Locomotion Inhibition of Cimex lectularius L. Following Topical, Sublethal Dose Application of the Chitin Synthesis Inhibitor Lufenuron
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Insecticide Dilutions
2.3. Insect Growth Regulator Topical Application Bioassay
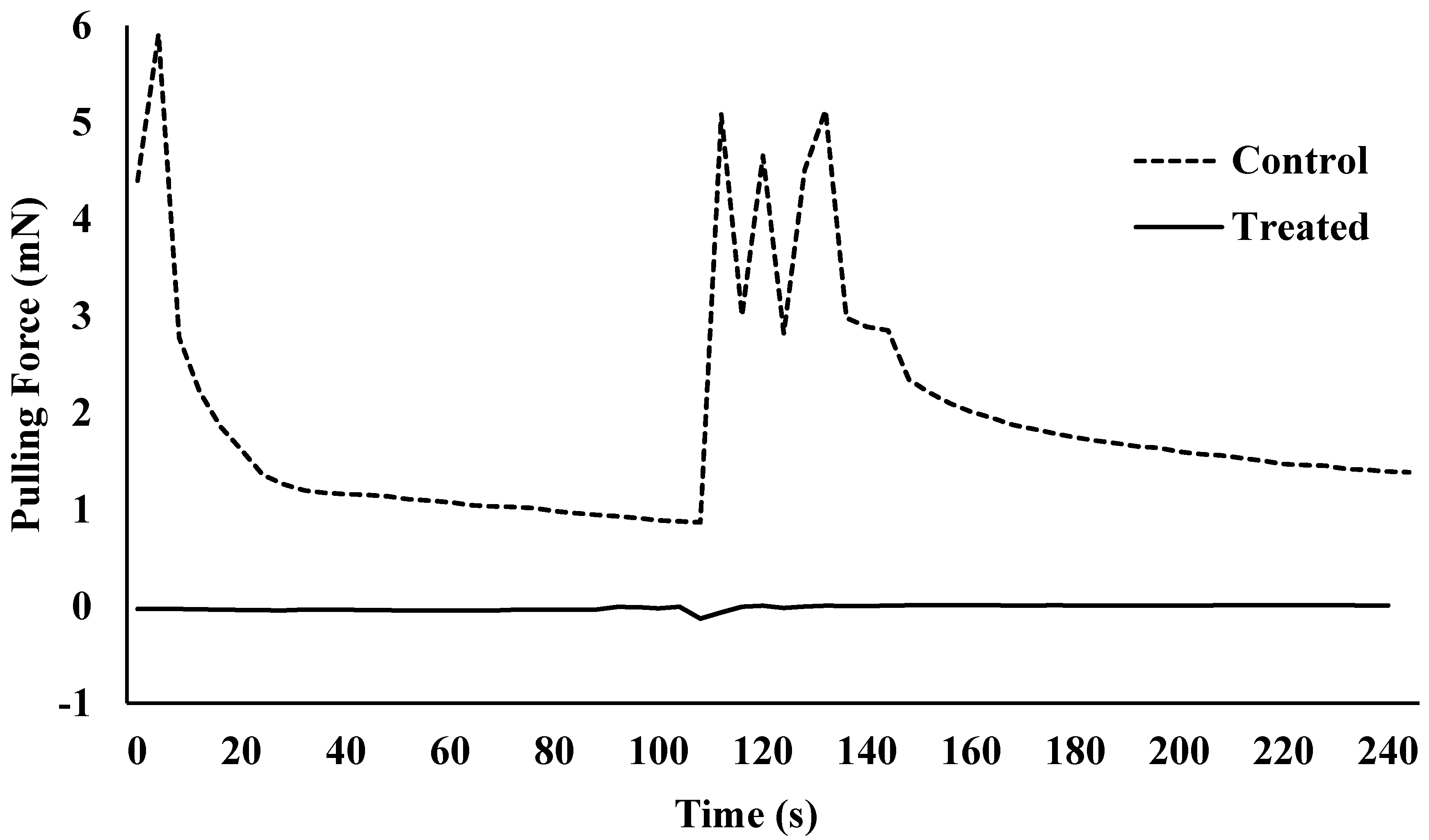
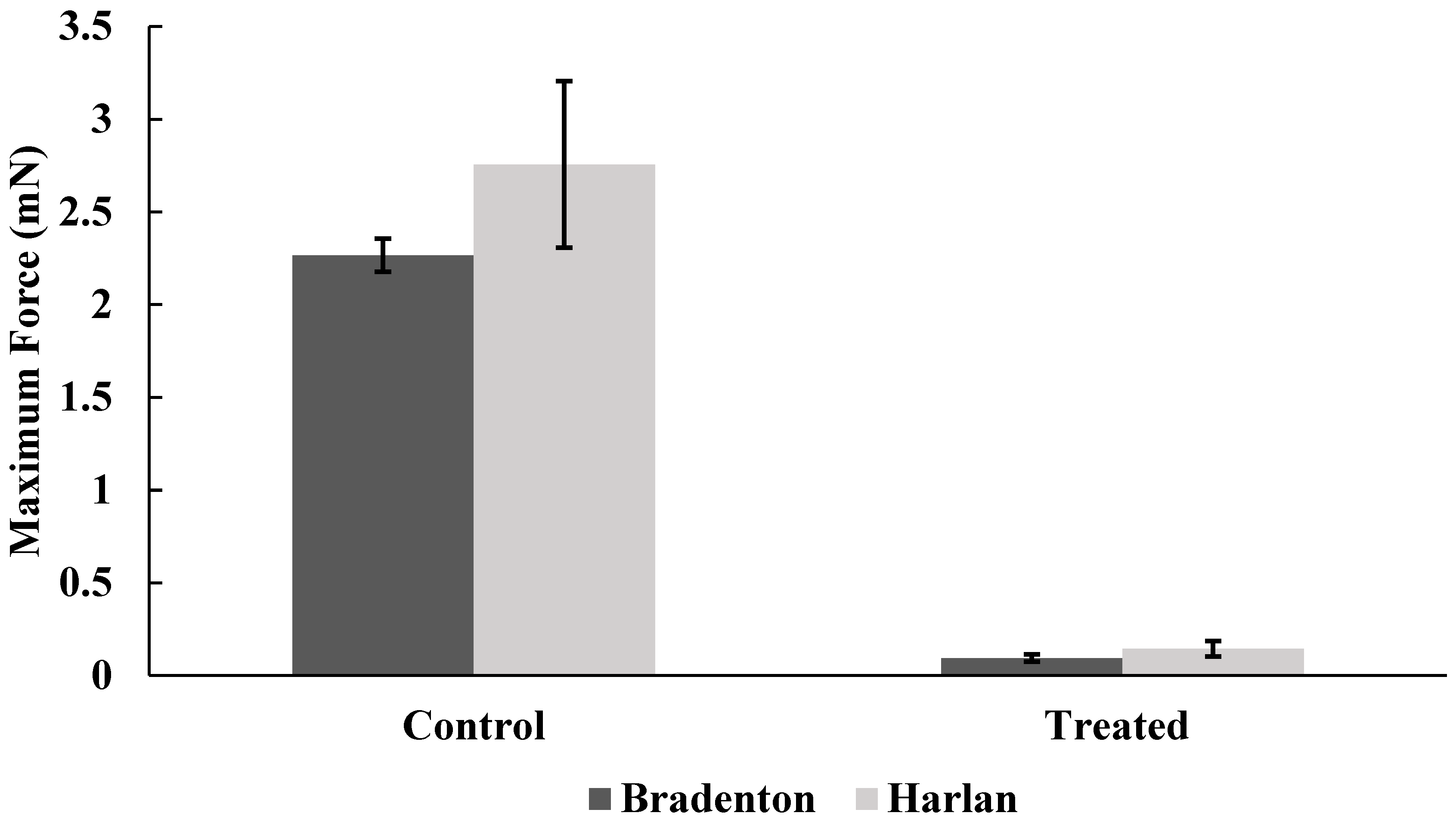
2.4. Locomotion Inhibition Quantified Using a Pulling Force Assay
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gangloff-Kaufmann, J.; Hollingsworth, C.; Hahn, J.; Hansen, L.; Kard, B.; Waldvogel, M. Bed bugs in America: A pest management industry survey. Am. Entomol. 2006, 52, 105–106. [Google Scholar] [CrossRef]
- Romero, A.; Potter, M.F.; Potter, D.A.; Haynes, K.F. Insecticide resistance in the bed bug: A factor in the pest’s sudden resurgence? J. Med. Entomol. 2007, 44, 175–178. [Google Scholar] [PubMed]
- Moore, D.J.; Miller, D.M. Laboratory evaluations of insecticide product efficacy for control of Cimex lectularius. J. Econ. Entomol. 2006, 99, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.S.; Kwon, D.H.; Strycharz, J.P.; Hollingsworth, C.S.; Lee, S.H.; Clark, J.M. Biochemical and molecular analysis of deltamethrin resistance in the common bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 2008, 45, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Adelman, Z.N.; Kilcullen, K.A.; Koganemaru, R.; Anderson, M.A.; Anderson, T.D.; Miller, D.M. Deep sequencing of pyrethroid-resistant bed bugs reveals multiple mechanisms of resistance within a single population. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed]
- Koganemaru, R.; Miller, D.M.; Adelman, Z.N. Robust cuticular penetration resistance in the common bed bug (Cimex lectularius L.) correlates with increased steady-state transcript levels of CPR-type cuticle protein genes. Pest. Biochem. Physiol. 2013, 106, 190–197. [Google Scholar] [CrossRef]
