Efficacy of Chlorantraniliprole in Controlling Structural Infestations of the Eastern Subterranean Termite in the USA
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. College Station, TX Properties (Monolithic Slab Foundation)
3.2. Raleigh, NC Properties (Crawl Space Foundation)
3.3. Columbus, OH Properties (Basement Foundation)
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nutting, W.L. Insecta: Isoptera. In Soil Biology Guide; Dindal, D.L., Ed.; John Wiley & Sons: New York, NY, USA, 1990; pp. 997–1032. [Google Scholar]
- Austin, J.W.; Szalanski, A.L.; Scheffrahn, R.H.; Messenger, M.T. Genetic variation of Reticulitermes flavipes (Isoptera: Rhinotermitidae) in North America applying the mitochondrial rRNA 16S gene. Ann. Entomol. Soc. Am. 2005, 98, 980–988. [Google Scholar] [CrossRef]
- Scherer, C.W.; Coffelt, M.A.; Saran, R. Changing Face of Urban Pest Management: Importance of Product Innovation. In Proceedings of the 2010 National Conference on Urban Entomology, Portland, OR, USA, 16–19 May 2010. [Google Scholar]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Insecticidal anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S.; et al. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Lahm, G.P.; Stevenson, T.M.; Selby, T.P.; Freudenberger, J.H.; Cordova, D.; Flexner, L.; Bellin, C.A.; Dubas, C.M.; Smith, B.K.; Hughes, K.A.; et al. Rynaxypyr™: A new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorg. Med. Chem. Lett. 2007, 17, 6274–6279. [Google Scholar] [CrossRef] [PubMed]
- Hannig, G.T.; Ziegler, M.; Marҫon, P.G. Feeding cessation effects of chlorantraniliprole, a new anthranilic diamide insecticide, in comparison with several insecticides in distinct chemical classes and mode-of-action groups. Pest Manag. Sci. 2009, 65, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Wang, M.; Xiong, L.; Liu, Z.; Li, Z. Synthesis and insecticidal activities of novel analogues of chlorantraniliprole containing nitro group. Chem. Res. Chin. Univ. 2011, 27, 610–613. [Google Scholar]
- Spomer, N.A.; Kamble, S.T.; Siegfried, B.D. Bioavailability of chlorantraniliprole and indoxacarb to eastern subterranean termites (Isoptera: Rhinotermitidae) in various soils. J. Econ. Entomol. 2009, 102, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Quarcoo, F.Y.; Appel, A.G.; Hu, X.P. Descriptive study of non-repellent insecticide-induced behaviors in Reticulitermes flavipes (Isoptera: Rhinotermitidae). Sociobiology 2010, 55, 217–227. [Google Scholar]
