J.J. The origin of chemical profiles of fungal symbionts and their significance for nestmate recognition in Acromyrmex leaf-cutting ants. Behav. Ecol. Sociobiol. 2007, 61, 1637–1649. [Google Scholar] [CrossRef]
- Heinze, J.; Foitzik, S.; Hippert, A.; Hölldobler, B. Apparent dear-enemy phenomenon and environment-based recognition cues in the ant Leptothoraxnylanderi. Ethology 1996, 102, 510–522. [Google Scholar] [CrossRef]
- Richard, F.J.; Poulsen, M.; Drijfhout, F.; Jones, G.R.; Boomsma, J.J. Specificity in chemical profiles of workers, brood and mutualistic fungi in Atta, Acromyrmex and Sericomyrmex fungus-growing ants. J. Chem. Ecol. 2003, 33, 2281–2292. [Google Scholar] [CrossRef] [PubMed]
- Meskali, M.; Bonavita-Cougourdan, A.; Provost, E.; Bagneres, A.G.; Dusticier, G.; Clement, J.L. Mechanism underlying cuticular hydrocarbon homogeneity in the ant Camponotusvagus (Scop.) (Hymenoptera: Formicidae): Role of post-pharyngeal glands. J. Chem. Ecol. 1995, 21, 1127–1148. [Google Scholar]
- Camargo, R.S.; Forti, L.C.; Lopes, J.F.; Andrade, A.P.P.; Raetano, C.G.; Mendonça, C.G. The role of workers in transferring queen substances and the differences between worker castes in the leaf-cutting ant, Acromyrmex subterraneus brunneus. Sociobiology 2006, 48, 503–513. [Google Scholar]
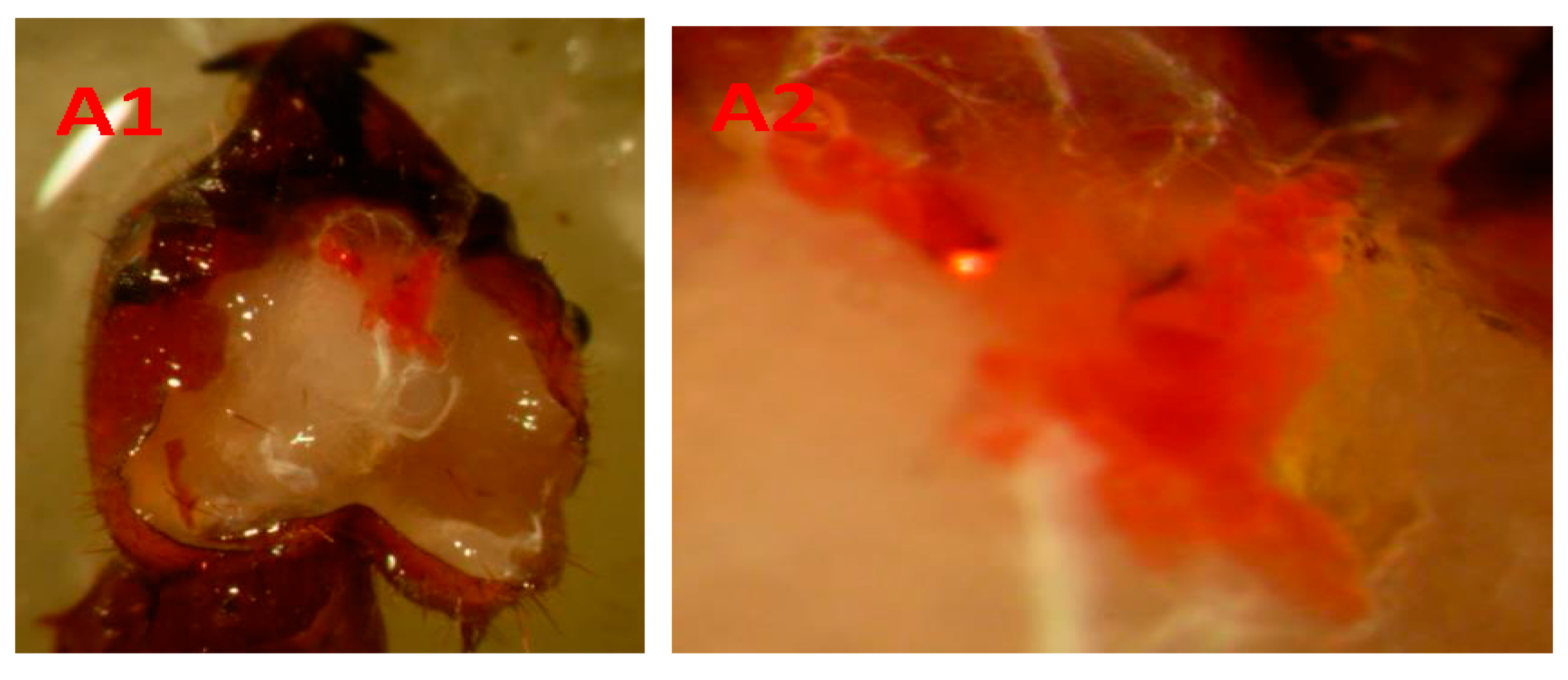
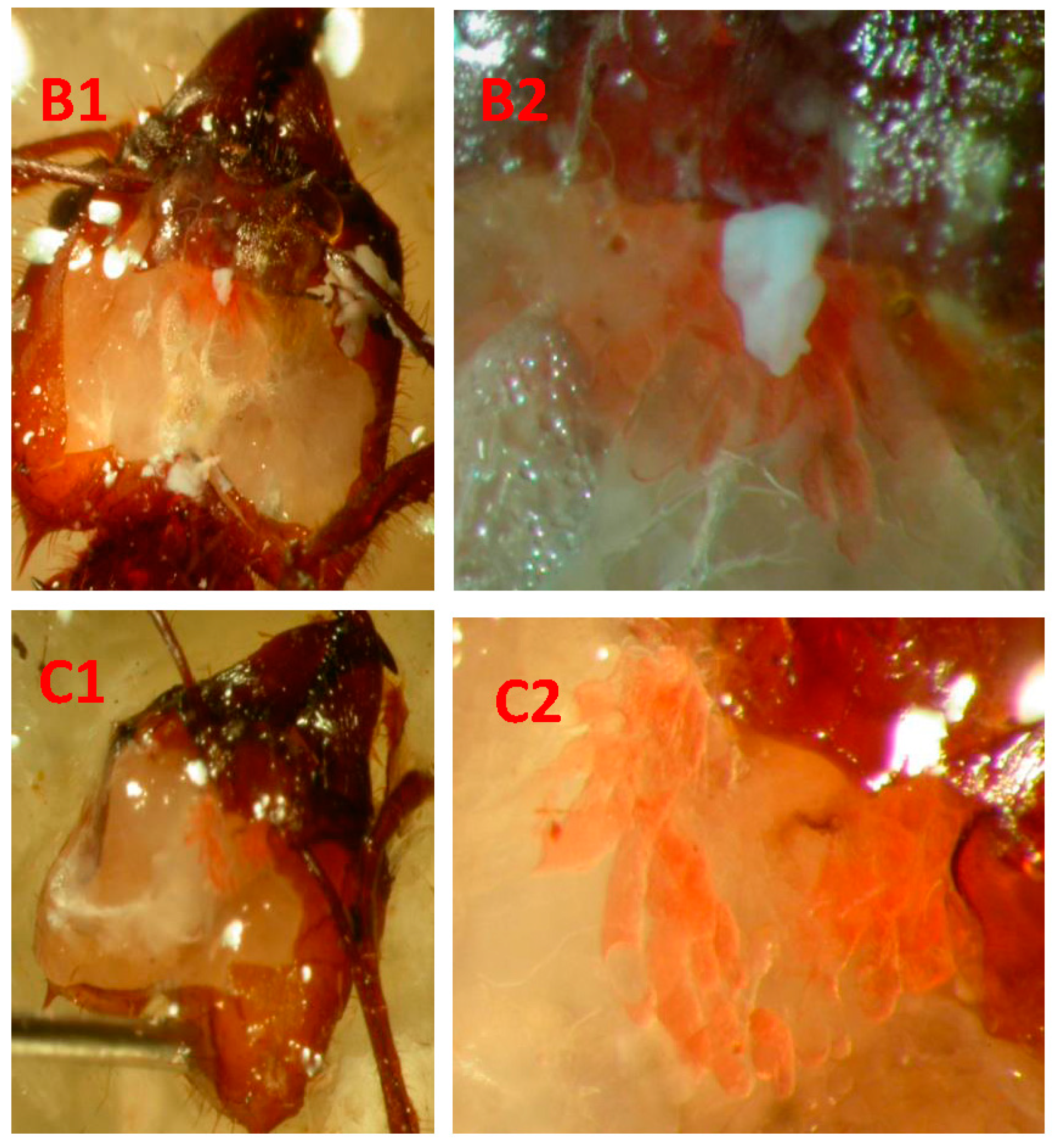
| Self-Grooming | Self-Grooming 2 | Worker–Worker Contact | ||||||
| 19-1 | 70 | a | 19-1 | 791.33 | a | 19-1 | 428.66 | a |