- Lilly, D.G.; Latham, S.L.; Webb, C.E.; Doggett, S.L. Cuticle thickening in a pyrethroid- resistant strain of the common bed bug, Cimex lectularius L. (Hemiptera: Cimicidae). PLoS ONE 2016. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Anderson, T.D. High levels of resistance in the common bed bug, Cimex lectularius (Hemiptera: Cimicidae), to neonicotinoid insecticides. J. Med. Entomol. 2016, 53, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Geden, C.J.; Devine, G.J. Pyriproxyfen and house flies (Diptera: Muscidae): Effects of direct exposure and autodissemination to larval habitats. J. Med. Entomol. 2012, 49, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Lohmeyer, K.H.; Pound, J.M.; Yeater, K.M.; May, M.A. Efficacy of Novaluron as a Feed-Through for Control of Immature Horn Flies, House Flies, and Stable Flies (Diptera: Muscidae) Developing in Cow Manure. J. Med. Entomol. 2014, 51, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, A.; Scott, J.M.; Qualls, W.A.; Müller, G.C.; Xue, R.D. Attractive toxic sugar baits mixed with pyriproxyfen sprayed on plants against adult and larval Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Arthur, F.H.; Fontenot, E.A. Residual activity of methoprene and novaluron as surface treatments to manage the flour beetles, Tribolium castaneum and Tribolium confusum. J. Insect Sci. 2012, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kavallieratos, N.G.; Athanassiou, C.G.; Vayias, B.J.; Tomanović, Ž. Efficacy of insect growth regulators as grain protectants against two stored-product pests in wheat and maize. J. Food Prot. 2012, 75, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Meola, R.; Meier, K.; Dean, S.; Bhaskaran, G. Effect of pyriproxyfen in the blood diet of cat fleas on adult survival, egg viability, and larval development. J. Med. Entomol. 