- Mao, L.; Henderson, G.; Scherer, C.W. Toxicity of seven termiticides on the Formosan and eastern subterranean termites. J. Econ. Entomol. 2011, 104, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Spomer, N.A.; Kamble, S.T. Temporal changes in chlorantraniliprole and indoxacarb in four Midwestern soils and bioefficacy against the eastern subterranean termite (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2011, 104, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Buczkowski, G.; Scherer, C.W.; Bennett, G.W. Toxicity and horizontal transfer of chlorantraniliprole in the eastern subterranean termite. J. Econ. Entomol. 2012, 105, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.K.; Ziegler, M.; Kudlie, S.; Harrison, D.; Leva, D.M.; Scherer, C.; Coffelt, M.A. Behavioral effects and tunneling responses of eastern subterranean termites (Isoptera: Rhinotermitidae) exposed to chlorantraniliprole-treated soils. J. Econ. Entomol. 2014, 107, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Thorne, B.L.; Breisch, N.L.; Scherer, C.W. Impacts on Reticulitermes flavipes (infraorder Isoptera: Rhinotermitidae) by chlorantraniliprole applied to soil surrounding established tunnels. J. Econ. Entomol. 2015, 108, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Puckett, R.T.; Keefer, T.C.; Gold, R.E. Performance of Altriset™ (chlorantraniliprole) termiticide against Formosan subterranean termites, Coptotermes formosanus Shiraki, in laboratory feeding cessation and collateral transfer trials, and field applications. Sociobiology 2012, 59, 531–548. [Google Scholar] [CrossRef]
- Yeoh, B.H.; Lee, C.-Y. Tunneling responses of the Asian subterranean termite, Coptotermes gestroi, in termiticide-treated sand (Isoptera: Rhinotermitidae). Sociobiology 2007, 50, 457–468. [Google Scholar]
- Neoh, K.-B.; Hu, J.; Yeoh, B.-H.; Lee, C.-Y. Toxicity and horizontal transfer of chlorantraniliprole against the Asian subterranean termite Coptotermes gestroi (Wasmann): effects of donor:recipient ratio, exposure duration and soil type. Pest Manag. Sci. 2012, 68, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Scheffrahn, R.H.; Scherer, C.W. Efficacy of Altriset® on the tropical arboreal termite, Nasutitermes corniger (Isoptera: Termitidae: Nasutitermitinae). Fla. Entomol. 2013, 96, 249–251. [Google Scholar] [CrossRef]
- Syngenta. Altriset Federal Label EPA Reg. No.100-1503; Altriset Termiticide, Syngenta Crop Protection: Greensboro, NC, USA, 2015; Available online: http://www.syngentapmp.com/current-label/altriset (accessed on 1 August 2017).
