Systematics of the Ceracis furcifer Species-Group (Coleoptera: Ciidae): The Specialized Consumers of the Blood-Red Bracket Fungus Pycnoporus sanguineus
Abstract
:1. Introduction
2. Material and Methods
| ANIC | Australian National Insect Collection (Canberra, Australia) |
| BMNH | Natural History Museum (London, UK) |
| CAMB | Coleção Ayr de Moura Bello (Rio de Janeiro, RJ, Brazil) |
| CMN | Canadian Museum of Nature (Ottawa, Ontario, Canada) |
| CNCI | Canadian National Collection of Insects (Ottawa, Ontario, Canada) |
| CELC | Coleção Entomológica do Laboratório de Sistemática e Biologia de Coleoptera da UFV (Viçosa, MG, Brazil) |
| CERPE | Coleção Entomológica da Universidade Federal Rural de Pernambuco (Recife, PE, Brazil) |
| CEMT | Seção de Entomologia da Coleção Zoológica, Departamento de Biologia e Zoologia, Instituto de Biociências, Universidade Federal de Mato Grosso (Cuiabá, MT, Brazil) |
| DZUP | Coleção Entomologica Pe. J. S. Moure, Universidade Federal do Paraná,(Curitiba, PR, Brazil) |
| FMNH | Field Museum of Natural History (Chicago, Illinois, USA) |
| MCNZ | Museu de Ciências Naturais da Fundação Zoobotânica do Rio Grande do Sul (Porto Alegre, RS, Brazil) |
| MFN | Museum für Naturkunde (Berlin, Germany) |
| MHNG | Muséum d’Histoire Naturelle (Géneve, Switzerland) |
| MNHN | Muséum National d’Histoire Naturelle (Paris, France) |
| MNRJ | Museu Nacional do Rio de Janeiro (Rio de Janeiro, RJ, Brazil) |