2000, 37, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, N.C.; Koehler, P.G.; Patterson, R.S. Residual effectiveness of insect growth regulators applied to carpet for control of cat flea (Siphonaptera: Pulicidae) larvae. J. Econ. Entomol. 1995, 88, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.; Paul, A.J.; Kitron, U.D.; Philip, J.R.; Barnett, S.; Piel, M.J.; Ness, R.W.; Evilsizer, M. Impact of an orally administered insect growth regulator (lufenuron) on flea infestations of dogs in a controlled simulated home environment. Am. J. Vet. Res. 1996, 57, 502–504. [Google Scholar] [PubMed]
- Kawada, H.; Hirano, M. Insecticidal effects of the insect growth regulators methoprene and pyriproxyfen on the cat flea (Siphonaptera: Pulicidae). J. Med. Entomol. 2014, 33, 819–822. [Google Scholar] [CrossRef]
- Jones, S.C. Evaluation of two insect growth regulators for the bait-block method of subterranean termite (Isoptera: Rhinotermitidae) control. J. Econ. Entomol. 1984, 77, 1086–1091. [Google Scholar] [CrossRef]
- Su, N.Y.; Thoms, E.M.; Ban, P.M.; Scheffrahn, R.H. Monitoring/baiting station to detect and eliminate foraging populations of subterranean termites (Isoptera: Rhinotermitidae) near structures. J. Econ. Entomol. 1995, 88, 932–936. [Google Scholar] [CrossRef]
- Su, N.Y.; Scheffrahn, R.H. Laboratory evaluation of two chitin synthesis inhibitors, hexaflumuron and diflubenzuron, as bait toxicants against Formosan and eastern subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 1993, 86, 1453–1457. [Google Scholar] [CrossRef]
- Su, N.Y. Field evaluation of a hexaflumuron bait for population suppression of subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 1994, 87, 389–397. [Google Scholar] [CrossRef]
