Minimal Pruning and Reduced Plant Protection Promote Predatory Mites in Grapevine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grapevine Leaf Fauna
2.2. Fungal Disease
3. Results
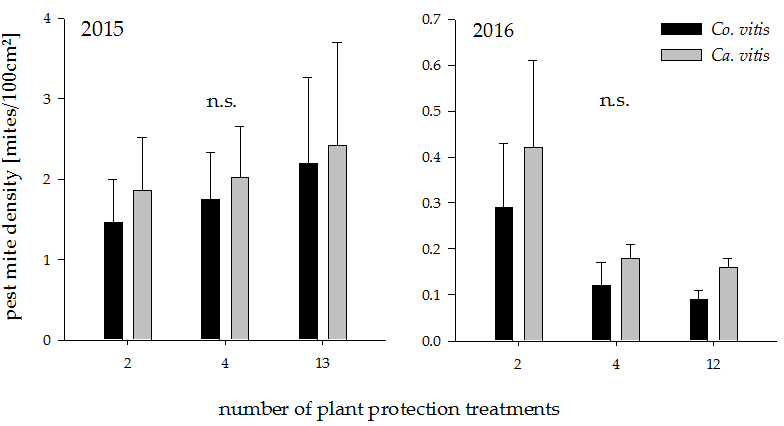
3.1. Effects of Reduced Plant Protection
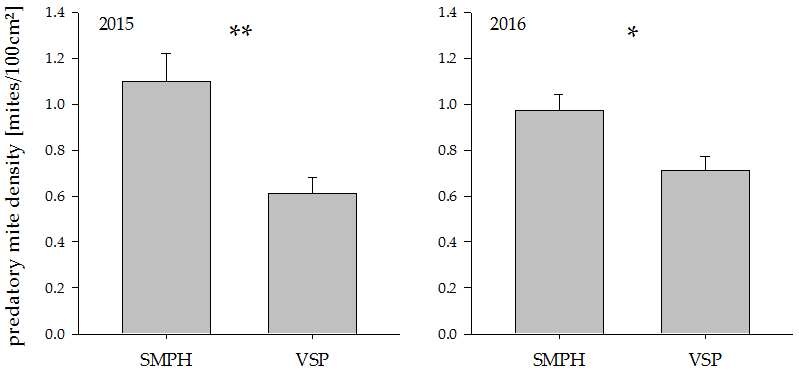
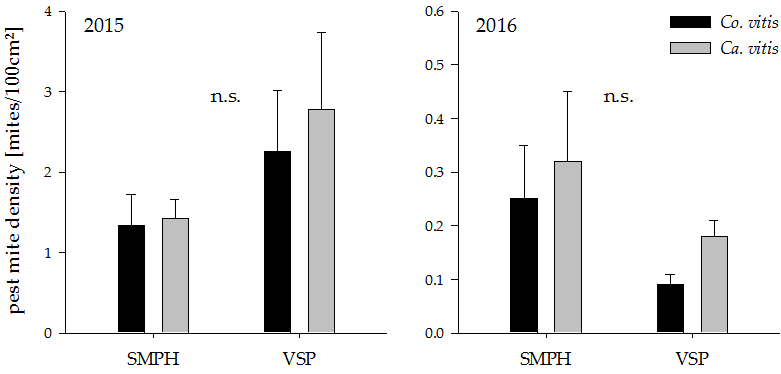
3.2. Effects of Minimal Pruning
3.3. Fungal Disease
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nidumolu, R.; Prahalad, C.; Rangaswami, M. Why sustainability is now the key driver of innovation. IEEE Eng. Manag. Rev. 2015, 43, 85–91. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, R.; Hausmann, L.; Harst, M.; Maul, E.; Zyprian, E.; Eibach, R. New horizons for grapevine breeding. Methods Temp. Fruit Breed. 2011, 5, 79–100. [Google Scholar]
- Howell, G.S. Sustainable grape productivity and the growth-yield relationship: A review. Am. J. Enol. Vitic. 2001, 52, 165–174. [Google Scholar]
- James, D.; Coyle, J. Which pesticides are safe to beneficial insects and mites. Agric. Environ. News 2001, 178, 12–14. [Google Scholar]
- Duso, C.; Pozzebon, A.; Kreiter, S.; Tixier, M.-S.; Candolfi, M. Management of phytophagous mites in european vineyards. In Arthropod Management in Vineyards; Springer: Houten, The Netherlands, 2012; pp. 191–217. [Google Scholar]
- Gerson, U.; Smiley, R.L.; Ochoa, R. Mites (Acari) for Pest Control; Wiley-Blackwell: Oxford, UK, 2003. [Google Scholar]
- Flechtmann, C.H.; McMurtry, J.A. Studies on how phytoseiid mites feed on spider mites and pollen. Int. J. Acarol. 1992, 18, 157–162. [Google Scholar] [CrossRef]
- Pozzebon, A.; Duso, C. Grape downy mildew plasmopara viticola, an alternative food for generalist predatory mites occurring in vineyards. Biol. Control 2008, 45, 441–449. [Google Scholar] [CrossRef]
- McMurtry, J.A.; De Moraes, G.J.; Sourassou, N.F. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 2013, 18, 297–320. [Google Scholar] [CrossRef]
- Symondson, W.; Sunderland, K.; Greenstone, M. Can generalist predators be effective biocontrol agents? Ann. Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [PubMed]
- Duso, C.; Camporese, P.; Van der Geest, L. Toxicity of a number of pesticides to strains of Typhlodromus pyri and Amblyseius andersoni (Acari: Phytoseiidae). Entomophaga 1992, 37, 363–372. [Google Scholar] [CrossRef]
- Gadino, A.N.; Walton, V.M.; Dreves, A.J. Impact of vineyard pesticides on a beneficial arthropod, Typhlodromus pyri (Acari: Phytoseiidae), in laboratory bioassays. J. Econ. Entomol. 2011, 104, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Schruft, G.; Wohlfarth, P.; Wegner, G. Studies on the side-effect of fungicides to the predacious mite Typhlodromus pyri in viticulture. Z. Pflanzenkr. Pflanzenschutz 1992, 99, 101–108. [Google Scholar]
- Pozzebon, A.; Tirello, P.; Moret, R.; Pederiva, M.; Duso, C. A fundamental step in IPM on grapevine: Evaluating the side effects of pesticides on predatory mites. Insects 2015, 6, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C. How organic grapewine protection affects field populations of the predatory mite Typhlodromus pyri. Julius Kühn Archiv 2010, 428, 377–378. [Google Scholar]
- Pozzebon, A.; Borgo, M.; Duso, C. The effects of fungicides on non-target mites can be mediated by plant pathogens. Chemosphere 2010, 79, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kraus, C.; Pennington, T.; Hecht, A.; Fischer, M.; Voegele, R.T.; Hoffmann, C.; Töpfer, R.; Kicherer, A. Effects of canopy architecture and microclimate on grapevine health in two training systems. 2017; in press. [Google Scholar]