- Parman, V.; Vargo, E.L. Population density, species abundance and breeding structure of subterranean termite colonies in and around infested houses in central North Carolina. J. Econ. Entomol. 2008, 101, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Vargo, E.L.; Parman, V. Effect of fipronil on subterranean termite (Isoptera: Rhinotermitidae) colonies in the field. J. Econ. Entomol. 2012, 105, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.; Rousset, F. GENEPOP, version 1.2: Population genetics software for exact tests and ecumenicism. J. Heredity 1995, 86, 248–249. [Google Scholar] [CrossRef]
- Vargo, E.L. Genetic structure of Reticulitermes flavipes and R. virginicus (Isoptera: Rhinotermitidae) colonies in an urban habitat and tracking of colonies following treatment with hexaflumuron bait. Environ. Entomol. 2003, 32, 1271–1282. [Google Scholar] [CrossRef]
- Gallagher, N.T.; Jones, S.C. Moisture augmentation of food items by Reticulitermes flavipes (Isoptera: Rhinotermitidae). Sociobiology 2010, 55, 735–748. [Google Scholar]
- Parman, V.; Vargo, E.L. Colony-level effects of imidacloprid in subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2010, 103, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Bulmer, M.S.; Adams, E.S.; Traniello, J.F.A. Variation in colony structure in the subterranean termite Reticulitermes flavipes. Behav. Ecol. Sociobiol. 2001, 49, 236–243. [Google Scholar] [CrossRef]


| Property {Area of Structure (sq m)}, Study Dates 1 | IGMs 2 | Cumulative Pre-Treatment Termite Activity 3 {Estimated Foraging Area (sq m)} | Treatment Date, Volume (L) | Post-Treatment Termite Activity 4 {Estimated Foraging Area (sq m) at Final Inspection} |
|---|---|---|---|---|
| TX-1 5 {109}, 6-VIII-12 – 7-V-14 | #1–#27 + #28–#40 | Infesting Colony {138} 1 tube on foundation; IGMs #14, #22–#25, #27, #32, #35 Landscape Colonies in IGMs Colony A {13}: #1, #27 | 15-X-12, 307 | Infesting Colony {0} No evidence in structure; IGMs #117MAT, #3217,18MAT, #399MAT Landscape Colonies in IGMs Colony A {2}: #17,8,18,19MAT; Colony B {0}: #317,8MAT; Colony C {0}: #329MAT; Colony D {2}: #2017MAT |
| TX-2 {146}, 6-VIII-12 – 4-XII-14 | #1–#27 + #28–#36 | Infesting Colony {36} 2 tubes on foundation; IGMs #1, #4, #6 | 9-XI-12, 265 6 | Infesting Colony {0} In window 7 @ 5 MAT; None in IGMs |
| TX-3 {278}, 10-VIII-12 – 4-XII-14 | #1–#25 + #26–#44 | Infesting Colony {36} 1 tube on foundation; IGMs #1, #4, #5 Landscape Colonies in IGMs Colony A {22}: #9, #15, #18; Colony B {12}: #17–#19; Colony C {2}: #10 | 21-IX-12, 337 | Infesting Colony {0} No evidence in structure; None in IGMs Landscape Colonies in IGMs {0} None detected |
