Aquatic Insects and their Potential to Contribute to the Diet of the Globally Expanding Human Population
Abstract
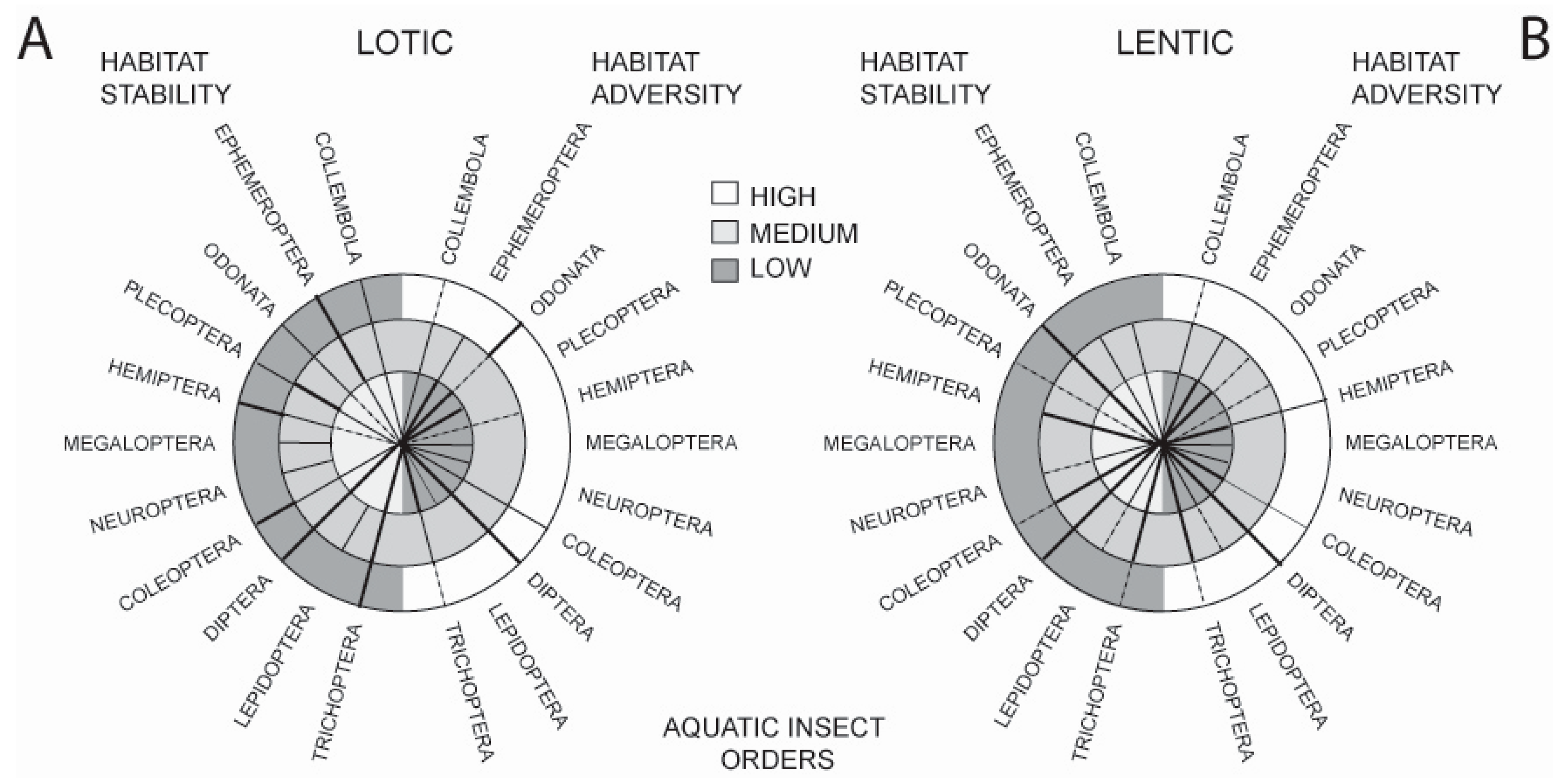
:1. Introduction
2. Background
3. Details of Those Insect Orders Having Greatest Entomophagous and/or Bulk Harvesting Potential
- Ephemeroptera—There are more than 3000 species of mayfly. The majority are lotic, but while many species live in small to medium-sized streams, huge, natural, mass emergences have been recorded from large rivers (e.g., the Mississippi; [18]). These events are seasonal but, as the adults are attracted to lights and bridges, there are opportunities to gather them, in bulk, for processing and storage. Large lentic species, such as Hexagenia limbata (Ephemeridae), perhaps have the potential to be raised in culture as their development is highly temperature dependent and they feed by collecting fine-particle organic detritus. The species has been observed to complete its life cycle in 17 weeks in warm canals in Utah and, in laboratory tanks, this has been reduced to 13 weeks [19]. The possibility of rearing lotic species has been trialed, with some success, using a low-cost, ‘reversed-funnel’ method to provide water circulation [20].
- Odonata—There are almost 6000 species of odonate, distributed from the tropics, where the greatest numbers and diversity occur, to the tree-line in polar regions [13]. Nymphs of six to seven species are eaten in China, with the most common being Crocothemis servilia, Gomphus cuneatus, and Lestes praemorsa ([24]; see also the Appendix). In Thailand, Hanboonsong [25] recorded species from four genera as being commonly eaten (Aeshna, Ceriagrion, Epophtalmia, and Rhyothemis). In total, some 26 species of odonate are known to be eaten in the Oriental Region (see the Appendix). Of note is the preference for species of Libellulidae (17) in the Orient but for species of Aeschnidae (7) in the Neotropics (see Appendix). The crude protein content of dried dragonfly nymphs has been measured at between 40 and 65% [7].
