Effects of Wildflower Strips and an Adjacent Forest on Aphids and Their Natural Enemies in a Pea Field
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Setup
2.2. Insect Monitoring
2.3. Statistical Analyses
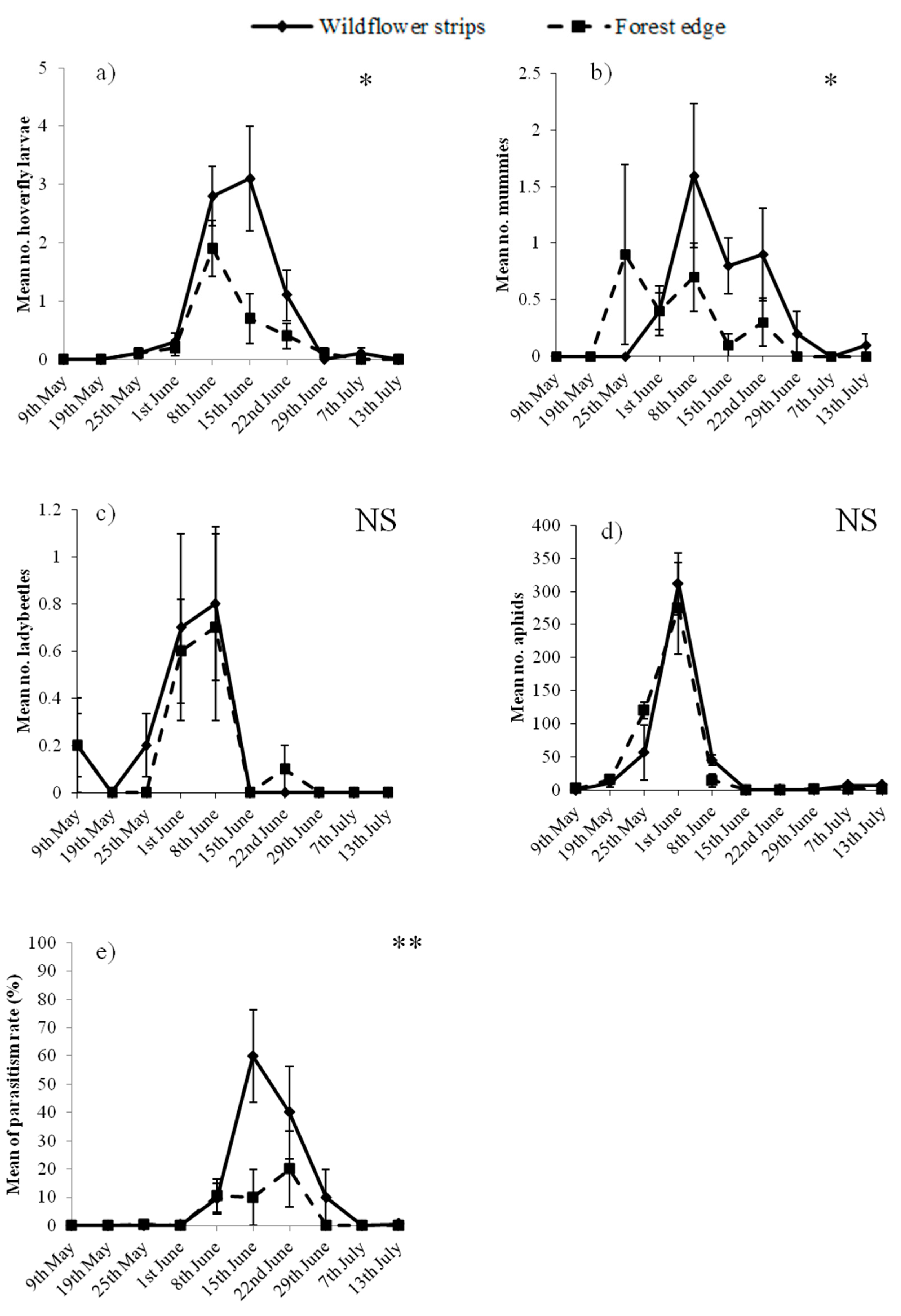
3. Results
4. Discussion
4.1. Aphid Predators
4.2. Aphid Parasitism
4.3. Perspectives
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robinson, R.A.; Sutherland, W.J. Post-war changes in arable farming and biodiversity in Great Britain. J. Appl. Ecol. 2002, 39, 157–176. [Google Scholar] [CrossRef]
- Baldi, I.; Cordier, S.; Coumoul, X.; Elbaz, A.; Gamet-Payrastre, L.; Le Bailly, P.; Multigner, L.; Rahmani, R.; Spinosi, J.; Van Maele-Fabry, G. Pesticides: Effets Sur la Santé; INSERM, Institut national de la santé et de la Recherche Médicale: Paris, France, 2013; ISBN 978-2-85598-905-1. [Google Scholar]
- Devine, G.J.; Furlong, M.J. Insecticide use: Contexts and ecological consequences. Agric. Hum. Values 2007, 24, 281–306. [Google Scholar] [CrossRef]
- Krebs, J.R.; Wilson, J.D.; Bradbury, R.B.; Siriwardena, G.M. The second Silent Spring? Nature 1999, 400, 611–612. [Google Scholar] [CrossRef]
- Hatt, S.; Artru, S.; Brédart, D.; Lassois, L.; Francis, F.; Haubruge, E.; Garré, S.; Stassart, P.M.; Dufrêne, M.; Monty, A.; Boeraeve, F. Towards sustainable food systems: The position of agroecology and how it questions current research practices (Review). Biotechnol. Agron. Soc. Environ. 2016, 20, 215–224. [Google Scholar]
- Foster, S.P.; Devine, G.J.; Devonshire, A.L. Insecticide resistance. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CABI: Cambridge, MA, USA, 2007; pp. 261–286. [Google Scholar]
- Skevas, T.; Lansink, A.O.; Stefanou, S.E. Designing the emerging EU pesticide policy: A literature review. Wagening. J. Life Sci. 2013, 64–65, 95–103. [Google Scholar] [CrossRef]
- Zehnder, G.; Gurr, G.M.; Kühne, S.; Wade, M.R.; Wratten, S.D.; Wyss, E. Arthropod pest management in organic crops. Annu. Rev. Entomol. 2007, 52, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.A. Conservation Biological Control; Academic Press: San Diego, CA, USA, 1998; ISBN 978-0-08-052980-6. [Google Scholar]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Agri-Environment Measures. Overview on General Principles, Types of Measures and Application; European Commission, Directorate General for Agriculture and Rural Development: Brussels, Belgium, 2005; p. 24. [Google Scholar]
- Holland, J.M.; Bianchi, F.J.; Entling, M.H.; Moonen, A.C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest Manag. Sci. 2016, 72, 1638–1651. [Google Scholar] [CrossRef] [PubMed]