- Mosson, H.J.; Short, J.E.; Schenkerb, R.; Edwardsa, J.P. The effects of the insect growth regulator lufenuron on Oriental cockroach, Blatta orientalis, and German cockroach, Blattella germanica, populations in simulated domestic environments. Pest Manag. Sci. 1995, 45, 237–246. [Google Scholar] [CrossRef]
- Koehler, P.G.; Patterson, R.S. Incorporation of pyriproxyfen in a German cockroach (Dictyoptera: Blattellidae) management program. J. Econ. Entomol. 1991, 84, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Ameen, A.; Wang, C.; Kaakeh, W.; Bennett, G.W.; King, J.E.; Karr, L.L.; Xie, J. Residual activity and population effects of noviflumuron for German cockroach (Dictyoptera: Blattellidae) control. J. Econ. Entomol. 2005, 98, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.L.; Appel, A.G.; Demark, J.J.; Bennett, G.W. Oral toxicity, formulation effects, and field performance of flufenoxuron against the German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 1992, 85, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.G. Efficacy of bed bug control products in lab bioassays: Do they make it past the starting gate? Am. Entomol. 2006, 52, 113–116. [Google Scholar] [CrossRef]
- Goodman, M.H.; Potter, M.F.; Haynes, K.F. Effects of juvenile hormone analog formulations on development and reproduction in the bed bug Cimex lectularius (Hemiptera: Cimicidae). Pest Manag. Sci. 2013, 69, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Demark, J.J.; Bennett, G.W. Efficacy of chitin synthesis inhibitors on nymphal German cockroaches (Dictyoptera: Blattellidae). J. Econ. Entomol. 1989, 82, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, J. Effect of Flufenoxurom Growth Regulator in Cimex lectularius L. Ph.D. Thesis, Universidade Estadual Paulista, Sao Paulo, Brazil, 2015. [Google Scholar]