- Hemiptera—Globally, there are around 3800 known species of aquatic and semiaquatic Hemiptera (grouped by some into the suborder Heteroptera) and, of these, a number are eaten. For example, there is a long history of consuming corixids in Mexico where their eggs are harvested as ‘Ahuautle’ and command high prices. Some six species (collectively known as ‘Axayacatl’) are eaten although these are now under threat due to habitat destruction [17]. The relative proportions of terrestrial and aquatic species eaten in this region are 79% and 21%, respectively.
- Coleoptera—Of the close to 400,000 species of beetle, roughly 5000 (1.3%) are considered to be aquatic. The latter live in a very wide spectrum of habitats (coldwater springs to salt-marshes) but, while they may be important to these ecosystems, they do not reach the levels of density or biomass seen in other orders, such as the Trichoptera and Diptera [35]. Lotic species are unlikely candidates for mass harvesting due to typically small, dispersed natural populations (although riffle beetles, Elmidae, may be an exception; [36]). However, some lentic species can occur in quite high densities (e.g., gyrinids and dytiscids), although their abundance is often seasonal, due to life cycle characteristics and habitat availability (e.g., temporary ponds and puddles; [37]). Globally, upwards of 78 species, in 22 genera, have been recorded as being edible. Mexico leads with 36 species eaten, followed by China (26) and Japan (15) [36]. Certain genera are consumed more than others, with 22 species within the dytiscid genus Cybister confirmed as eaten, worldwide, and also 12 species of the hydrophilid genus Hydrophilus [9]. In Thailand, Hanboonsong [25] reports three species of Hydrophilidae and eight species of Dytiscidae as commonly eaten. In China, they are consumed more for their anti-diuretic effect, although Cybister tripunctatus has a high fat content (21.6%), which can contribute significantly as a source of oil in the diet, and also strong antioxidant properties [32]. However, this species, like a number of other exploited aquatic insects, is on the decline, and is on the Red Data List in Japan [38]. Ramos-Elorduy [17] has reported that, in Mexico, 14 insect species are considered to be threatened. In large part, this decline is due to over-harvesting of wild populations. Clearly, culturing techniques need to be developed to compensate. Such an approach has multiple benefits for local populations, not only directly through food but also for local economies, as excess beetle biomass can be sold to ready national and international markets. As a comparison, as early as 1994 in South Africa, Van der Waal showed the sale of grasshoppers to be a business worth over 1 million dollars, annually [39]. More recently in Uganda, Agea et al. [40] reported on a thriving trade based on the sale of the wild-caught grasshopper, Ruspolia nitidula, with the average retail price per kilogram being $2.80 U.S., comparable to the price of goat meat ($2.13). Such data are not readily available for aquatic insects. However, in Guangdong, China, water beetles sold in local markets are now being hatched in special nurseries [30,41]. Two problems hinder the mass rearing of water beetles: provision of an ample and continuous supply of live food; and surface-rippling resulting from tank aeration requirements that interfere with the respiration of small beetle larvae. Using Dytiscus sharpi as a model species, Inoda and Kamimura [42] have designed a new open-aquarium system that largely addresses the second issue. Adequate supply of live food can be achieved through parallel mass rearing of mosquito larvae (see next section).
- Diptera—There are around 120,000 known species of true (two-winged) fly, with many more thought yet to be described. Within the order are several large aquatic families that are important to natural and human-centric ecosystems: Tipulidae (craneflies); Culicidae (mosquitoes); Chironomidae (non-biting midges); and Simuliidae (blackflies). In the life cycle, the adults are typically terrestrial, with the larvae and pupae living in water [13]. In some species and habitats, population sizes can be vast and affect humans in diverse ways—for example, negatively, as vectors of disease, and, positively, as a food source.
- Trichoptera—Of the approximately 7000 known species of caddisfly, all but a few live in freshwater lotic or lentic habitats. Within these, they have become adapted to a wide range of conditions and, where favorable, their larvae can occur at high densities. Consequently, the adults often emerge synchronously and in large numbers, and are strongly attracted to lights. These mass emergences can be a nuisance around urban rivers and lakes (e.g., the ‘shad-fly’ emergences that occur annually, in May and June, from the St. Lawrence, Winnipeg, and Niagara rivers in Canada [59]. However, such events provide opportunities for harvesting—although, again, there are few records of this happening. A particular habitat that promotes very high larval densities of net-spinning families (e.g., the Hydropsychidae) is the fast water flowing over flat concrete surfaces at dam outflows or around hydroelectric generation stations [60]. In a manner similar to that described, above, for blackflies, concrete slabs or slates inserted into suitable rivers can replicate such habitats from which late-instar larvae and pupae can be gathered. An alternative artificial substrate is sheets of artificial turf (‘astro-turf’) to which net-spinning larvae readily attach. There is also the potential for larger, lentic species to be raised in tanks or small artificial ponds—especially those that emulate vernal woodland pools.
