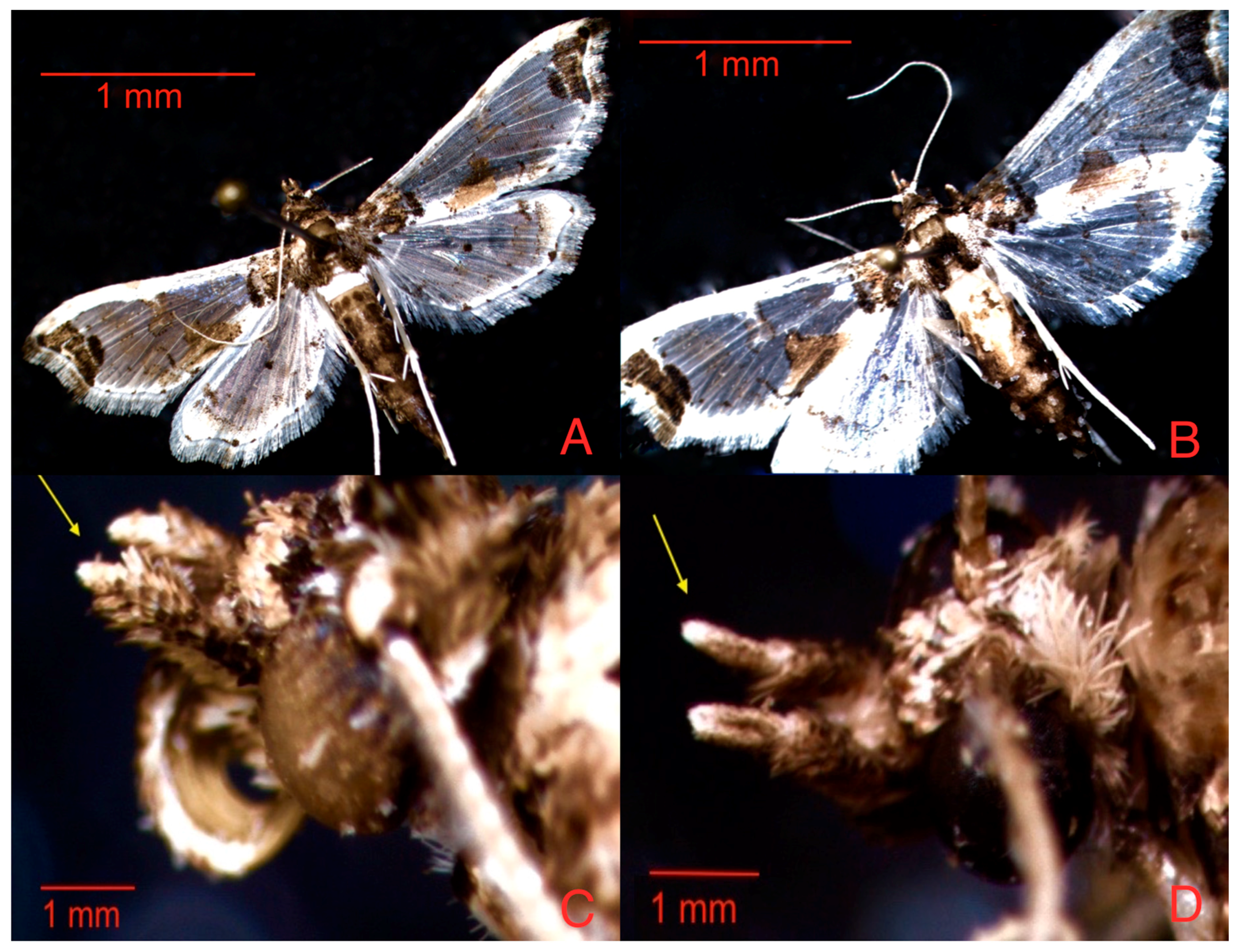
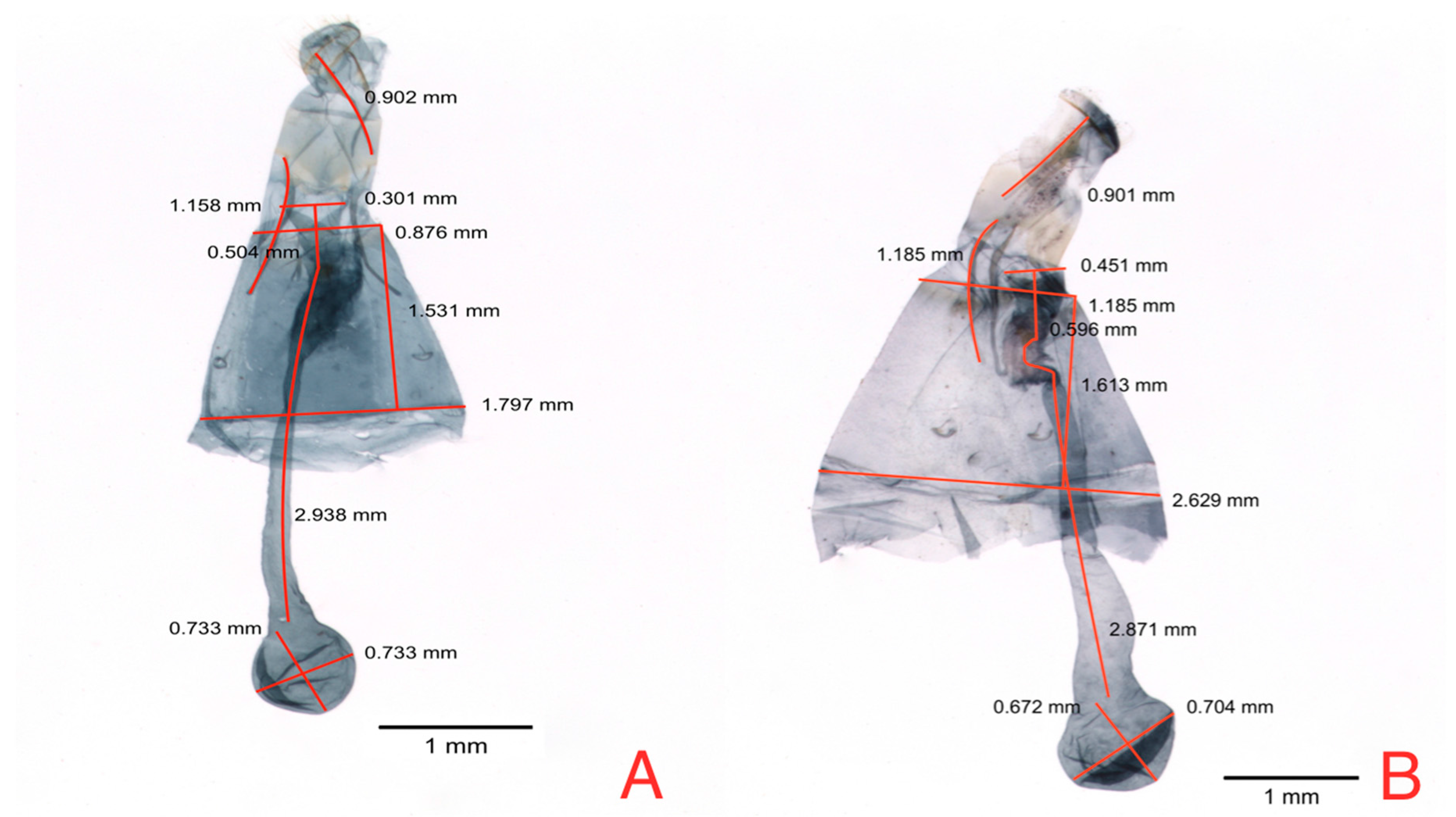
3.1. Analysis of Variance for Solanaceae Plant and Zone
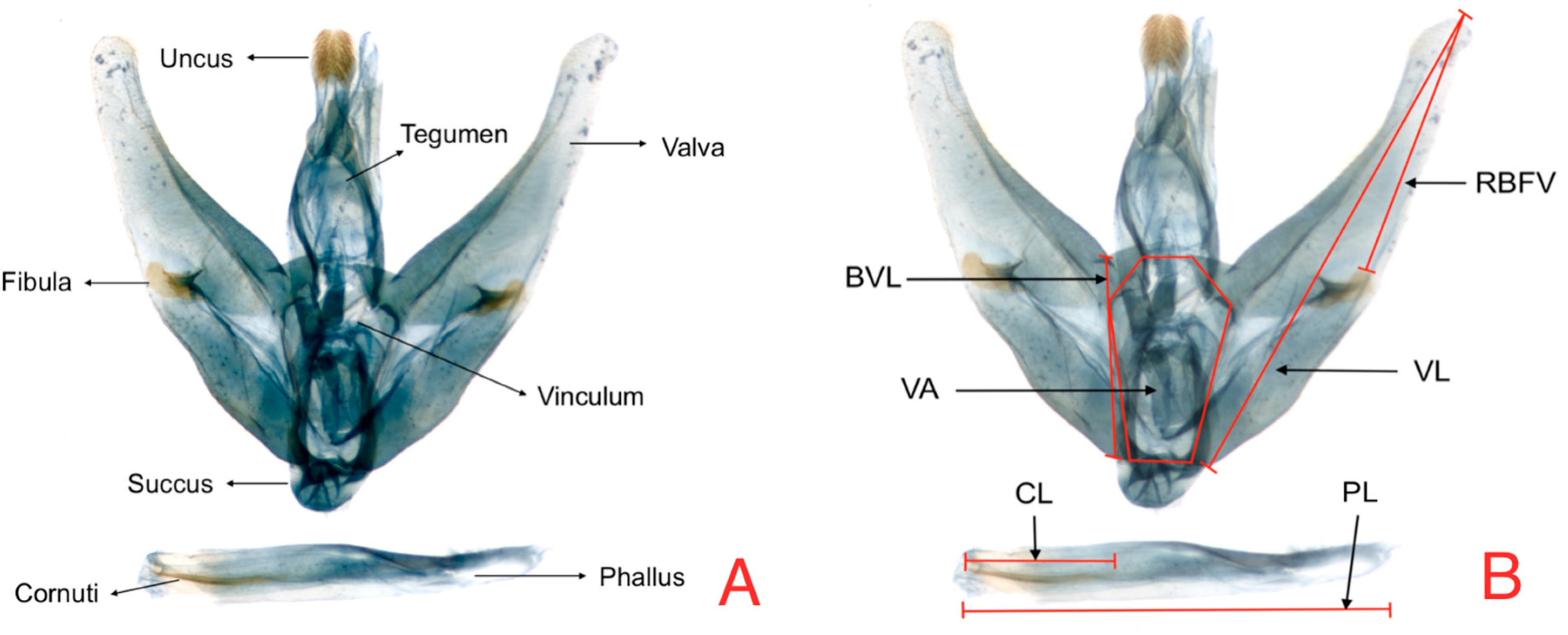
For male genitalia, significant differences were found for host plant factor in the cornuti length (RCL) in the ANOVA analysis (
Table 2). Additionally, there were differences for the zone factor in the variable RCL where specimens collected in Eastern Andes lands presented a more reduced length for this variable (
Figure 4).
MANOVA analysis of males of
N. elegantalis found highly significant differences between host plant and zone variables, (
Table 3). These findings are consistent with the study by Díaz et al. 2015 in Colombia [
6], where the source of food was the main factor that discriminated the morphological differentiation among the female population of
N. elegantalis, giving support to sympatric differentiation. In this study, zone was also found as an important factor for male differentiation. In both cases, sympatric differentiation seems to be the main force in the genetic dynamics of this species, as the source of food seems to determinate the size of the morphological structures. Food source represented the highest source of variation among the populations studied and differentiated four population groups of