- Hottel, B.A.; Pereira, R.M.; Gezan, S.A.; Qing, R.; Sigmund, W.M.; Koehler, P.G. Climbing ability of the common bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 2015, 52, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Billen, J.; Doggett, S.L.; Lee, C.Y. Differences in Climbing Ability of Cimex lectularius and Cimex hemipterus (Hemiptera: Cimicidae). J. Econ. Entomol. 2017, 110, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Montes, C.; Cuadrillero, C.; Vilella, D. Maintenance of a Laboratory Colony of Cimex lectularius (Hemiptera: Cimicidae) Using an Artificial Feeding Technique. J. Med. Entomol. 2002, 39, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H. Chitin synthesis inhibitors: old molecules and new developments. Insect Sci. 2013, 20, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H. Insect chitin synthases: A review. J. Comp. Physiol. B 2006, 176, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, F. Studies on the action mechanism of benzoylurea insecticides to inhibit the process of chitin synthesis in insects: A review on the status of research activities in the past, the present and the future prospects. Pestic. Biochem. Physiol. 2010, 97, 133–139. [Google Scholar] [CrossRef]
- Redfern, R.E.; Kelly, T.J.; Bořkovec, A.B.; Hayes, D.K. Ecdysteroid titers and molting aberrations in last-stage Oncopeltus nymphs treated with insect growth regulators. Pestic. Biochem. Physiol. 1982, 18, 351–356. [Google Scholar] [CrossRef]
- Cutler, G.C.; Scott-Dupree, C.D.; Tolman, J.H.; Harris, C.R. Toxicity of the insect growth regulator novaluron to the non-target predatory bug Podisus maculiventris (Heteroptera: Pentatomidae). Biol. Control 2006, 38, 196–204. [Google Scholar] [CrossRef]