4. Aquatic Insects: Taste Versus Nutrition
5. Aquatic Insects and Animal Feed
6. Harvesting Versus Culturing
7. Dangers in Eating Aquatic Insects
8. Environmental Change
9. Conclusions
Author Contributions
Conflicts of Interest
Appendix
| Order | Family | Genus | Species | Region |
|---|---|---|---|---|
| Coleoptera | Dytiscidae | Cybister | distinctus | Trop. Africa |
| Cybister | hova | Trop. Africa | ||
| Cybister | owas | Trop. Africa | ||
| Eretes | sticticus | Trop. Africa | ||
| Rhantus | latus | Trop. Africa | ||
| Hydrophilidae | Hydrophilus | senegalensis | Trop. Africa | |
| Diptera | Chaoboridae | Chaoborus | edulis | Trop. Africa |
| Chaoborus | pallidipes | Trop. Africa | ||
| Chironomidae | unident. | Trop. Africa | ||
| Culicidae | unident. | Trop. Africa | ||
| Ephemeroptera | Caenidae | Caenis | kungu | Trop. Africa |
| Polymitarcidae | Povilla | adusta | Trop. Africa | |
| Hemiptera | Belostomatidae | Belostoma | unident. | Trop. Africa |
| Limnogeton | fieberi | Trop. Africa | ||
| Nepidae | Nepa | unident. | Trop. Africa | |
| unident. | Trop. Africa | |||
| Odonata | Libellulidae | Trithemis | arteriosa | Trop. Africa |
| unident. | Trop. Africa | |||
| Coleoptera | Gyrinidae | Aulonogyrus | strigosus | Australian |
| Ephemeroptera | Palingeniidae | Plethogenesia | Australian | |
| unident. | Australian | |||
| Odonata | Libellulidae | unident. | Australian | |
| (Zygoptera) | unident. | Australian | ||
| Coleoptera | Dytiscidae | Cybister | ellipticus | Nearctic |
| Cybister | explanatus | Nearctic | ||
| Diptera | Ephydridae | Ephydra | cinerea | Nearctic |
| Ephydra | hians | Nearctic | ||
| Ephydra | macellaria | Nearctic | ||
| Rhagionidae | Atherix | unident. | Nearctic | |
| Tipulidae | Holorusia | hespera | Nearctic | |
| Tipula | derbyi | Nearctic | ||
| Tipula | quaylii | Nearctic | ||
| Tipula | simplex | Nearctic | ||
| Hemiptera | Belostomatidae | Lethocerus | americanus | Nearctic |
| Odonata | Aeschnidae | Rhionaeschna | multicolor | Nearctic |
| Plecoptera | Perlodidae | Isoperla | unident. | Nearctic |
| Coleoptera | Dytiscidae | Cybister | explanatus | Neotropical |
| Cybister | fimbriolatus | Neotropical | ||
| Cybister | flavocinctus | Neotropical | ||
| Cybister | occidentalis | Neotropical | ||
| Dytiscus | habilis | Neotropical | ||
| Dytiscus | marginicollis | Neotropical | ||
| Laccophilus | apicalis | Neotropical | ||
| Laccophilus | fasciatus | Neotropical | ||
| Megadytes | giganteus | Neotropical | ||
| Rhantus | atricolor | Neotropical | ||
| Rhantus | consimilis | Neotropical | ||
| Thermonectus | basilaris | Neotropical | ||
| Thermonectus | marmoratus | Neotropical | ||
| Elmidae | Austrelmis | chilensis | Neotropical | |
| Austrelmis | codimentarius | Neotropical | ||
| Gyrinidae | Gyrinus | parcus | Neotropical | |
| Gyrinus | plicatus | Neotropical | ||
| Haliplidae | Haliplus | punctatus | Neotropical | |
| Peltodytes | mexicanus | Neotropical | ||
| Peltodytes | ovalis | Neotropical | ||
| Histeridae | Hololepta | guidonis | Neotropical | |
| Hydrophilidae | Berosus | uniden. | Neotropical | |
| Hydrophilus | uniden. | Neotropical | ||
| Tropisternus | mexicanus | Neotropical | ||
| Tropisternus | sublaevis | Neotropical | ||
| Tropisternus | tinctus | Neotropical | ||
| Diptera | Chironomidae | unident. | Neotropical | |
| Culicidae | unident. | Neotropical | ||
| Ephydridae | Ephydra | hians | Neotropical | |
| Mosillus | tibialis | Neotropical | ||
| Simuliidae | Simulium | rubithorax | Neotropical | |
| Stratiomyidae | Chrysoclorina | unident. | Neotropical | |
| Syrphidae | Copestylum | anna | Neotropical | |
| Eristalis | unident. | Neotropical | ||
| Ephemeroptera | Baetidae | Baetis | unident. | Neotropical |
| Ephemeridae | Ephemera | unident. | Neotropical | |
| Hemiptera | Belostomatidae | Abedus | dilatutus | Neotropical |
| Abedus | ovatus | Neotropical | ||
| Belostoma | micantulum | Neotropical | ||
| Lethocerus | unident. | Neotropical | ||
| Corixidae | Corisella | edulis | Neotropical | |
| Corisella | mercenaria | Neotropical | ||
| Corisella | texcocana | Neotropical | ||
| Graptocorixa | abdominalis | Neotropical | ||
| Graptocorixa | bimaculata | Neotropical | ||
| Krisousacorixa | Azteca | Neotropical | ||
| Krisousacorixa | femorata | Neotropical | ||
| Naucoridae | Ambrysus | stali | Neotropical | |
| Ambrysus | usingeri | Neotropical | ||
| Limnocorus | ? minutus | Neotropical | ||
| Notonectidae | Notonecta | unifasciata | Neotropical | |
| Megaloptera | Corydalidae | Corydalus | cornutus | Neotropical |
| Corydalus | unident. | Neotropical | ||
| Odonata | Aeschnidae | Aeschna | unident. | Neotropical |
| Anax | unident. | Neotropical | ||
| Coryphaeschna | adnexa | Neotropical | ||