| TX-4 {278}, 3-XII-12 – 30-VII-15 | #1–#26 + #27–#44 | Infesting Colony {192} 1 tube on foundation; IGMs #1–#4, #14 | 12-XI-13, 326 | Infesting Colony {0} No evidence in structure; None in IGMs |
| Property {Area of Structure (sq m)}, Study Dates 1 | IGMs 2 | Cumulative Pre-Treatment Termite Activity 3 {Estimated Foraging Area (sq m)} | Treatment Date, Volume (L) | Post-Treatment Termite Activity 4 {Estimated Foraging Area (sq m) at Final Inspection} |
|---|---|---|---|---|
| NC-1 {190}, 8-VIII-12 – 17-IV-15 | #1–#21 + #22–#39 | Infesting Colony {44} Shelter tubes in crawl space (1, 2, 3, 4); IGMs #5, #7 Landscape Colonies in IGMs Colony A {2}: #4 | 26-IV-13, 587 5,6 | Infesting Colony {0} Tubes 13MAT, 22MAT, 34,6MAT, 42–4MAT, 53MAT, 63MAT, 73–6MAT, 85MAT; Joists 12MAT, 2–34MAT, 48MAT Landscape Colonies in IGMs {0} None detected |
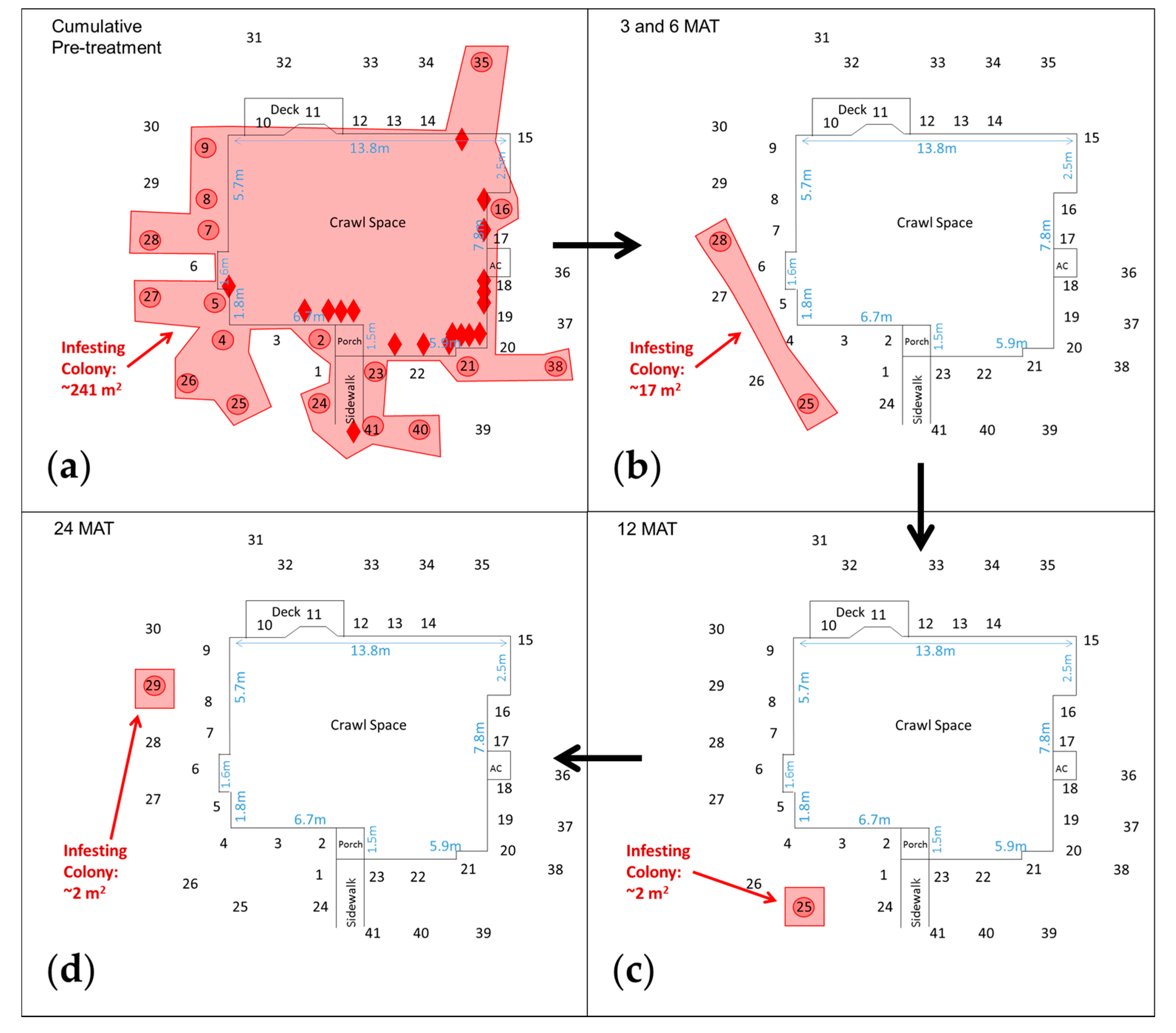
| NC-2 {129}, 7-VIII-12 – 22-IV-15 | #1–#23 + #24–#41 | Infesting Colony {241} 4 auxiliary wooden stakes; 14 tubes on interior and exterior of foundation; IGMs #2, #4, #5, #7–#9, #16, #21, #23–#28, #35, #38, #40, #41 | 18-IV-13, 776 7 | Infesting Colony {2} No evidence in structure; IGMs #251–3,6,9,12MAT, #261MAT, #281–3,6,15,18MAT, #2918,21,24MAT, #351MAT, #401MAT |
| NC-3 8 {136}, 12-VI-12 – 17-X-14 | #1–#16 + #17–#35 | Infesting Colony {40} Tubes in crawl space (2, 3, 4); 2 floor joists in crawl space; IGM #22 Landscape Colonies in IGMS Colony A {4}: #22; tree stump Colony B {2}: #23 | 19-IV-13, 598 5 | Infesting Colony {2} Tube 21MAT; tree stump18MAT Landscape Colonies in IGMs Colony A {0}: #221,2,6,9,12MAT; Colony B {2}: #231-18MAT; Colony C {0}: #201MAT; Colony D {2}: #272,3MAT, #252,3,6,12,15,18MAT, #266,9MAT Colony E {2}: #2818MAT |
| NC-4 {106}, 13-VI-13 – 8-VIII-15 | #1–#14 + #15–#31 | Infesting Colony {74} 3 tubes in crawl space; IGMs #2, #9, #13, #22, #25, #26 Landscape Colonies in IGMs Colony A {2}: #27 | 27-VIII-13, 731 7 | Infesting Colony {0} No evidence in structure; IGMs #251-3MAT, #261–3,6MAT Landscape Colonies in IGMs Colony A {2}: #41MAT, #112,3,MAT, #1212MAT, #271–3,9,12,15,24MAT, #281–3,6,12,21MAT; Colony B {2}: #301–24MAT |
| Property {Area of Structure (sq m)}, Study Dates 1 | IGMs 2 | Cumulative Pre-Treatment Termite Activity 3 {Estimated Foraging Area (sq m)} | Treatment Date, Volume (L) | Post-Treatment Termite Activity 4 {Estimated Foraging Area (sq m) at Final Inspection} |