| 9-1 | 64.33 | a b | 9-1 | 434 | b | 9-1 | 228.66 | b |
| 4-1 | 53 | b | 4-1 | 270.66 | c | 4-1 | 144.66 | c |
| 1-1 | 7.66 | c | 1-1 | 3 | d | 1-1 | 5.33 | d |
| Worker–Worker Contact | Allogrooming | Allogrooming 2 | ||||||
| 19-1 | 2693 | a | 19-1 | 25.66 | a | 19-1 | 138.66 | a |
| 9-1 | 698 | b | 9-1 | 21 | a b | 9-1 | 57.66 | b |
| 4-1 | 252.33 | c | 4-1 | 16.33 | b | 4-1 | 24 | c |
| 1-1 | 0 | c | 1-1 | 0.33 | c | 1-1 | 0.33 | d |
| 1-1 | 9-1 | 19-1 | 4-1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Self-Grooming | 7.67 | A | Worker–Worker contact 2 | 698 | A | Worker–Worker contact 2 | 2693 | A | Self-Grooming 2 | 270.67 | a |
| Worker–Worker contact | 5.33 | A b | Self-Grooming 2 | 434 | B | Self-Grooming 2 | 791.33 | B | Worker–Worker contact 2 | 252.33 | a |
| Self-Grooming 2 | 3.0 | B | Worker–Worker contact | 228.67 | C | Worker–Worker contact | 428.67 | C | Worker–Worker contact | 144.67 | b |
| Allogrooming | 0.33 | C | Self-Grooming | 64.33 | D | Allogrooming 2 | 138.67 | D | Self-Grooming | 53 | c |
| Allogrooming 2 | 0.33 | C | Allogrooming 2 | 57.67 | D | Self-Grooming | 70 | E | Allogrooming 2 | 24 | d |
| Worker–Worker contact2 | 0 | C | Allogrooming | 16.33 | E | Allogrooming | 25.67 | F | Allogrooming | 21 | d |
| Coefficients: Estimate Std. Error z Value Pr (>|z|) | ||||
|---|---|---|---|---|
| (Intercept) | 0.6931 | 0.866 | 0.8 | 0.423 |
| 19:1 | −0.5596 | 0.9039 | −0.619 | 0.536 |
| 4:1 | 0.3185 | 1.0445 | 0.305 | 0.76 |