| MPEG | Museu Paraense Emílio Goeldi (Belém, PA, Brazil) |
| MZSP | Museu de Zoologia da Universidade de São Paulo (São Paulo, SP, Brazil) |
| NMNH | National Museum of Natural History, Smithsonian Institution (Washington, D. C., USA) |
| SNSD | Senckenberg Naturhistorische Sammlungen Dresden (Dresden, Germany) |
3. Results
3.1. Taxonomic synopsis
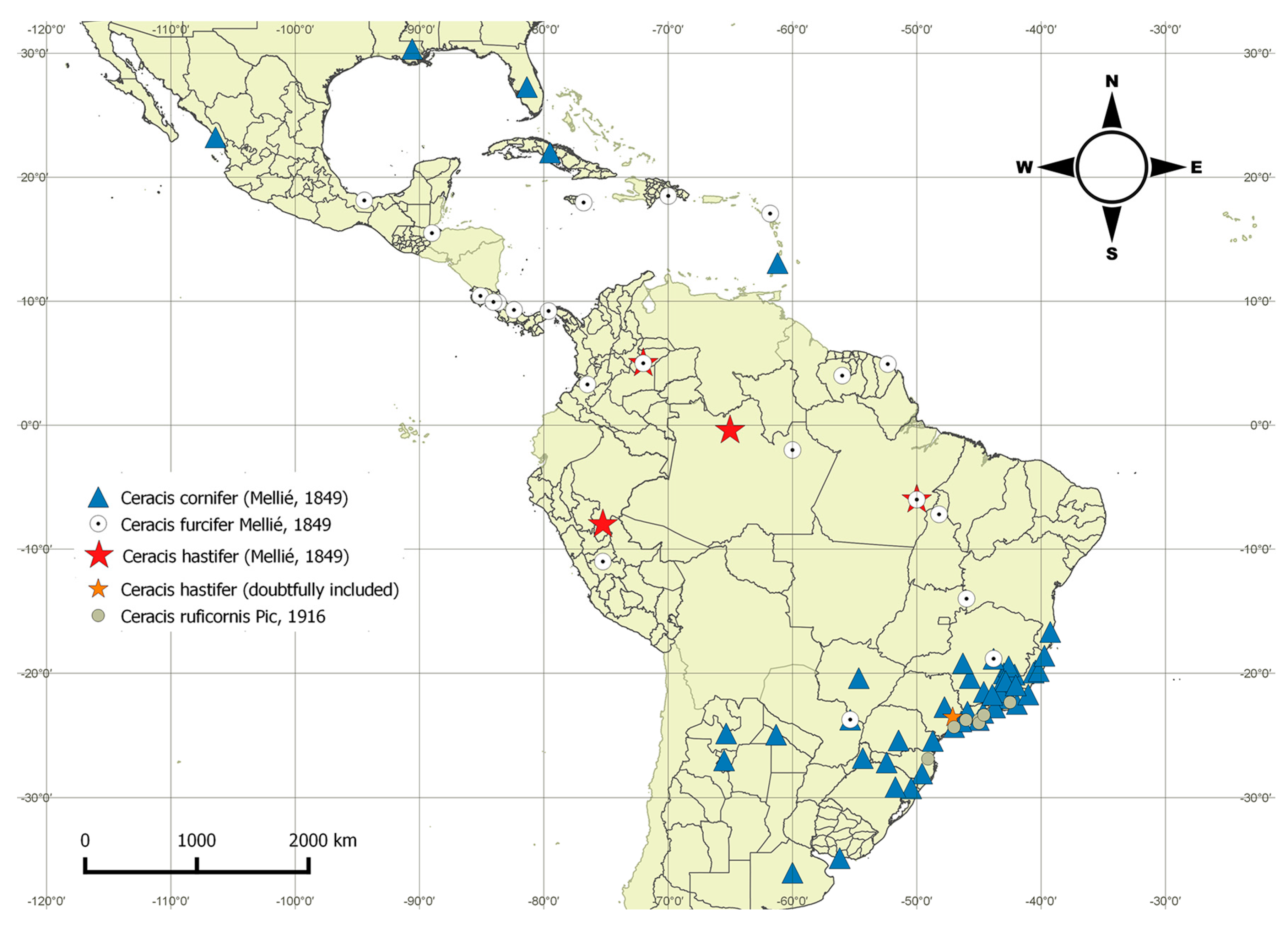
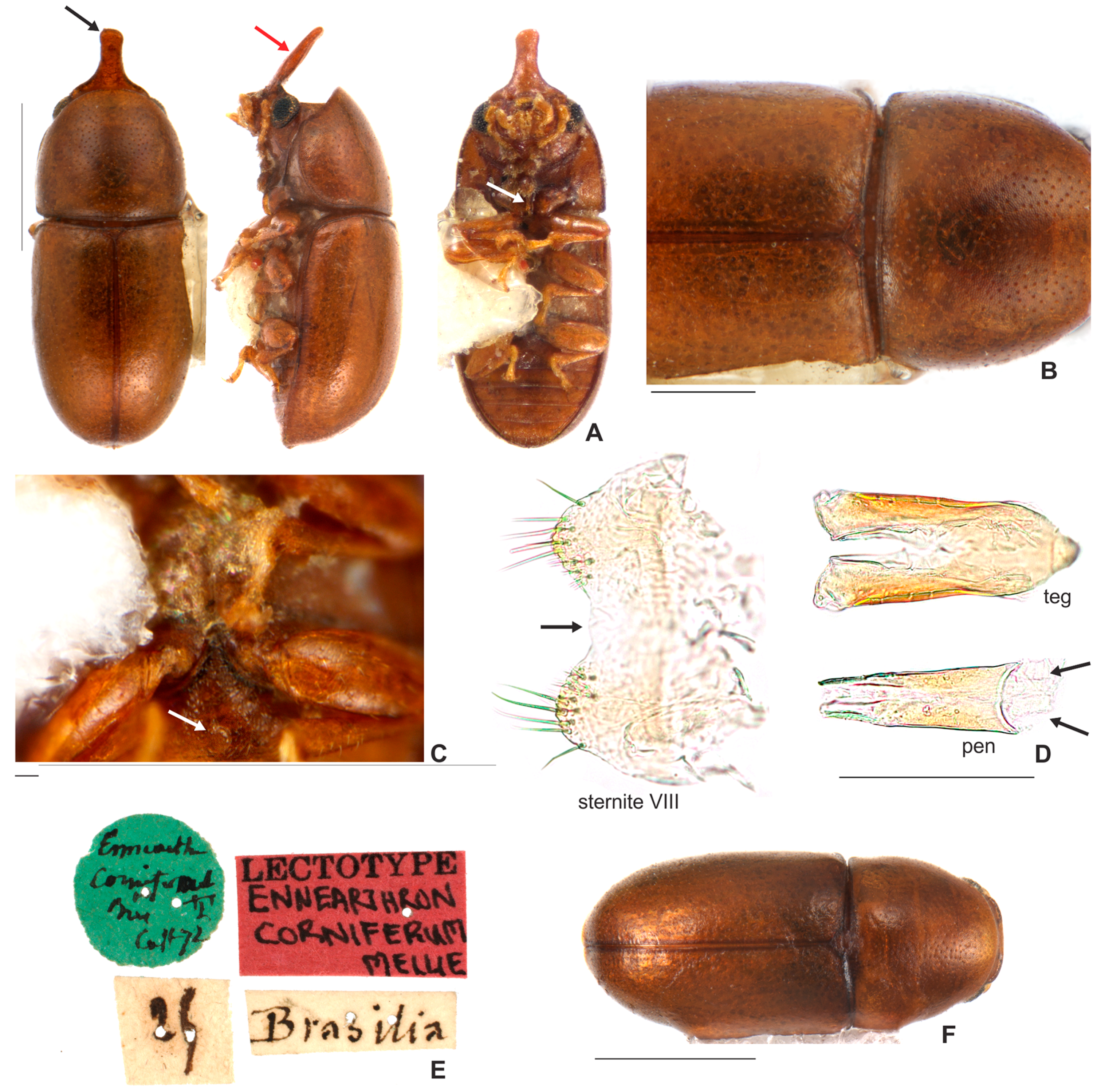
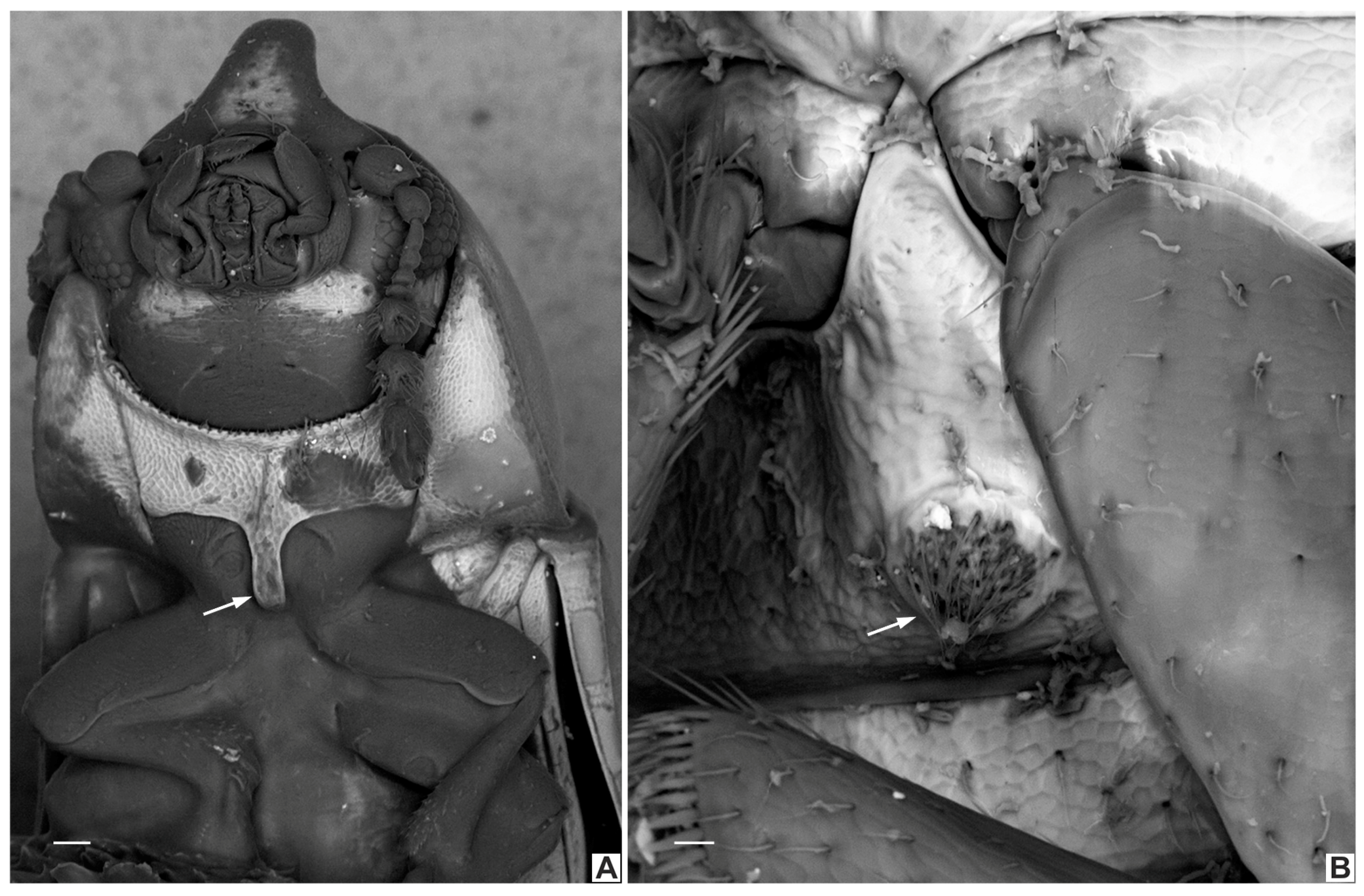
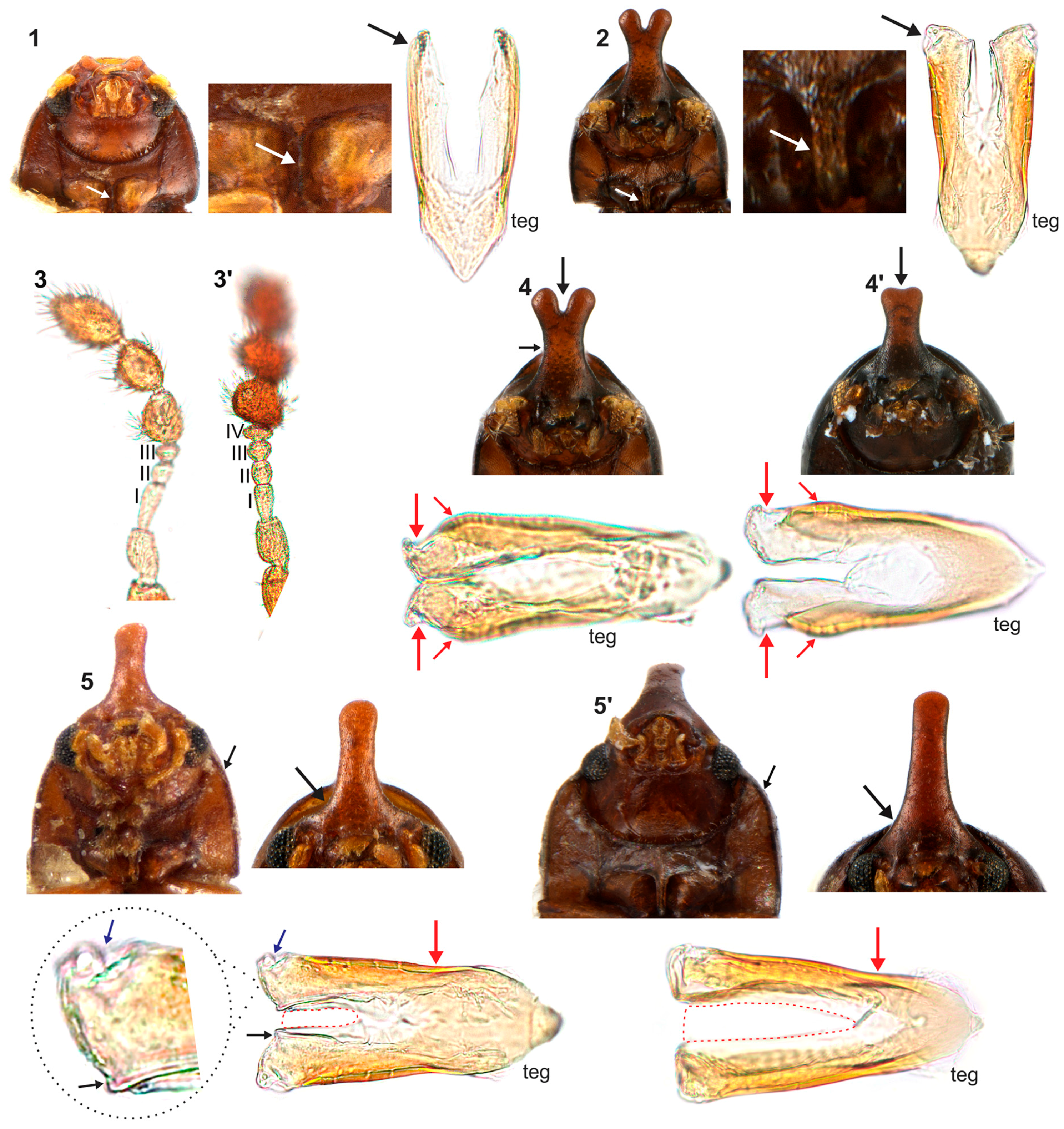
3.1.1. Ceracis cornifer (Mellié, 1849)
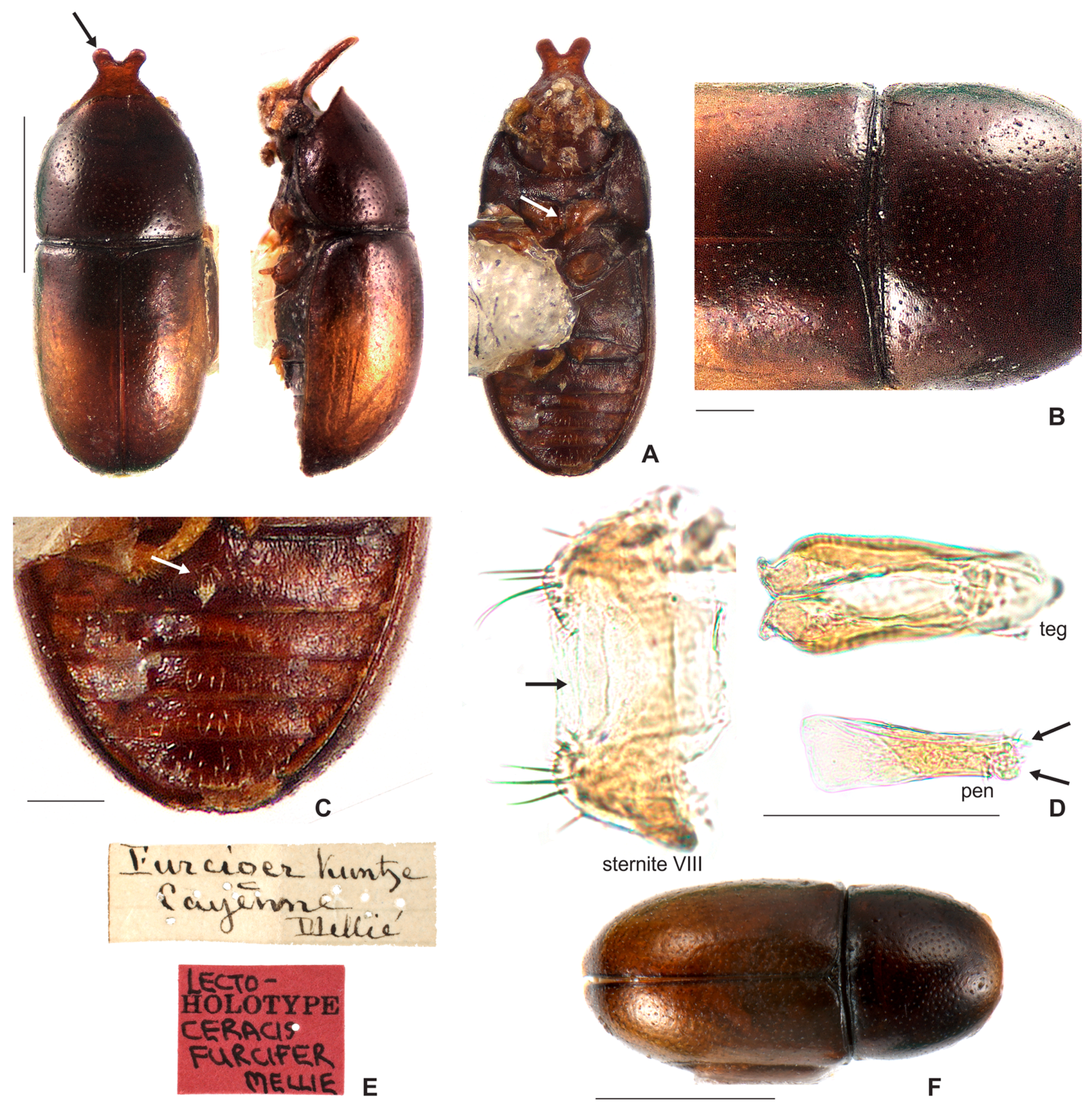
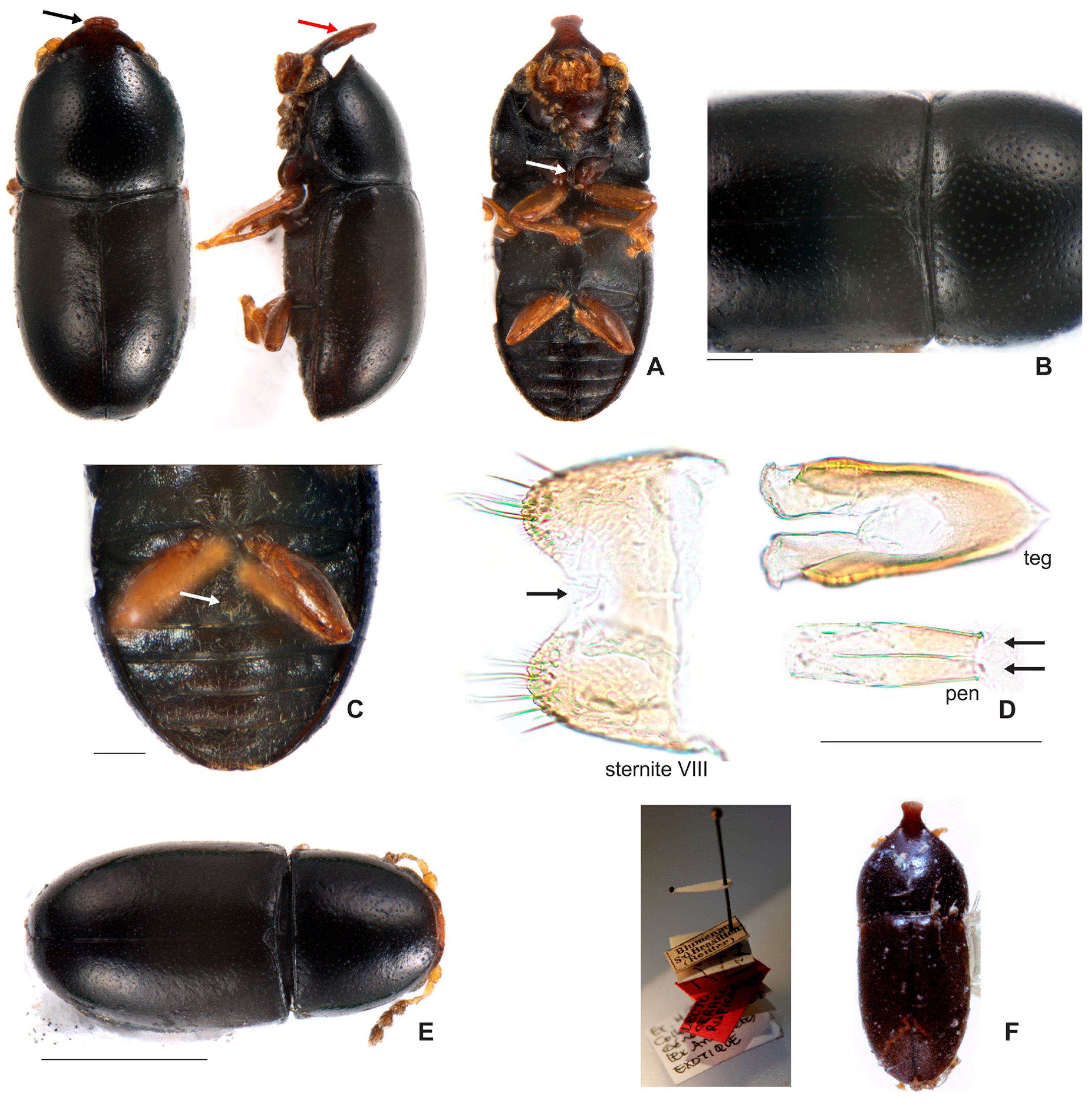
3.1.2. Ceracis furcifer Mellié, 1849
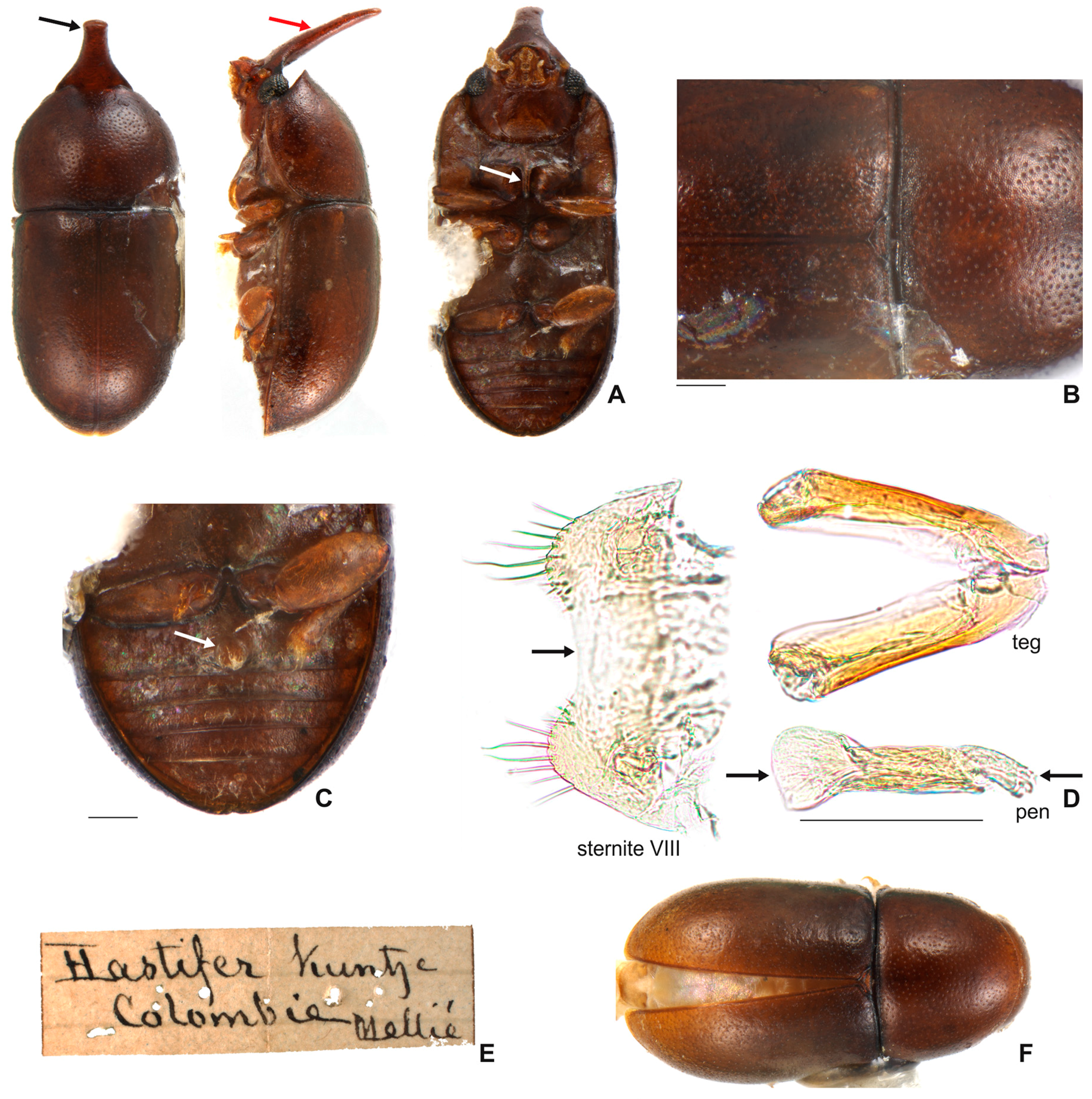
3.1.3. Ceracis hastifer (Mellié, 1849)
3.1.4. Ceracis ruficornis Pic, 1916
3.2. Species Accounts
3.2.1. Ceracis cornifer (Mellié, 1849)
3.2.2. Ceracis furcifer Mellié, 1849
3.2.3. Ceracis hastifer (Mellié, 1849)
3.2.4. Ceracis ruficornis Pic, 1916
3.2.5. Identification Key to Males of the furcifer Group
4. Discussion
4.1. Intraspecific Variation: Coloration and Horn Shape in Males
4.2. Distribution Patterns of the furcifer Group
4.3. Specialization on the Host Fungus Pycnoporus sanguineus
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Species | Record | Breeding record | Source |
|---|---|---|---|
| Ceracis cornifer (Mellié) | 84 | 40 | [9,10,21,64] and present work |