N. elegantalis in Ecuador.
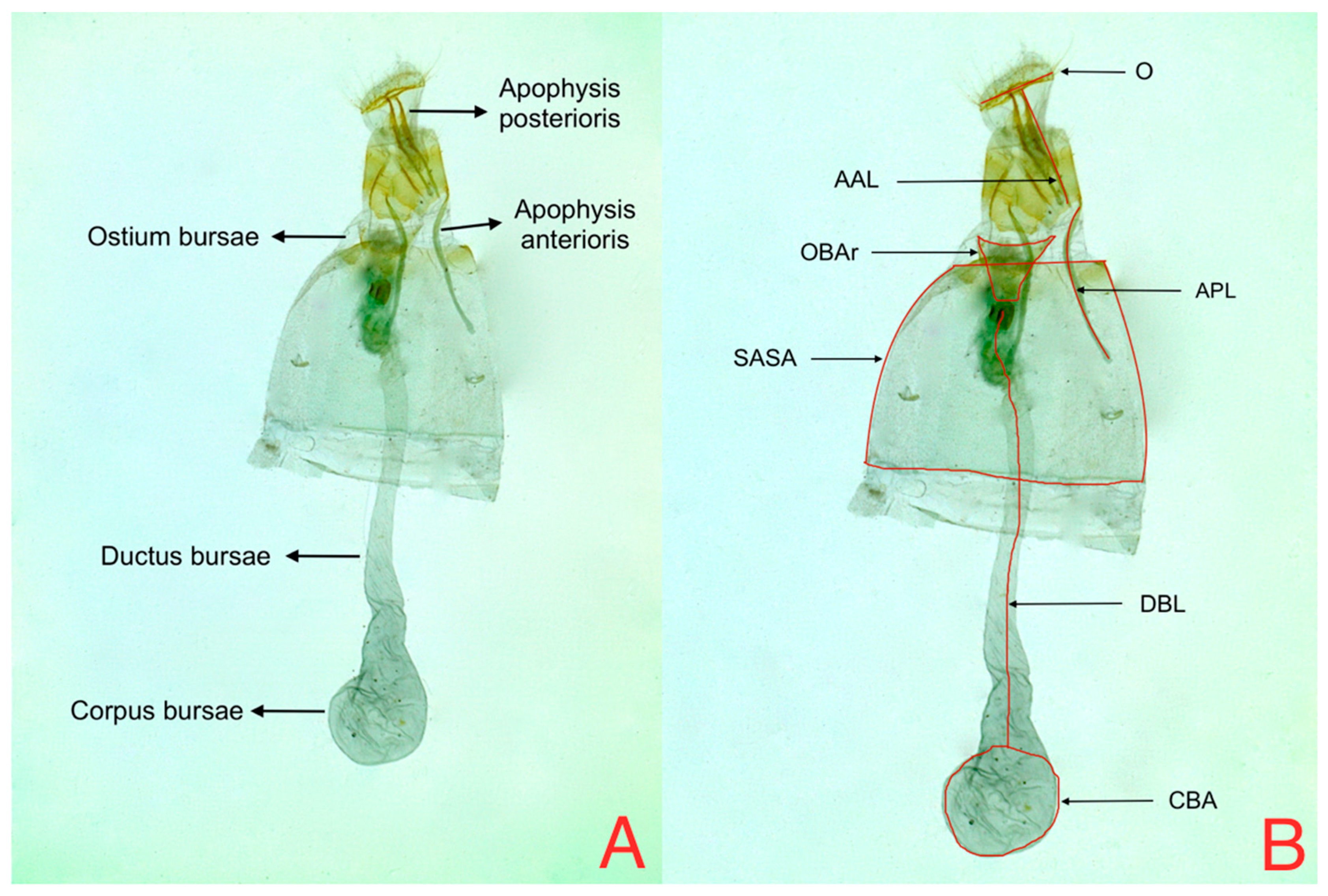
ANOVA analysis of female genitalia (
Table 4) variables found statistical differences (
p < 0.05) for Solanaceous host for two out of six evaluated variables, OBAr and SASA (
Figure 5). These results are consistent with the results obtained by Díaz et al. 2015, which mention that SASA shows the highest variability, suggesting that this variable might be useful as a morphological marker to differentiate from which host the individuals of this species come [
5,
12].
On the other hand, the CBA variable showed statistical differences (p < 0.05) for the interaction between host and zone effect. For the zone, there were no mean differences, which suggests that climatological similarities between zones do not cause differentiation among individuals.