| Rhionaeschna | brevifrons | Neotropical | ||
| Rhionaeschna | marchali | Neotropical | ||
| Rhionaeschna | multicolor | Neotropical | ||
| Rhionaeschna | peralta | Neotropical | ||
| Coenagrionidae | Argia | unident. | Neotropical | |
| Corduliidae | Lauramacromia | dubitalis | Neotropical | |
| Gomphidae | Agriogomphus | unident. | Neotropical | |
| Progomphus | unident. | Neotropical | ||
| Zonophora | unident. | Neotropical | ||
| Libellulidae | Brechmorhoga | unident. | Neotropical | |
| Dasythemis | unident. | Neotropical | ||
| Megapodagrionidae | Oxystigma | unident. | Neotropical | |
| Trichoptera | Calamoceratidae | Phylloicus | unident. | Neotropical |
| Hydropsychidae | Leptonema | unident. | Neotropical | |
| Leptoceridae | Oecetis | disjuncta | Neotropical | |
| Triplectides | unident. | Neotropical | ||
| Odontoceridae | Marilia | unident. | Neotropical | |
| Coleoptera | Dytiscidae | Copelatus | unident. | Oriental |
| Cybister | guerini | Oriental | ||
| Cybister | limbatus | Oriental | ||
| Cybister | rugosus | Oriental | ||
| Cybister | tripunctatus | Oriental | ||
| Dytiscus | unident. | Oriental | ||
| Eretes | sticticus | Oriental | ||
| Hydaticus | rhantoides | Oriental | ||
| Laccophilus | pulicarius | Oriental | ||
| Rhantaticus | congestus | Oriental | ||
| Haliplidae | unident. | Oriental | ||
| Hydrophilidae | Hydrobiomorpha | spinicollis | Oriental | |
| Hydrophilus | acuminatus | Oriental | ||
| Hydrophilus | bilineatus | Oriental | ||
| Hydrophilus | cavisternum | Oriental | ||
| Hydrophilus | hastatus | Oriental | ||
| Hydrophilus | olivaceus | Oriental | ||
| Hydrophilus | picicornis | Oriental | ||
| Hydrophilus | pallidipalpis | Oriental | ||
| Ephemeroptera | Baetidae | Cloeon | kimminsi | Oriental |
| Ephemeridae | Ephemera | unident. | Oriental | |
| Hemiptera | Belostomatidae | Diplonychus | unident. | Oriental |
| Lethocerus | indicus | Oriental | ||
| Sphaerodema | molestum | Oriental | ||
| Sphaerodema | rusticum | Oriental | ||
| Gerridae | Cylindrostethus | scrutator | Oriental | |
| Gerris | spinole | Oriental | ||
| Nepidae | Laccotrephes | griseus | Oriental | |
| Laccotrephes | maculatus | Oriental | ||
| Laccotrephes | robustus | Oriental | ||
| Laccotrephes | ruber | Oriental | ||
| Nepa | unident. | Oriental | ||
| Ranatra | longipes thai | Oriental | ||
| Ranatra | varipes | Oriental | ||
| Notonectidae | Anisops | barbata | Oriental | |
| Anisops | bouvieri | Oriental | ||
| Notonecta | unident. | Oriental | ||
| Odonata | Aeschnidae | Aeschna | unident. | Oriental |
| Anax | guttatus | Oriental | ||
| Coenagrionidae | Ceriagrion | unident. | Oriental | |
| Enallagma | unident. | Oriental | ||
| Corduliidae | Epophthalmia | vittigera | Oriental | |
| Gomphidae | Ictinogomphus | rapax | Oriental | |
| ? Stylurus | unident. | Oriental | ||
| unident. | Oriental | |||
| Libellulidae | Acisoma | panorpoides | Oriental | |
| Brachythemis | contaminate | Oriental | ||
| Cratilla | lineata | Oriental | ||
| Crocothemis | servillia | Oriental | ||
| Diplacodes | trivialis | Oriental | ||
| Libellula | puchella | Oriental | ||
| Neurothemis | ramburii | Oriental | ||
| Orthetrum | glaucum | Oriental | ||
| Orthetrum | sabina | Oriental | ||
| ? Pachydiplax | Oriental | |||
| Pantala | flavicens | Oriental | ||
| Potamarcha | obscura | Oriental | ||
| Rhyothemis | unident. | Oriental | ||
| Sympetrum | unident. | Oriental | ||
| Tramea | transmarina | Oriental | ||
| Trithemis | aurora | Oriental | ||
| ? Urothemis | Oriental | |||
| Macromiidae | Macroma | unident. | Oriental | |
| Plecoptera | Pteronarcyidae | Pteronarcys | dorsata | Oriental |
| Nemouridae | Nemoura | unident. | Oriental | |
| Coleoptera | Dytiscidae | Agabus | fulvipennis | Palaearctic |
| Cybister | bengalensis | Palaearctic | ||
| Cybister | brevis | Palaearctic | ||
| Cybister | guerini | Palaearctic | ||
| Cybister | japonicas | Palaearctic | ||
| Cybister | lewisianus | Palaearctic | ||
| Cybister | limbatus | Palaearctic | ||
| Cybister | sugillatus | Palaearctic | ||
| Cybister | tripunctatus | Palaearctic | ||
| Dytiscus | habilis | Palaearctic | ||
| Dytiscus | marginalis | Palaearctic | ||
| Dytiscus | validus | Palaearctic | ||
| Platambus | guttulus | Palaearctic | ||
| Rhantus | pulverosus | Palaearctic | ||
| Gyrinidae | Gyrinus | curtus | Palaearctic | |
| Gyrinus | japonicas | Palaearctic | ||
| Dineutes | marginatus | Palaearctic | ||
| Hydrophilidae | Hydrophilus | acuminatus | Palaearctic | |
| Hydrophilus | bilineatus | Palaearctic | ||
| Hydrophilus | cavisternum | Palaearctic | ||
| Hydrophilus | hastatus | Palaearctic | ||
| Hydrophilus | pallidipalpes | Palaearctic | ||
| Tropisternus | collaris | Palaearctic | ||
| Diptera | Tipulidae | Tipula | paludosa | Palaearctic |