| Cis aff. pusillus (comptus group) | Undetermined | Undetermined | [65] |
| Ceracis minutus Dury | 3 (3) | 3 | [21,66] |
| Cis neserorum Souza-Gonçalves & Lopes-Andrade (as Cis sp. E) | 1 | Undetermined | [67,68] |
| Ceracis furcifer Mellié | 12 | 6 | [1] and present work |
| Ceracis hastifer (Mellié) | 1 | 0 | Present work |
| Ceracis ruficornis Pic | 1 | 1 | Present work |
| Homo sapiens L. | 4 | NA ** | [57,58,59,60] |
| Insects (without details) | 1 | Undetermined | [69] |
| Acromyrmex lundi (Guérin-Méneville) * | 1 | Undetermined | [56] |
| Ceracis sp.2 | 1 | Undetermined | [64] |
| Cis sp.3 (taurus group) | 1 | Undetermined | [64] |
| Strigocis vicosensis Lopes-Andrade | 1 | Undetermined | [64] |
| Xylographus contractus Mellié | 1 | Undetermined | [64] |
| Xylographus rufipes Pic | 1 | Undetermined | [64] |
| Ceracis tabellifer (Mellié) | 3 | Undetermined | [6] |
| Strigocis sp. | 3 | Undetermined | [10] |
| Cis sp.4 (tricornis group) | 1 | Undetermined | [10] |
| Grossicis diadematus (Mellié) | 1 | Undetermined | [10] |
| Cis sp.2 (vitulus group) | 1 | Undetermined | [10] |
| Cis sp.3 (comptus group) | 1 | Undetermined | [10] |
| Ceracis cucullatus (Mellié) | 1 | Undetermined | [6] |
| Ceracis punctulatus Casey | 2 | Undetermined | [21,47] |
| Cis creberrimus Mellié | 1 | Undetermined | [21,47] |
| Cis subfuscus Gorham | Undetermined | Undetermined | [47] |
| Strigocis opacicollis Dury | 1 | Undetermined | [21,47] |