MANOVA analysis found significant differences only for the Solanaceous host between female populations from
S. betaceum and
S. quitoense (
Table 5), suggesting that sympatric differentiation may take place in contrast to allopatric differentiation. A possible explanation for this nonstructural geographical differentiation of
N. elegantalis might be the relative facility and connectivity between Eastern and Western sides of the Andes in Ecuador. According to Díaz et al. 2013 [
1], in Colombia, there were geographic differentiations of
N. elegantalis likely due to deeper isolation of different biotypes in comparison with the mobility among different areas that exist in Ecuador.
3.2. Principal Component Host Classification
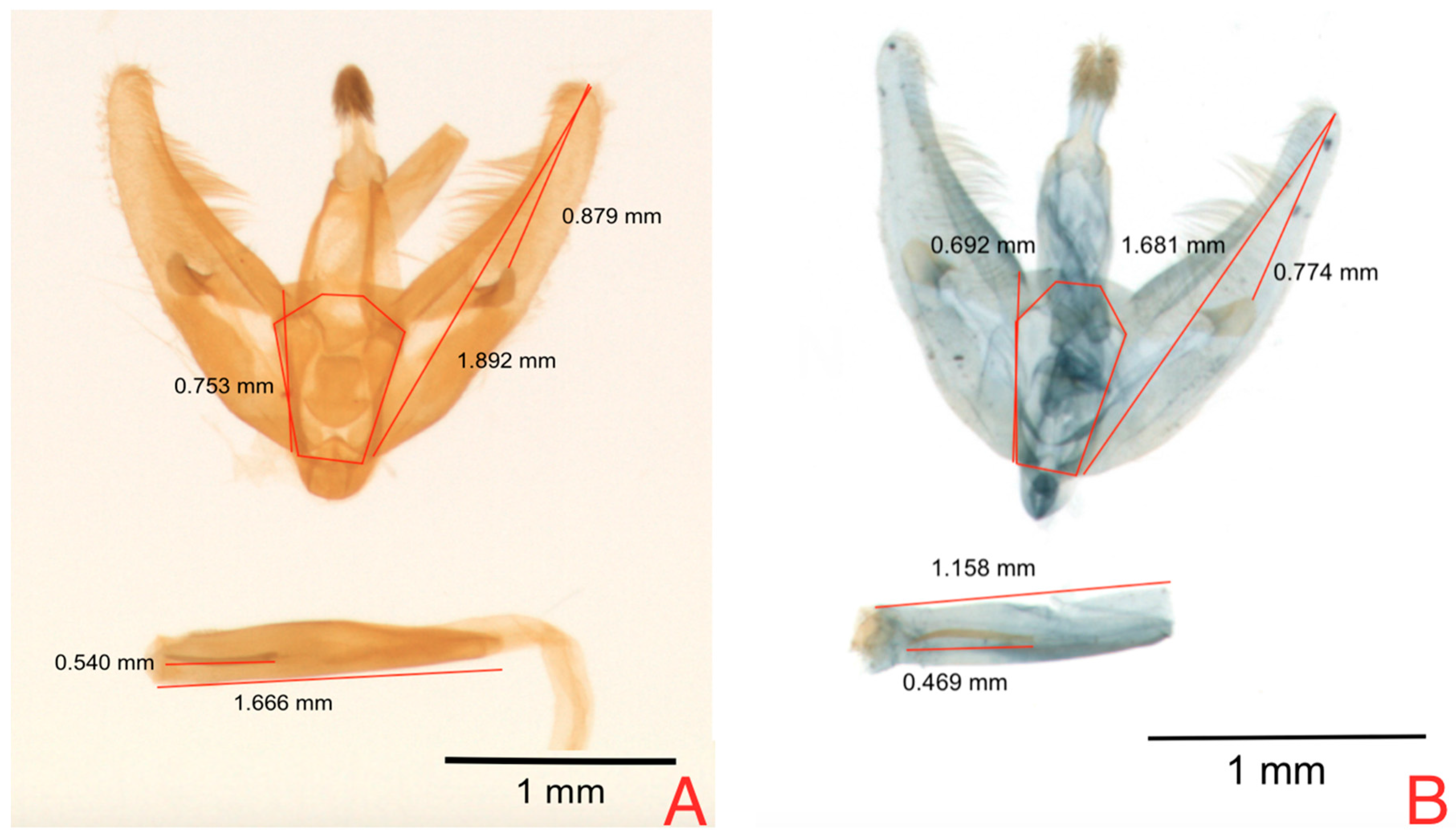
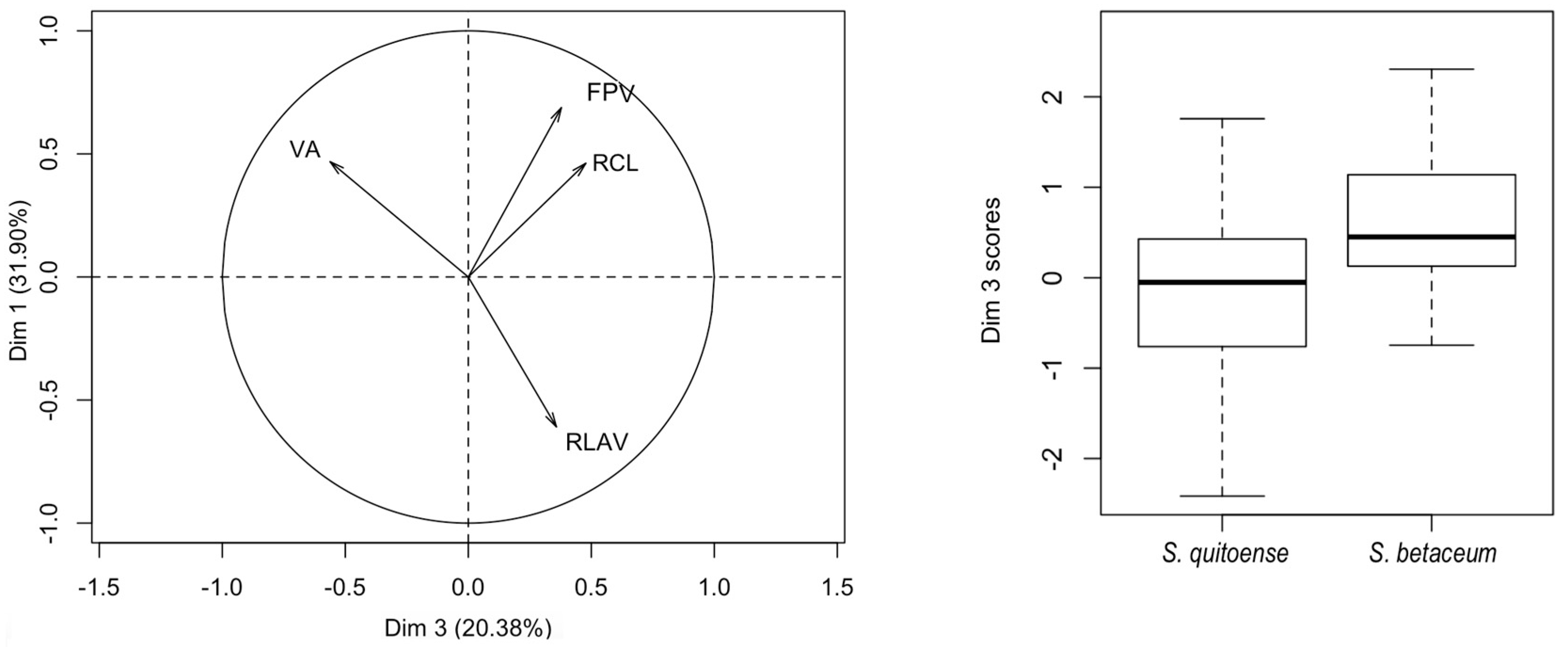
Principal component logistic regression for male genitalia of
N. elegantalis used in the classification of specimens coming from the two Solanaceae hosts (
Table 6), resulted in statistical significance for PC3.
Table 7 presents the loadings of this component, which shows the contrast between VA and RBFV.
Figure 6 represents the dispersion of measures for this component of the individuals in both hosts and clearly shows that individuals from tree tomato have larger size than individuals from naranjilla.