|---|---|---|---|---|
| OH-1 {185}, 25-VI-12 – 12-XII-14 | #1–#32 + #33–#61 | Infesting Colony {205} Wood debris near house; 2 tubes; 3 auxiliary monitors in basement; IGMs #24‒#29 Landscape Colonies in IGMs Colony A {2}: Near #34 | 20-IX-12, 1079 | Infesting Colony {0} No evidence in structure; IGMs #181MAT, #251MAT, #261,2,7,9,12MAT Landscape Colonies in IGMs Colony A {0}: #17MAT |
| OH-2 {119}, 13-V-13 – 12-VIII-15 | #1–#20 + #21–#40 | Infesting Colony {132} Kitchen wall; 6 tubes on foundation; IGMs #6, #11, #28, #31, #35, #37 | 25-VII-13, 757 | Infesting Colony {31} No evidence in structure; IGMs #72,12MAT, #1325MAT, #16–#1712MAT, #2912MAT, #3025MAT, #3117MAT, #35–362MAT, #3717,21,25MAT |
| OH-3 {136}, 26-VII-13 – 15-IX-15 | #1–#17 + #18–#27 | Infesting Colony {118} Alates in upstairs bathroom; 2 auxiliary monitors in basement; tubes on foundation; tree stump near structure; IGMs #1, #3, #5, #10, #11, #21–#23 | 13-IX-13, 908 | Infesting Colony {32} No evidence in structure; IGMs #31,2,3,8,19,21,24MAT, #48,10,15,21,24MAT, #62,3MAT, #51,2,3,8,10MAT, #710,15,24MAT, #81,2,3,10,19,21MAT, #91,2,21MAT, #108MAT, #111,8,10,15,21,24MAT, #1210MAT, #1821MAT, #211MAT, #2219,21,24MAT, #231MAT |
| OH-4 {91}, 13-VIII-13 – 15-IX-15 | #1–#21 + #22–#36 | Infesting Colony {110} Auxiliary monitors in basement (1, 2, 4, 5); tubes on foundation; IGMs #8, #12, #16–#19, #30, #31, #33 Landscape Colonies in IGMs Colony A {2}: #7 | 13-IX-13, 757 | Infesting Colony {24} Auxiliary monitors 5 4–51MAT; IGMs #121MAT, #1721,24MAT, #301MAT, #3118,21MAT, #3224MAT, #331,15MAT Landscape Colonies in IGMs Colony A {0}: #71MAT |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, S.C.; Vargo, E.L.; Keefer, T.C.; Labadie, P.; Scherer, C.W.; Gallagher, N.T.; Gold, R.E. Efficacy of Chlorantraniliprole in Controlling Structural Infestations of the Eastern Subterranean Termite in the USA. Insects 2017, 8, 92. https://doi.org/10.3390/insects8030092
Jones SC, Vargo EL, Keefer TC, Labadie P, Scherer CW, Gallagher NT, Gold RE. Efficacy of Chlorantraniliprole in Controlling Structural Infestations of the Eastern Subterranean Termite in the USA. Insects. 2017; 8(3):92. https://doi.org/10.3390/insects8030092
Chicago/Turabian StyleJones, Susan C., Edward L. Vargo, T. Chris Keefer, Paul Labadie, Clay W. Scherer, Nicola T. Gallagher, and Roger E. Gold. 2017. "Efficacy of Chlorantraniliprole in Controlling Structural Infestations of the Eastern Subterranean Termite in the USA" Insects 8, no. 3: 92. https://doi.org/10.3390/insects8030092