| Species | Host fungus | Record | Source |
|---|---|---|---|
| Ceracis cornifer (Mellié) | Lenzites betulina (L.) Fr. | 1 | [10] |
| Ceracis cornifer (Mellié) | Trametes villosa (Sw.) Kreisel | 3 | [64] |
| Ceracis cornifer (Mellié) | Phellinus gilvus (Schwein.) Pat. | 1 | [64] |
| Ceracis cornifer (Mellié) | Stereum sp. | 1 | [64] |
| Ceracis cornifer (Mellié) | Schizophyllum commune Fr. | 1 | [64] |
| Ceracis cornifer (Mellié) | Trametes sp. | 1 | [65] |
| Ceracis cornifer (Mellié) | Trametes sp. | 1 (one breeding record) | Present work |
| Ceracis cornifer (Mellié) | Trametes hispida Bagl. | 1 | Present work |
| Ceracis furcifer Mellié | Coriolus sp. and Lenzites sp. | 1 | [21] |
| Ceracis furcifer Mellié | Daedalea elegans Spreng. | 1 | Present work |
| Ceracis furcifer Mellié | Polyporus maximus (Brot.) Fr. | 1 | Present work |
| Ceracis furcifer Mellié | Daedalea elegans Spreng. | 1 | Present work |
| Ceracis ruficornis Pic | Coriolus sp. and Lenzites sp. | 1 | [21] |
References
- Mellié, M.J. Monographie de I’ ancien genre Cis des auters. Ann. Soc. Entomol. Fr. 1849, 6, 205–400. [Google Scholar]
- Lacordaire, J.T. Histoire Naturelle des Insectes. Genera des Coléoptères ou Exposé Méthodique et Critique de Tous les Genres proposés Jusqu’ici dans cet Ordre D’insectes. Tome IV; Librairie Encyclopédique de Roret: Paris, France, 1857. [Google Scholar]