| Ephemeroptera | Baetidae | Cloeon | dipterum | Palaearctic |
| Ephemerellidae | Ephemerella | jinghongensis | Palaearctic | |
| Hemiptera | Belostomatidae | Lethocerus | deyrollei | Palaearctic |
| Lethocerus | indicus | Palaearctic | ||
| Sphaerodema | rusticum | Palaearctic | ||
| Nepidae | Laccotrephes | japonensis | Palaearctic | |
| Ranatra | chinensis | Palaearctic | ||
| Ranatra | unicolor | Palaearctic | ||
| Megaloptera | Corydalidae | Acanthacorydalis | orientalis | Palaearctic |
| Protohermes | grandis | Palaearctic | ||
| Odonata | Gomphidae | Gomphus | cuneatus | Palaearctic |
| Lestidae | Lestes | praemorsus | Palaearctic | |
| Libellulidae | Crocothemis | servilia | Palaearctic | |
| Sympetrum | darwinianum | Palaearctic | ||
| Sympetrum | eroticum | Palaearctic | ||
| Sympetrum | infuscatum | Palaearctic | ||
| Plecoptera | Perlidae | Kamimuria | tibialis | Palaearctic |
| Paragnetina | tinctpennis | Palaearctic | ||
| Trichoptera | Hydropsychidae | Cheumatopsyche | brevilineata | Palaearctic |
| Stenopsychidae | Parastenopsyche | sauteri | Palaearctic | |
| Stenopsyche | griseipennis | Palaearctic |
References
- Elias, S.A. The use of insect fossils in archeology. Advan. Quatern. Entomol. 2009, 12, 89–112. [Google Scholar]
- Chou, Y. The history of entomology in China. Entomotaxonomia 1980, 3, 50–51. [Google Scholar]
- Holt, V.M. Why Not Eat Insects? E.W. Classey, Ltd.: London, UK, 1885; p. 99. [Google Scholar]
- Bodenheimer, F.S. Insects as Human Food; W. Junk: Hague, The Netherlands, 1951; p. 352. [Google Scholar]
- Mitsuhashi, J. Edible Insects of the World; CRC Press, Taylor and Francis Group: London, UK, 2016; p. 296. [Google Scholar]
- Kohl, A. Business Potential of Insect Food. B.A. Thesis, School of Business, JAMK University of Applied Sciences, Jyväskylä, Finland, 2016; p. 94. [Google Scholar]
- Chen, X.; Feng, Y.; Chen, Z. Common edible insects and their utilization in China. Entomol. Res. 2009, 39, 299–303. [Google Scholar] [CrossRef]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible insects; future prospects for food and feed security. Int. Forestry Rev. 2013. [Google Scholar] [CrossRef]
- Jongema, Y. World List of Edible Insects. 2015. Available online: http://www.wur.nl/en/Expertise-Services/Chair-groups/Plant-Sciences/Laboratory-of-Entomology/Edible-insects/Worldwide-species-list.htm (accessed on 1 April 2017).
- Ramos-Elorduy, J. Creepy Crawly Cuisine: The Gourmet Guide to Edible Insects; Park Street Press: Rochester, VT, USA, 1998; p. 150. [Google Scholar]
- Christiansen, K.A.; Snider, R.J. Aquatic Collembola. In An Introduction to the Aquatic Insects of North America; Meritt, R.W., Cummins, K.W., Berg, M.B., Eds.; Kendall Hunt: Dubuque, IA, USA, 2008; pp. 165–179. [Google Scholar]
- Scudder, G.G.E.; McE Kevan, D.K.; Bousfield, E.L. Higher Classification. In Canada and Its Insect Fauna; Danks, H.V., Ed.; Entomological Society of Canada: Ottawa, Canada, 1979; Volume 108, pp. 235–240. [Google Scholar]
- Williams, D.D.; Feltmate, B.W. Aquatic Insect; CAB International: Wallingford, Oxford, UK, 1994; p. 358. [Google Scholar]
- Mitsuhashi, J. Insects as traditional foods in Japan. Ecol. Food Nutr. 2010, 36, 187–199. [Google Scholar] [CrossRef]
- POGOGI. World’s Sushi Database. 2012. Available online: http://pogogi.com/interesting-japanese-delicacies (accessed on 12 March 2017).
- Feng, Y.; Chen, X.-M.; Ye, S.-D.; Wang, S.-Y.; Chen, Y.; Wang, Z.-L. Note on two species of edible insects in Yunnan and their nutritious analysis. In Research and Development of Resource Insects; Chen, X.-M., Ed.; Yunnan Science and Technology Press: Kunming, China, 1999; pp. 125–127. [Google Scholar]
- Ramos-Elorduy, J. Threatened edible insects in Hildago, Mexico and some measures to preserve them. J. Ethnobiol. Ethnomed. 2006, 2, 51. [Google Scholar] [CrossRef] [PubMed]
- Fremling, C.R. Factors influencing the distribution of mayflies along the Mississippi River. In Proceedings of the 1st International Conference on Ephemeroptera; Peters, W.L., Peters, J.G., Eds.; Brill: Leiden, The Netherlands, 1973; pp. 12–25. [Google Scholar]
- Waltz, R.D.; Burian, S.K. Ephemeroptera. In An Introduction to the Aquatic Insects of North America; Meritt, R.W., Cummins, K.W., Berg, M.B., Eds.; Kendall Hunt: Dubuque, IA, USA, 2008; pp. 181–236. [Google Scholar]
- Vancsa, E.; Csata, Z; Rakosy, L. Adaptation of a simple technique for rearing lotic mayflies (Insecta: Ephemeroptera) nymphs. Entomol. Romanica 2014, 19, 5–12. [Google Scholar]
- Bergeron, D.; Bushway, R.J.; Roberts, F.L.; Kornfield, I.; Okedi, J.; Bushway, A.A. The nutrient composition of an insect flour sample from Lake Victoria, Uganda. J. Food Compos. Anal. 1988, 1, 371–377. [Google Scholar] [CrossRef]