- Balzan, M.V.; Bocci, G.; Moonen, A.-C. Landscape complexity and field margin vegetation diversity enhance natural enemies and reduce herbivory by Lepidoptera pests on tomato crop. BioControl 2016, 61, 141–154. [Google Scholar] [CrossRef]
- Martin, E.A.; Seo, B.; Park, C.-R.; Reineking, B.; Steffan-Dewenter, I. Scale-dependent effects of landscape composition and configuration on natural enemy diversity, crop herbivory, and yields. Ecol. Appl. 2016, 26, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.M.; Landis, D.A.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C.; et al. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef]
- Thies, C.; Roschewitz, I.; Tscharntke, T. The landscape context of cereal aphid-parasitoid interactions. Proc. R. Soc. B 2005, 272, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Tschumi, M.; Albrecht, M.; Collatz, J.; Dubsky, V.; Entling, M.H.; Najar-Rodriguez, A.J.; Jacot, K. Tailored flower strips promote natural enemy biodiversity and pest control in potato crops. J. Appl. Ecol. 2016, 53, 1169–1176. [Google Scholar] [CrossRef]
- Hatt, S.; Lopes, T.; Boeraeve, F.; Chen, J.; Francis, F. Pest regulation and support of natural enemies in agriculture: Experimental evidence of within field wildflower strips. Ecol. Eng. 2017, 98, 240–245. [Google Scholar] [CrossRef]
- Morandin, L.A.; Long, R.F.; Kremen, C. Hedgerows enhance beneficial insects on adjacent tomato fields in an intensive agricultural landscape. Agric. Ecosyst. Environ. 2014, 189, 164–170. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Goedhart, P.W.; Baveco, J.M. Enhanced pest control in cabbage crops near forest in The Netherlands. Landsc. Ecol. 2008, 23, 595–602. [Google Scholar] [CrossRef]
- Tscharntke, T.; Karp, D.S.; Chaplin-Kramer, R.; Batáry, P.; DeClerck, F.; Gratton, C.; Hunt, L.; Ives, A.R.; Jonsson, M.; Larsen, A.; et al. When natural habitat fails to enhance biological pest control—Five hypotheses. Biol. Conserv. 2016, 204, 449–458. [Google Scholar] [CrossRef]
- Van Emden, H.F.; Harrington, R. (Eds.) Aphids as Crop Pests; CABI: Cambridge, MA, USA, 2007. [Google Scholar]
- Katis, N.I.; Tsitsipis, J.A.; Stevens, M.H.H.; Powell, G. Transmission of plant viruses. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CABI: Cambridge, MA, USA, 2007; pp. 353–390. [Google Scholar]
- Alignier, A.; Raymond, L.; Deconchat, M.; Menozzi, P.; Monteil, C.; Sarthou, J.P.; Vialatte, A.; Ouin, A. The effect of semi-natural habitats on aphids and their natural enemies across spatial and temporal scales. Biol. Control 2014, 77, 76–82. [Google Scholar] [CrossRef]