- Lawrence, J.F. Delimitation of the Genus Ceracis (Coleoptera: Ciidae) with a revision of North America Species. Bull. Mus. Comp. Zool. 1967, 136, 91–144. [Google Scholar]
- Lawrence, J.F. The Australian Ciidae (Coleoptera: Tenebrionoidea): A Preliminary Revision. Zootaxa 2016, 4198, 1–208. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Carvalho, C.; Lopes-Andrade, C. Two new Neotropical species of Ceracis Mellié (Coleoptera, Ciidae) and redefinition of the cucullatus group. ZooKeys 2011, 132, 51–64. [Google Scholar] [CrossRef]
- Antunes-Carvalho, C.; Lopes-Andrade, C. Two Invaders Instead of One: The True Identity of Species under the Name Ceracis cucullatus (Coleoptera: Ciidae). PLoS ONE 2013, 8, e72319. [Google Scholar] [CrossRef] [PubMed]
- Pecci-Maddalena, I.S.C.; Sandoval-Gómez, V.E.; Lopes-Andrade, C. Ceracis zarathustrai sp. nov. (Coleoptera: Ciidae) from the Atlantic Forest biome. Zoologia (Curitiba) 2014, 31, 482–488. [Google Scholar] [CrossRef]
- Lopes-Andrade, C.; Madureira, M.; Zacaro, A.A. Delimitation of the Ceracis singularis group (Coleoptera: Tenebrionoidea: Ciidae), with the description of a new Neotropical species. Dugesiana 2002, 9, 59–63. [Google Scholar]
- Gumier-Costa, F.; Lopes-Andrade, C.; Zacaro, A.A. Association of Ceracis cornifer (Mellié) (Coleoptera: Ciidae) with the Bracket Fungus Pycnoporus sanguineus (Basidiomycetes: Polyporaceae). Neotrop. Entomol. 2003, 32, 359–360. [Google Scholar] [CrossRef]