- Langellotto, G.A.; Denno, R.F. Responses of invertebrate natural enemies to complex-structured habitats: A meta-analytical synthesis. Oecologia 2004, 139, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Obermaier, E.; Heisswolf, A.; Poethke, H.J.; Randlkofer, B.; Meiners, T. Plant architecture and vegetation structure: Two ways for insect herbivores to escape parasitism. Eur. J. Entomol. 2008, 105, 233–240. [Google Scholar] [CrossRef]
- Andow, D.; Prokrym, D. Plant structural complexity and host-finding by a parasitoid. Oecologia 1990, 82, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.; Nickel, H.; Grützner, T.; Platner, C. Habitat structure mediates top–down effects of spiders and ants on herbivores. Basic Appl. Ecol. 2008, 9, 152–160. [Google Scholar] [CrossRef]
- Hill, K.; Schlamp, H. Einsatz der waschmethode zur ermittlung des raubmilbenbesatzes auf rebblattern. Weinwissenschaft 1984, 4, 255–262. [Google Scholar]
- Krantz, G.W. A Manual of Acarology; Oregon State University Book Stores Inc.: Corvallis, OR, USA, 1978. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014; Volume 2014, pp. 3–36. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. R Core Team (2014) Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-117. Available online: http://CRAN.R-project.org/package=nlme (accessed on 17 August 2017).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.; Zalom, F.; Wilson, L.; Leavitt, G. Sulfur can suppress mite predators in vineyards. Calif. Agric. 1997, 51, 19–21. [Google Scholar] [CrossRef]
- Goodwin, W.; Martin, H. The action of sulphur as a fungicide and as an acaricide. Ann. Appl. Biol. 1929, 16, 93–103. [Google Scholar] [CrossRef]
- Hassan, S.; Bigler, F.; Bogenschütz, H.; Boller, E.; Brun, J.; Calis, J.; Coremans-Pelseneer, J.; Duso, C.; Grove, A.; Heimbach, U. Results of the sixth joint pesticide testing programme of the iobc/wprs-working group «pesticides and beneficial organisms». Entomophaga 1994, 39, 107–119. [Google Scholar] [CrossRef]
- Pozzebon, A.; Loeb, G.M.; Duso, C. Role of supplemental foods and habitat structural complexity in persistence and coexistence of generalist predatory mites. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed]

| Year | Measure for Fungal Disease | Number of Plant Protection Treatments | Pruning Method | |||
|---|---|---|---|---|---|---|
| 2 | 4 | 12/13 | VSP | SMPH | ||
| 2015 | infection level ± SE [%] | 0 | 0 | 0 | 0 | 0 |
| incidence rate ± SE [%] | 0 | 0 | 0 | 0 | 0 | |
| 2016 | infection level ± SE [%] | 11.8 ± 2.9 a | 5.0 ± 1.2 b | 2.5 ± 1.4 b | 5.7 ± 1.4 | 7.1 ± 2.4 |
| incidence rate ± SE [%] | 65.0 ± 7.2 a | 49.1 ± 6.6 b | 31.8 ± 7.0 c | 43.4 ± 8.0 a | 53.9 ± 5.1 b | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennington, T.; Kraus, C.; Alakina, E.; Entling, M.H.; Hoffmann, C. Minimal Pruning and Reduced Plant Protection Promote Predatory Mites in Grapevine. Insects 2017, 8, 86. https://doi.org/10.3390/insects8030086
Pennington T, Kraus C, Alakina E, Entling MH, Hoffmann C. Minimal Pruning and Reduced Plant Protection Promote Predatory Mites in Grapevine. Insects. 2017; 8(3):86. https://doi.org/10.3390/insects8030086
Chicago/Turabian StylePennington, Theresa, Christian Kraus, Ekatarina Alakina, Martin H. Entling, and Christoph Hoffmann. 2017. "Minimal Pruning and Reduced Plant Protection Promote Predatory Mites in Grapevine" Insects 8, no. 3: 86. https://doi.org/10.3390/insects8030086