- Hottel, B.A.; Pereira, R.M.; Koehler, P.G. The influence of roughness and pyrethroid formulations on bed bug (Cimex lectularius L.) resting preferences. Insects 2015, 6, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Haynes, K.F. Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.G.; Scott-Dupree, C.D.; Tolman, J.H.; Harris, R.C. Acute and sublethal toxicity of novaluron, a novel chitin synthesis inhibitor, to Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Pest Manag. Sci. 2005, 61, 1060–1068. [Google Scholar] [CrossRef] [PubMed]

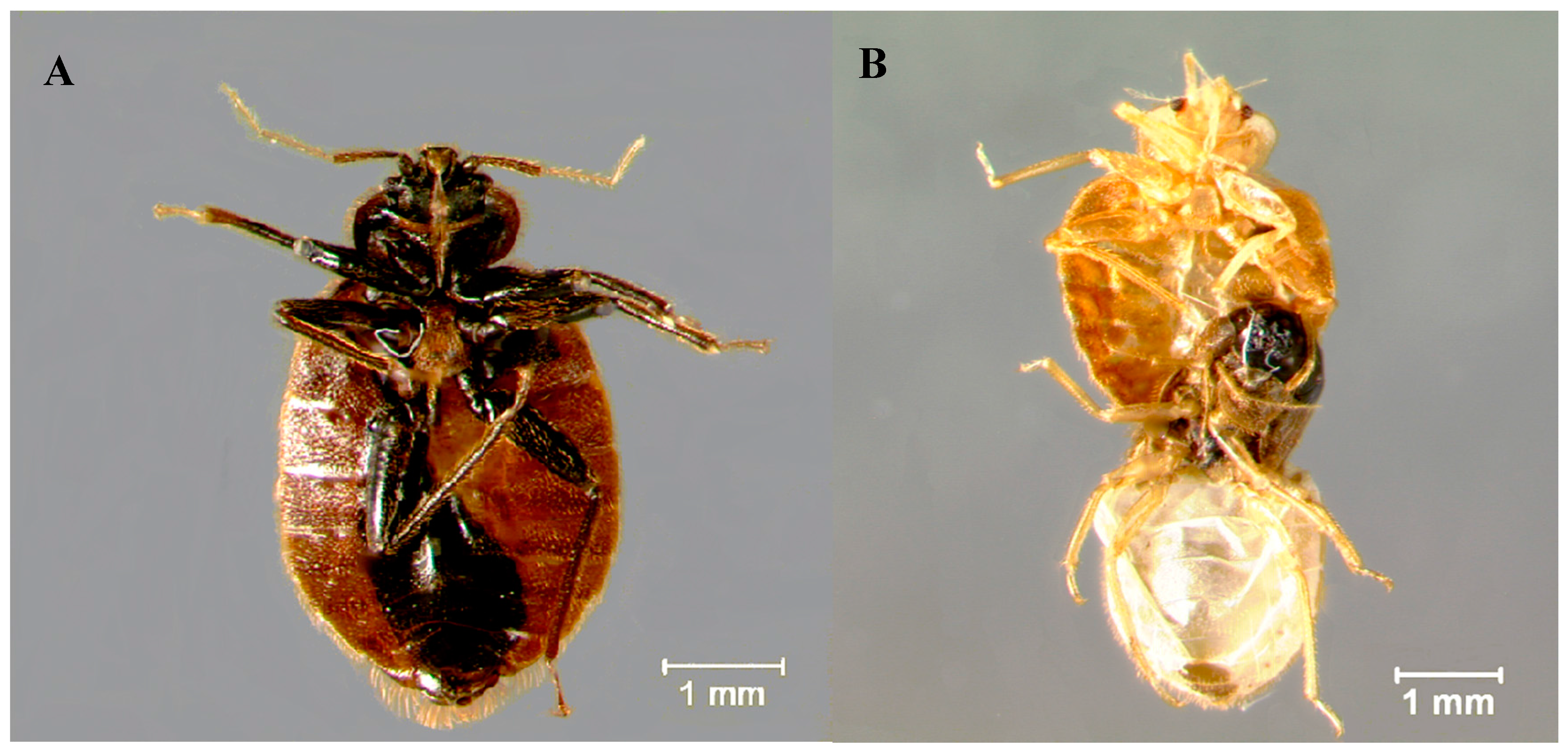



| Strain | Dose (% w/v) | n | # Dead | # Malformed | # Affected 1 | % Affected 2 | % Malformed/Affected 3 |
|---|---|---|---|---|---|---|---|
| Harlan | |||||||
| 0.000016 | 55 | 4 | 0 | 4 | 7 | 0 | |
| 0.00016 | 55 | 1 | 0 | 1 | 2 | 0 | |
| 0.0016 | 55 | 4 | 33 | 37 | 67 | 89 | |
| 0.016 | 55 | 20 | 18 | 38 | 69 | 47 | |
| 0.16 | 85 | 36 | 16 | 52 | 61 | 31 | |
| Bradenton | |||||||
| 0.32 | 55 | 8 | 18 | 26 | 47 | 69 | |
| 0.63 | 55 | 21 | 19 | 40 | 73 | 48 | |
| 1.25 | 55 | 28 | 15 | 43 | 78 | 35 | |
| 2.5 | 55 | 17 | 12 | 29 | 53 | 42 | |
| 5.0 | 85 | 28 | 10 | 38 | 45 | 26 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, B.; Baldwin, R.; Koehler, P. Locomotion Inhibition of Cimex lectularius L. Following Topical, Sublethal Dose Application of the Chitin Synthesis Inhibitor Lufenuron. Insects 2017, 8, 94. https://doi.org/10.3390/insects8030094
Campbell B, Baldwin R, Koehler P. Locomotion Inhibition of Cimex lectularius L. Following Topical, Sublethal Dose Application of the Chitin Synthesis Inhibitor Lufenuron. Insects. 2017; 8(3):94. https://doi.org/10.3390/insects8030094
Chicago/Turabian StyleCampbell, Brittany, Rebecca Baldwin, and Philip Koehler. 2017. "Locomotion Inhibition of Cimex lectularius L. Following Topical, Sublethal Dose Application of the Chitin Synthesis Inhibitor Lufenuron" Insects 8, no. 3: 94. https://doi.org/10.3390/insects8030094