| 9:1 | −0.2877 | 0.9428 | −0.305 | 0.76 |
| Coefficients: Estimate Std. Error z Value Pr (>|z|) | ||||
|---|---|---|---|---|
| (Intercept) | 0.2877 | 0.5 | 0.575 | 0.56504 |
| 04:01 | 1.0116 | 0.5839 | 1.733 | 0.08317 |
| 09:01 | 1.5041 | 0.5528 | 2.721 | 0.00651 |
| 19:01 | 2.0794 | 0.5303 | 3.921 | 8.82 × 10−5 |
| Coefficients: Estimate Std. Error z Value Pr (>|z|) | ||||
|---|---|---|---|---|
| (Intercept) | −0.4055 | 0.7071 | −0.573 | 0.56636 |
| 04:01 | 0.6931 | 0.866 | 0.8 | 0.42349 |
| 09:01 | 1.7918 | 0.7638 | 2.346 | 0.018978 |
| 19:01 | 2.6391 | 0.7319 | 3.606 | 0.000311 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camargo, R.D.S.; Puccini, C.; Forti, L.C.; De Matos, C.A.O. Allogrooming, Self-Grooming, and Touching Behavior: Contamination Routes of Leaf-Cutting Ant Workers Using a Fat-Soluble Tracer Dye. Insects 2017, 8, 59. https://doi.org/10.3390/insects8020059
Camargo RDS, Puccini C, Forti LC, De Matos CAO. Allogrooming, Self-Grooming, and Touching Behavior: Contamination Routes of Leaf-Cutting Ant Workers Using a Fat-Soluble Tracer Dye. Insects. 2017; 8(2):59. https://doi.org/10.3390/insects8020059
Chicago/Turabian StyleCamargo, Roberto Da Silva, Carolina Puccini, Luiz Carlos Forti, and Carlos Alberto Oliveira De Matos. 2017. "Allogrooming, Self-Grooming, and Touching Behavior: Contamination Routes of Leaf-Cutting Ant Workers Using a Fat-Soluble Tracer Dye" Insects 8, no. 2: 59. https://doi.org/10.3390/insects8020059