- Graf-Peters, L.V.; Lopes-Andrade, C.; Silveira, R.M.B.; Moura, L.A.; Reck, M.A.; Nogueira-de-Sá, F. Host Fungi and Feeding habits of Ciidae (Coleoptera) in a Subtropical Rainforest in Southern Brazil, with an overview of Host-Fungi of Neotropical Ciids. Fla. Entomol. 2011, 94, 553–566. [Google Scholar] [CrossRef]
- Brèthes, J. Descripción de varios coleópteros de Buenos Aires. Anales Soc. Cient. Argent. 1922, 94, 263–305. [Google Scholar]
- Gorham, H.S. On the serricorn Coleoptera of St. Vincent, Grenada and the Grenadines (Malacodermata, Ptinidae, Bostrychidae) with descriptions of new species. Proc. Zool. Soc. 1898, 27, 315–343. [Google Scholar]
- Pic, M. Diagnoses Spécifiques. Mélanges Exotico-Entomologiques. 1916, 17, 1–22. [Google Scholar]
- Blackwelder, R.E. Checklist of the coleopterous insects of Mexico, Central America, the West Indies, and South America. Part 3. Bull. US Nat. Hist. Mus. 1945, 185, 343–550. [Google Scholar] [CrossRef]
- Abdullah, M. The systematic position of Cisidae (Heteromera) including a catalogue of the world and comments on central European families of Cucujoidea (Coleoptera). Zool. Beitr. (NF). 1973, 19, 189–246. [Google Scholar]
- Peck, S.B. The beetles of the Lesser Antilles (Insecta, Coleoptera): Diversity and distributions. Insecta Mundi 2015, 0460, 1–360. Available online: http://digitalcommons.unl.edu/insectamundi/967/ (accessed on 30 May 2017).