- Gillies, M.T. Mayflies as food: A confused story from South America. Mayfly Newsl. 1996, 6, 1. [Google Scholar]
- Grant, P.M. Mayflies as food. In Trends Research Ephemeroptera Plecoptera; Dominguez, E., Ed.; Kluwer: Dordrecht, The Netherlands, 2001; pp. 107–124. [Google Scholar]
- Feng, Y.; Chen, X.-M.; Wang, S.-Y.; Ye, S.-D.; Chen, Y. Three edible Odonata species and their nutritive value. Forest Res. 2001, 14, 421–424. [Google Scholar]
- Hanboonsong, Y. Edible Insects and Associated Food Habits in Thailand. In Forest Insects as Food: Humans Bite Back; Proceedings of a Workshop on Asia-Pacific Resources and Their Potential for Development; Durst, P.B., Johnson, D.V., Leslie, R.L., Shono, K., Eds.; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2010; pp. 173–182. [Google Scholar]
- Pemberton, R.W. Catching and eating dragonflies in Bali and elsewhere in Asia. Am. Entomol. 1995, 41, 97–99. [Google Scholar] [CrossRef]
- Locklin, J.L.; Huckabee, J.S.; Gering, E.J. A method for rearing large quantities of the damselfly Ischnura ramburii (Odonata: Coenagrionidae), in the laboratory. Fla. Entomol. 2012, 95, 273–277. [Google Scholar] [CrossRef]
- Polhemus, J.T. Aquatic and Semiaquatic Hemiptera. In An Introduction to the Aquatic Insects of North America; Meritt, R.W., Cummins, K.W., Berg, M.B., Eds.; Kendall Hunt: Dubuque, IA, USA, 2008; pp. 385–423. [Google Scholar]
- Johnson, D.V. The contribution of edible forest insects to human nutrition and to forest management. In Forest Insects as Food: Humans Bite Back; Proceedings of a Workshop on Asia-Pacific Resources and Their Potential for Development; Durst, P.B., Johnson, D.V., Leslie, R.L., Shono, K., Eds.; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2010; pp. 5–22. [Google Scholar]
- Yhoung-aree, J. Edible insects in Thailand; nutritional values and health concerns. In Forest Insects as Food: Humans Bite Back; Proceedings of a Workshop on Asia-Pacific Resources and Their Potential for Development; Durst, P.B., Johnson, D.V., Leslie, R.L., Shono, K., Eds.; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2010; pp. 201–216. [Google Scholar]
- Inoda, T. Giant Water Bug. 2017. Available online: http://www5f.biglobe.ne.jp/~Dytiscus/TAGAME/intro.htm (accessed on 15 January 2017).
- Shantibala, T.; Lokeshwari, R.K.; Debara, H. Nutritional and antinutritional composition of the five species of aquatic edible insects consumed in Manipur, India. J. Insect Sci. 2014, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, Y.; Hagizuka, C.; Ohshima, I. The effect of combinations of food insects for continuous rearing of the wing polymorphic water strider Limnogonus fossarum fossarum (Hemiptera: Gerridae). J. Insect Sci. 2016, 16. [Google Scholar] [CrossRef]
- McPherson, J.E. Notes on the laboratory rearing of Notonecta hoffmanni (Hemiptera: Notonectidae). Pan-Pacific Entomol. 1966, 42, 54–56. [Google Scholar]
- White, D.S.; Roughley, R.E. Aquatic Coleoptera. In An Introduction to the Aquatic Insects of North America; Meritt, R.W., Cummins, K.W., Berg, M.B., Eds.; Kendall Hunt: Dubuque, IA, USA, 2008; pp. 385–423. [Google Scholar]
- Ramos-Elorduy, J.; Moreno, J.M.P.; Camacho, V.H.M. Edible aquatic Coleoptera of the world with an emphasis on Mexico. J. Ethnobiol. Ethnomed. 2009, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.D. The Biology of Temporary Waters; Oxford University Press: Oxford, UK, 2006; p. 337. [Google Scholar]
- Japan Environment Agency. Threatened Wildlife of Japan, Red Data Book, 2nd ed.; Environment Agency of Japan: Tokyo, Japan, 2000. [Google Scholar]
- Van der Waal, B.C.W. The importance of grasshoppers (Fam. Acrididae) as traditional food in villages in northern Transvaal, South Africa. In Proceedings of the Fourth International Congress of Ethnobiology Abstracts, Lucknow, India, 17–21 November 1994; p. 140. [Google Scholar]
- Agea, J.G.; Biryomumaisho, D.; Buyinza, M.; Nabanoga, G.N. Commercialization of Ruspolia nitidula (Nsenene grasshoppers) in Central Uganda. Afr. J. Food Agri. Nutr. Dev. 2008, 8, 319–332. [Google Scholar] [CrossRef]
- Jach, M.A. Fried water beetles—Cantonese style. Am. Ethnol. 2003, 49, 34–37. [Google Scholar] [CrossRef]
- Inoda, T.; Kamimura, S. New open aquarium system to breed larvae of water beetles (Coleoptera: Dytiscidae). Coleopterists Bull. 2004, 58, 37–43. [Google Scholar] [CrossRef]
- Byers, G.W.; Gelhaus, J.K. Tipulidae. In An Introduction to the Aquatic Insects of North America; Meritt, R.W., Cummins, K.W., Berg, M.B., Eds.; Kendall Hunt: Dubuque, IA, USA, 2008; pp. 773–800. [Google Scholar]
- Culler, L.E.; Ayres, M.P.; Virginia, R.A. In a warmer Arctic, mosquitoes avoid increased mortality from predators by growing faster. Proc. R. Soc. B. 2015. [Google Scholar] [CrossRef] [PubMed]