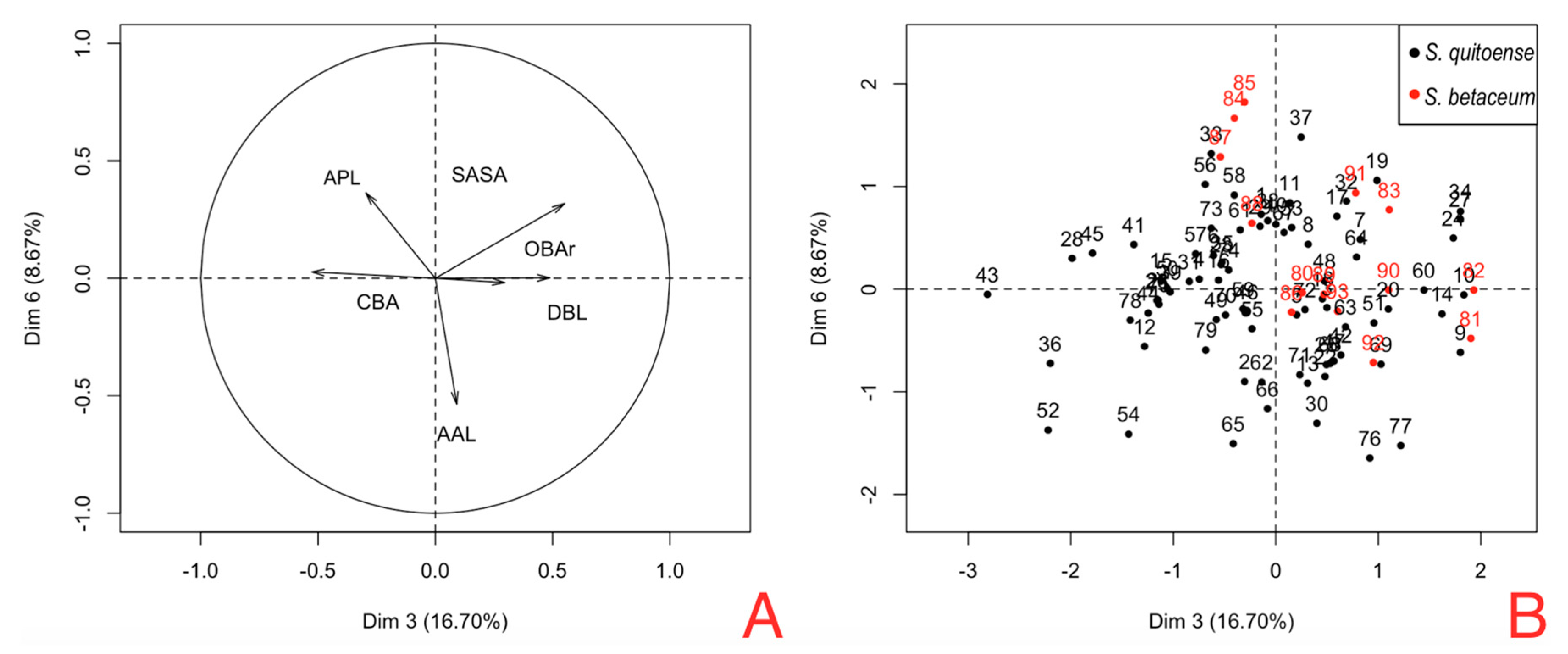
The principal component logistic regression for female genitalia of
N. elegantalis, in the two Solanaceous hosts (
Table 8), identified two significant components, PC 3 and PC 6, with the results of each variable shown in
Figure 7A. PC3 shows the contrast between CBA vs. OBAr, and DBL vs. SASA (
Table 9). PC6 shows the contrast of RAAL between females on naranjilla with bigger RAAL in comparison to females on tree tomato.
Figure 7B shows the position of females hosted in tree tomato (red dots) and females hosted in naranjilla (black dots); these components show that tree tomato females have a bigger SASA and OBAr and smaller CBA and RAPL.
Figure 5 shows that the areas occupied by OBAr and SASA are bigger than other structures, showing that females on tree tomato have a larger capacity in the abdominal region.