- Morrone, J.J. Biogeographic areas and transition zones of Latin America and the Caribbean Islands based on panbiogeographic and cladistics analyses of the entomofauna. Annu. Rev. Entomol. 2006, 51, 467–494. [Google Scholar] [CrossRef] [PubMed]
- Morrone, J.J. Cladistic biogeography of the Neotropical region: Identifying the main events in the diversification of the terrestrial biota. Cladistics 2014, 30, 202–214. [Google Scholar] [CrossRef]
- Oliveira, E.H.; Lopes-Andrade, C.; Lawrence, J.F. Review of the Neotropical Ciidae (Insecta: Coleoptera) in the Cis taurus species-group. Arthropod Syst. Phylogeny 2013, 71, 181–210. [Google Scholar]
- Escalante, T.; Morrone, J.J.; Rodríguez-Tapia, G. Biogeographic regions of North American mammals based on endemism. Biol. J. Linn. Soc. Lond. 2013, 110, 485–499. [Google Scholar] [CrossRef]
- Lawrence, J.F. Host preference in ciid beetles (Coleoptera: Ciidae) inhabiting the fruiting bodies of Basidiomycetes in North America. Bull. Mus. Comp. Zool. 1973, 145, 163–212. Available online: http://www.biodiversitylibrary.org/part/34211#/summary (accessed on 30 May 2017).
- Orledge, G.M.; Reynolds, S.E. Fungivore host-use groups from cluster analysis: Patterns of utilisation of fungal fruiting bodies by ciid beetles. Ecol. Entomol. 2005, 30, 620–641. [Google Scholar] [CrossRef]
- Roberts, P.; Evans, S. The Book of Fungi: A Life-Size Guide to Six Hundred Species from around the World, 1st ed.; Ivy press: Brighton, UK, 2011. [Google Scholar]
- Lesage-Meessen, L.; Haon, M.; Uzan, E.; Levasseur, A.; Piumi, F.; Navarro, D.; Taussac, S.; Favel, A.; Lomascolo, A. Phylogeographic relationships in the polypore fungus Pycnoporus inferred from molecular data. FEMS Microbiol. Lett. 2011, 325, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, R.M.B.; Reck, M.A.; Graf, L.V.; De Sá, F.N. Polypores from a Brazilian pine forest in Southern Brazil: Pileate species. Hoehnea 2008, 35, 619–630. [Google Scholar] [CrossRef]
- Smânia, A.; Marques, C.J.S.; Smânia, E.F.A.; Zanetti, C.R.; Carobrez, S.G.; Tramonte, R.; Loguercio-Leite, C. Toxicity and antiviral activity of cinnabarin obtained from Pycnoporus sanguineus (Fr.) Murr. Phytother. Res. 2003, 17, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.F.C.; Le Hyaric, M.; Berg, M.M.; Almeida, M.V.; Edwards, H.G.M. Raman spectroscopic characterization of cinnabarin produced by the fungus Pycnoporus sanguineus (Fr.) Murr. J. Raman. Spectrosc. 2007, 38, 1628–1632. [Google Scholar] [CrossRef]
- Borderes, J.; Costa, A.; Guedes, A.; Tavares, L.B.B. Antioxidant activity of the extracts from Pycnoporus sanguineus mycelium. Braz. Arch. Biol. Technol. 2011, 54, 1167–1174. [Google Scholar] [CrossRef]
- Modes, K.S.; Lazarotto, M.; Beltrame, R.; Vivian, M.A.; Santini, E.J.; Muniz, M.F.B. Resistência natural das madeiras de sete espécies florestais ao fungo Pyconoporus sanguineus causador da podridão-branca. Cerne Lavras 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Rohr, C.O.; Levin, L.N.; Mentaberry, A.N.; Wirth, S.A. A first insight into Pycnoporus sanguineus BAFC 2126 transcriptome. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Forootanfar, H.; Faramarzi, M.A. Insights into laccase producing organisms, fermentation states, purification strategies, and biotechnological applications. Biotechnol. Prog. 2015, 31, 1443–1463. [Google Scholar] [CrossRef] [PubMed]
- Vikineswary, S.; Abdullah, N.; Renuvathani, M.; Sekaran, M.; Pandey, A.; Jones, E.B.G. Productivity of laccase in solid substrate fermentation of selected agro-residues by Pycnoporus sanguineus. Bioresour. Technol. 2006, 97, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, C.; Liu, H.Z.; Liu, C.Z. Immobilization of Pycnoporus sanguineus laccase by metal affinity adsorption on magnetic chelator particles. J. Chem. Technol. Biotechnol. 2008, 83, 97–104. [Google Scholar] [CrossRef]
- Lopes-Andrade, C.; Grebennikov, V.V. First record and five new species of Xylographellini (Coleoptera: Ciidae) from China, with online DNA barcode library of the Family. Zootaxa 2015, 4006, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Evenhuis, N.L. A compendium of zoological type nomenclature: A reference source. Bish. Mus. Tech. Rep. 2008, 41, 1–23. [Google Scholar]
- Lopes-Andrade, C.; Lawrence, J.F. Phellinocis, a new genus of Neotropical Ciidae (Coleoptera: Tenebrionoidea). Zootaxa 2005, 1034, 43–60. [Google Scholar] [CrossRef]
- Lopes-Andrade, C. The first Strigocis Dury (Coleoptera: Ciidae) from the southern Neotropical region and a provisional key to world species. ZooKeys 2011, 81, 27–37. [Google Scholar] [CrossRef] [PubMed]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2016. Available online: http://qgis.osgeo.org (accessed on 30 May 2017).
- Wick, M. The GeoNames Geographical Database. 2012. Available online: www. geonames.org (accessed on 30 May 2017).