- Ayieko, M.; Oriaro, V. Consumption, indigenous knowledge and cultural values of the lake fly species within the Lake Victoria region. Afr. J. Environ. Sci. Tech. 2008, 2, 282–286. [Google Scholar]
- Vladimirova, V.V. Mass Breeding of Aedes Aegypti Mosquitoes. In Technical Translation FSTC-HT-23-603-68; Department of the Army, USSR Medical Parasitology and Parasitic Diseases, 1966; Volume 35, pp. 719–723. [Google Scholar]
- Das, S.; Garver, L.; Dimopoulos, G. Protocol for mosquito rearing (Anopheles gambiae). J. Visual. Exp. 2007. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G.; Mandal, S.K.; Ghosh, A.K. Biocontrol of larval mosquitoes by Acilius sulcatus (Coleoptera: Dytiscidae). BioMed Central Infect. Dis. 2008. [Google Scholar] [CrossRef] [PubMed]
- Ferrington, L.C.; Berg, M.B.; Coffman, W.P. Chironomidae. In An Introduction to the Aquatic Insects of North America; Meritt, R.W., Cummins, K.W., Berg, M.B., Eds.; Kendall Hunt: Dubuque, IA, USA, 2008; pp. 847–989. [Google Scholar]
- Kumar, D.; Ramesh, U. Rearing practices of live feedstuff animal midge fly larvae (Chironomus circumdatus) Kieffer (Diptera: Chironomidae). Int. J. Curr. Sci. 2014, 12, 170–177. [Google Scholar]
- Nandi, S.; Aditya, G.; Chowdhury, I.; Das, A.; Saha, G.K. Chironomid midges as allergens: Evidence from two species from West Bengal, Kolkata, India. Indian J. Med. Res. 2014, 139, 921–926. [Google Scholar]
- Bouguenac, V.; Giani, N. Mise en place d’un elevation de Chironomus riparius Meigen (Diptera, Chironomidae), l’aval d’une station d’epuration par lagunage. Ann. Limnol. 1992, 28, 233–243. [Google Scholar] [CrossRef]
- Shaw, P.C.; Mark, K.K. Chironomid farming—A means of recycling farm manure and potentially reducing water pollution in Hong Kong. Aquaculture 1980, 21, 155–163. [Google Scholar] [CrossRef]
- Gahukar, R. Entomophagy and human food security. Int. J. Trop. Insect Sci. 2011, 31, 129–144. [Google Scholar] [CrossRef]
- Malmqvist, B. Preimaginal blackflies (Diptera: Simuliidae) and their predators in a central Scandinavian lake outlet stream. Ann. Zool. Fennici 1994, 31, 245–255. [Google Scholar]
- Leksawasdi, P. Compendium of research on selected edible insects in northern Thailand. In Forest Insects as Food: Humans Bite Back; Proceedings of a Workshop on Asia-Pacific Resources and Their Potential for Development; Durst, P.B., Johnson, D.V., Leslie, R.L., Shono, K., Eds.; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2010; pp. 183–188. [Google Scholar]
- Raybould, J.N. A method of rearing Simulium damnosum Theobald (Diptera: Simuliidae) under artificial conditions. Bull. W.H.O. 1967, 37, 447–453. [Google Scholar] [PubMed]
- Marr, J.D.M. The use of an artificial breeding-site and cage in the study of Simulium damnosum Theobald. Bull. W.H.O. 1962, 27, 622–629. [Google Scholar] [PubMed]
- Resh, V.H.; Rosenberg, D.M. Economic Aspects of Freshwater Invertebrates. In Thorp and Covich’s Freshwater Invertebrates: Ecology and General Biology; Thorp, J.H., Rogers, D.C., Eds.; Elsevier: San Diego, CA, USA, 2015; pp. 98–109. [Google Scholar]
- Stiege, S. Abundance, Diversity and Seasonality of Adult Trichoptera in and around Hydroelectric Generating Stations along the Winnipeg River. Ph.D. Thesis, Department Entomology, University of Manitoba, Winnipeg, MB, Canada, 2004; p. 138. [Google Scholar]
- Cesard, N.; Komatsu, S.; Iwata, A. Processing insect abundance: Trading and fishing of zazamushi in Central Japan (Nagano Prefecture, Honshu Island). J. Ethnobiol. Ethnomed. 2015, 11, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutsch, J.; Murakhver, N. They Eat That? A Cultural Encyclopedia of Weird and Exotic Food from Around the World; ABC-CLIO: Oxford, UK, 2012; p. 234. [Google Scholar]
- Xiaoming, C.; Ying, F.; Hong, Z. Review of the nutritive value of edible insects. In Forest Insects as Food: Humans Bite Back, Proceedings of a Workshop on Asia-Pacific Resources and Their Potential for Development; Durst, P.B., Johnson, D.V., Leslie, R.L., Shono, K., Eds.; FAO Regional Office for Asia and the Pacific: Bangkok, Thailand, 2010; pp. 85–92. [Google Scholar]
- Okedi, J. Chemical evaluation of the Lake Victoria lakefly as nutrient source in animal feeds. Insect Sci. Appl. 1992, 13, 373–376. [Google Scholar] [CrossRef]
- Kourimska, L.; Adamkova, A. Nutritional and sensory quality of edible insects. Nutr. Food Sci. J. 2016, 4, 22–26. [Google Scholar]
- Ramos-Elorduy, J. Nutritional value of edible insects from the State of Oaxaca, Mexico. J. Food Compos. Anal. 1997, 10, 142–157. [Google Scholar] [CrossRef]
- Hensrud, D.D. The Mayo Clinic Diet, 2nd ed.; Mayo Clinic: Rochester, MN, USA, 2017; p. 334. [Google Scholar]
- NHS (National Health Service). Available online: www.nhs.uk/referenceintakes-dailyamounts (accessed on 16 December 2016).
- Bell, J.G.; Sargent, J.R.; Ghioni, C. Fatty acid compositions of 10 freshwater invertebrates which are natural food organisms of Atlantic salmon parr (Salmo salar): A comparison with commercial diets. Aquaculture 1994, 128, 301–313. [Google Scholar] [CrossRef]
- Fontaneto, D.; Tommaseo-Ponzetta, M.; Galli, C.; Rise, P.; Glew, R.H.; Paoletti, M.G. Differences in fatty acid composition between aquatic and terrestrial insects used as food in human nutrition. Ecol. Food Nutr. 2011. [Google Scholar] [CrossRef] [PubMed]
- Ayieko, M.; Oriaro, V.; Nyambuga, I.A. Processed products of termites and lake flies: improving entomophagy for food security within the Lake Victoria region. Afr. J. Food Agri. Nutr. Dev. 2010, 10. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuze, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Animal Feed Sci. Tech. 2014. [Google Scholar] [CrossRef]
- Schurr, K. Insects as a major protein source in sewage lagoon biomass usable as animal food. Proc. North Central Branch Entomol. Soc. Am. 1972, 27, 135–137. [Google Scholar]
- Ayieko, M.A.; Millicent, F.N. Climate change and the abundance of edible insects in the Lake Victoria Region. J. Cell Anim. Biol. 2010, 4, 112–118. [Google Scholar]
- Shike, D.W. Beef cattle feed efficiency. In Proceedings of the Driftless Region Beef Conference 2013; Available online: http://lib.dr.iastate.edu/cgi/viewcontent.cgi?article=1027&context=driftlessconference (accessed on 31 January 2013).
- Lunn, D. Improving Feed Efficiency in Feedlot Cattle. Nutrifax-Nutrition News and Information Update, 2006. Available online: http://www.nutrecocanada.com/docs/shur-gain---beef/improving-feed-efficiency-in-feedlot-cattle.pdf (accessed on 22 April 2017).
- Associated Press. U.N. Promotes Insects as Low-Fat, High-Protein Snack to Feed the Hungry. New York Daily News. 13 May 2013. Available online: http://www.nydailynews.com/life-style/eats/u-n-eat-bugs-good-good-world-article-1.1342532 (accessed on 20 July 2017).
- Meuwissen, P. Insecten als Nieuwe Eiwitbron: Een Scenarioverkenning van de Marktkansen; ZLTO Projecten: ‘s-Hertogenbosch, The Netherlands, 2011. (In Dutch) [Google Scholar]
- Henry, M.; Gasco, L.; Piccolo, G.; Fountoulaki, E. Review on the use of insects in the diet of farmed fish: Past and future. Animal Feed Sci. Tech. 2015, 203, 1–22. [Google Scholar] [CrossRef]
- Van Itterbeeck, J.; Van Huis, A. Environmental manipulation for edible insect procurement: A historical perspective. J. Ethnobiol. Ethnomed. 2012, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.R. The pastoral niche in Pre-Hispanic Mesoamerica. In Pre-Columbian Foodways: Interdisciplinary Approaches to Food, Culture, and Markets in Ancient Mesoamerica; Staller, J.E., Carrasco, M.D., Eds.; Springer: New York, NY, USA, 2010; pp. 109–136. [Google Scholar]
- KNA. ‘University Embarks on Edible Insects’ Project to Address Malnutrition in Africa’. Available online: http://kenyanewsagency.go.ke/en/university-embarks-on-edible-insects-project-to-address-malnutrition-in-africa/ (accessed on 22 April 2017).
- Dossey, A.T.; Tatum, J.T.; McGill, W.I. Current status, insect processing technology, and recommendations moving forward. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J.A., Guadalupe, M., Eds.; Elsevier: New York, NY, USA, 2016; pp. 113–152. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, A.C.; Penaflor, M.; Nardi, C.; Bezner, W.; McNeil, J.N. Weather forcasting by insects: Modified sexual behavior in response to atmospheric pressure changes. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
| Class Collembola [springtails] |
| Class Insecta |
| Subclass Ptilota |
| Infraclass Palaeopterygota |
| Order Ephemeroptera [mayflies] |
| Order Odonata [dragonflies/damselflies] |
| Infraclass Neopterygota |
| Order Plecoptera [stoneflies] |
| Order Orthoptera [grasshoppers/crickets] |
| Order Hemiptera [true bugs] |
| Order Megaloptera [Dobsonflies] |
| Order Neuroptera [lacewings] |
| Order Coleoptera [beetles] |
| Order Diptera [true flies] |
| Order Lepidoptera [butterflies/moths] |
| Order Trichoptera [caddisflies] |
| Order Hymenoptera [bees/wasps/ants] |
|
| Dietary Component Intake (g/day) | Recommended Adult Reference | Yield Potential from Dried Insect Bodies (Range) [100 g is Roughly ½ a Cup] |
|---|---|---|
| Protein | 50 g | 20–76 g/100 g |
| Carbohydrate | 260 g | 1–5 g/100 g |
| Total fat | 70 g | 10–60 g/100 g |
| Fiber | 30 g | 12–137 mg/kg |
| Energy | 2000 kcal/day | 293–762 kcal/100 g |
| Order/Family | Existing | Potential | Harvesting Protocol |
|---|---|---|---|
| Ephemeroptera | low | could be higher |
|
| Odonata | medium | could be higher |
|
| Hemiptera | med/high | could be higher |
|
| Coleoptera | med/high | could be higher |
|
| Diptera | |||
| Tipulidae | none | could be viable |
|
| Culicidae/ | none | could be viable |
|
| Chaoboridae | medium | viable |
|
| Chironomidae | low | very high |
|
| Simuliidae | very low | could be viable |
|
| Trichoptera | low | could be higher |
|
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, D.D.; Williams, S.S. Aquatic Insects and their Potential to Contribute to the Diet of the Globally Expanding Human Population. Insects 2017, 8, 72. https://doi.org/10.3390/insects8030072
Williams DD, Williams SS. Aquatic Insects and their Potential to Contribute to the Diet of the Globally Expanding Human Population. Insects. 2017; 8(3):72. https://doi.org/10.3390/insects8030072
Chicago/Turabian StyleWilliams, D. Dudley, and Siân S. Williams. 2017. "Aquatic Insects and their Potential to Contribute to the Diet of the Globally Expanding Human Population" Insects 8, no. 3: 72. https://doi.org/10.3390/insects8030072