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Antunes-Carvalho, C.; Sandoval-Gómez, V.E.; Lopes-Andrade, C.L. Grossicis, a new genus of Neotropical minute tree-fungus beetles (Coleoptera: Ciidae), with a detailed discussion on its systematic position in the family. C. R. Biol. 2012, 335, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Casey, T.L. Studies in the Ptinidae, Cioidae and Sphindidae of America. J. N. Y. Entomol. Soc. 1898, 6, 61–92. [Google Scholar]
- Lopes-Andrade, C. Recent advances in the study of the Brazilian Ciidae (Coleoptera: Tenebrionoidea). Dugesiana 2002, 9, 5–13. [Google Scholar]
- Gorham, H.S. Supplement to Malacodermata. In Biologia Centrali-Americana. Insecta. Coleoptera; Godman, F.D., Salvin, O., Eds.; Porter: London, UK, 1886; pp. 313–360. [Google Scholar]
- Mueller, C.; Jaeger, B.; Kompantsev, A.V.; Uhlig, M. Type and species catalogue of the minute tree-fungus beetles of the Museum für Naturkunde in Berlin, with general information on the Coleoptera collection, its curation and “Historical Collection” (Coleoptera, Polyphaga, Ciidae and Pterogeniidae). Zoosyst. Evol. 2001, 77, 303–323. [Google Scholar] [CrossRef]
- Pic, M. Noveautés diverses. Mélanges Exotico-Entomol. 1922, 35, 1–34. [Google Scholar]
- Lawrence, J.F. Revision of the North American Ciidae (Coleoptera). Bull. Mus. Comp. Zool. 1971, 142, 419–522. [Google Scholar]
- Paulian, R. Biologie des Coléoptères; Éditions Lechevalier: Paris, France, 1988. [Google Scholar]
- Da Silva, M.B.; Pinto-da-Rocha, R. História biogeográfica da Mata Atlântica: Opiliões (Arachinida) como modelo para sua inferência. In Biogeografia da América do Sul − Padrões e Processos; Carvalho, C.J.B., Almeida, E.A.B., Eds.; Editora Roca: São Paulo, Brazil, 2011; pp. 221–238. [Google Scholar]
- Costa, L.P. The historical bridge between the Amazon and the Atlantic Forest of Brazil: A study of molecular phylogeography with small mammals. J. Biogeogr. 2003, 30, 71–86. [Google Scholar] [CrossRef]
- Melo Santos, A.M.; Cavalcanti, D.R.; da Silva, J.M.C.; Tabarelli, M. Biogeographical relationships among tropical forests in north-eastern Brazil. J. Biogeogr. 2007, 34, 437–446. [Google Scholar] [CrossRef]
- Filgueiras, B.K.C.; Iannuzzi, L.; Vaz-de-Mello, F.Z. First report of Oxysternon silenus Castelnau (Scarabaeidae, Scarabaeinae, Phanaeini) in the Brazilian Atlantic forest. Rev. Bras. Entomol. 2011, 55, 283–284. [Google Scholar] [CrossRef]
- Lourenço, W.R. The disrupted pattern of distribution of the genus Hadrurochactas Pocock; evidence of past connections between Amazon and the Brazilian Atlantic forest. C. R. Biol. 2010, 333, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Batalha-Filho, H.; Fjeldsa, J.; Fabre, P.H.; Miyaki, C.Y. Connections between the Atlantic and the Amazonian forest avifaunas represent distinct historical events. J. Ornithol. 2013, 154, 41–50. [Google Scholar] [CrossRef]
- Silva, M.; Noll, F.B. Biogeography of the social wasp genus Brachygastra (Hymenoptera: Vespidade: Polistinae). J. Biogeogr. 2014, 42, 833–842. [Google Scholar] [CrossRef]
- Lechner, B.E.; Josens, R. Observation of leaf-cutting ants foraging on wild mushrooms. Insectes Soc. 2012, 59, 285–288. [Google Scholar] [CrossRef]
- Fidalgo, O.; Hirata, J.M. Etnomicologia Caiabi, Txicão e Txucarramãe. Rickia 1979, 8, 1–5. [Google Scholar]
- Scarpa, G.F. Medicinal plants used by the Criollos of Northwestern Argentine Chaco. J. Ethnopharmacol. 2004, 91, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, G.F. Etnobotánica médica de los Indígenas Chorote y su comparación con la de los Criollos del Chaco Semiárido (Argentina). Darwiniana 2009, 47, 92–107. [Google Scholar]
- Murrill, W.A. The Polyporaceae of North America-VIII. Hapalopilus, Pycnoporus, and New Monotypic Genera. Bull. Torrey Bot. Club. 1904, 31, 415–428. [Google Scholar] [CrossRef]
- Kayne, S.B. Traditional Medicine: A Global Perspective; Pharmaceutical Press: London, UK; Chicago, IL, USA, 2010. [Google Scholar]
- Zulfadhly, Z.; Mashitah, M.D.; Bhatia, S. Heavy metals removal in fixed-bed column by the macro fungus Pycnoporus sanguineus. Environ. Pollut. 2001, 112, 463–470. [Google Scholar] [CrossRef]
- Futuyma, D.J.; Moreno, G. The Evolution of ecological specialization. Annu. Rev. Ecol. Syst. 1988, 19, 207–233. [Google Scholar] [CrossRef]
- Souza, N.F.R. The fauna of Ciidae beetles (Insecta: Coleoptera) from Cerrado in Alto Paranaíba, MG. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, MG, Brazil, 2013. Available online: http://www.locus.ufv.br/handle/123456789/2273?show=full (accessed on 30 May 2017).
- Araujo, L.S. Ecologia de besouros micetócolos: Novas perspectivas para biomas da América do Sul e África. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2015. Available online: http://www.locus.ufv.br/handle/123456789/6382 (accessed on 30 May 2017).
- Lawrence, J.F. A catalog of the Coleoptera of America north of Mexico. Family Ciidae 1982, 529(105), 1–18. [Google Scholar]
- Neser, O.C. The first record of Astichus Förster (Hymenoptera: Eulophidae: Entiinae), parasitoids of Ciidae (Coleoptera) in bracket fungi, from the Afrotropical Region and the description of four new species from South Africa. Zootaxa 2012, 3183, 49–64. [Google Scholar]
- Souza-Gonçalves, I.; Lopes-Andrade, C. Seven new species of Cis Latreille (Coleoptera: Ciidae) from southern Africa. Entomol. Sci. 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Sarasija, P.; Remadevi, O.K. Insect communities associated with the fruiting bodies of wood decaying macro fungi in Rajiv Gandhi National Park, Nagarahole (Nilgiri Biosphere Reserve, Karnataka). J. Indian Acad. Wood Sci. 2011, 8, 106–111. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pecci-Maddalena, I.S.C.; Lopes-Andrade, C. Systematics of the Ceracis furcifer Species-Group (Coleoptera: Ciidae): The Specialized Consumers of the Blood-Red Bracket Fungus Pycnoporus sanguineus. Insects 2017, 8, 70. https://doi.org/10.3390/insects8030070
Pecci-Maddalena ISC, Lopes-Andrade C. Systematics of the Ceracis furcifer Species-Group (Coleoptera: Ciidae): The Specialized Consumers of the Blood-Red Bracket Fungus Pycnoporus sanguineus. Insects. 2017; 8(3):70. https://doi.org/10.3390/insects8030070
Chicago/Turabian StylePecci-Maddalena, Italo S. C., and Cristiano Lopes-Andrade. 2017. "Systematics of the Ceracis furcifer Species-Group (Coleoptera: Ciidae): The Specialized Consumers of the Blood-Red Bracket Fungus Pycnoporus sanguineus" Insects 8, no. 3: 70. https://doi.org/10.3390/insects8030070