- Schellhorn, N.; Bianchi, F.J.J.A.; Hsu, C.L. Movement of entomophagous arthropods in agricultural landscapes: Links to pest suppression. Annu. Rev. Entomol. 2014, 59, 559–581. [Google Scholar] [CrossRef] [PubMed]
- Uyttenbroeck, R.; Hatt, S.; Piqueray, J.; Paul, A.; Bodson, B.; Francis, F.; Monty, A. Creating perennial flower strips: Think functional! Agric. Agric. Sci. Procedia 2015, 6, 95–101. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. lme4: Linear mixed-effects models using Eigen and S4. R Package Version 2014, 1, 1–23. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Ver Hoef, J.M.; Boveng, P.L. Quasi-Poisson vs. Negative binomial regression: How should we model overdispersed count data? Ecology 2007, 88, 2766–2772. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. Lmer Test: Tests in Linear Mixed Effects Models. Available online: https://rdrr.io/cran/lmerTest/man/lmer.html (accessed on 8 September 2016).
- Xu, Q.; Hatt, S.; Lopes, T.; Yong, Z.; Bodson, B.; Chen, J.; Francis, F. A push-pull strategy to control aphids combines intercropping with semiochemical releases. J. Pest Sci. 2017, in press. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Sarthou, J.-P.; Ouin, A.; Arrignon, F.; Barreau, G.; Bouyjou, B. Landscape parameters explain the distribution and abundance of Episyrphus balteatus (Diptera: Syrphidae). Eur. J. Entomol. 2005, 102, 539–545. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Van Rijn, P.C.J. Pick and mix: Selecting flowering plants to meet the requirements of target biological control insects. In Biodiversity and Insect Pests: Key Issues for Sustainable Management; Gurr, G.M., Wratten, S.D., Snyder, W.E., Read, D.M.Y., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 139–165. [Google Scholar]
- Almohamad, R.; Verheggen, F.; Haubruge, É. Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrphidae): A review. Biotechnol. Agron. Soc. Environ. 2009, 13, 467–481. [Google Scholar]
- Lundgren, J.G. Nutritional aspects of non-prey foods in the life histories of predaceous Coccinellidae. Biol. Control 2009, 51, 294–305. [Google Scholar] [CrossRef]
- Sarthou, J.-P.; Badoz, A.; Vaissière, B.; Chevallier, A.; Rusch, A. Local more than landscape parameters structure natural enemycommunities during their overwintering in semi-natural habitats. Agric. Ecosyst. Environ. 2014, 194, 17–28. [Google Scholar] [CrossRef]
- Ville de Gembloux Relevés Faunistiques et Floristiques de la Réserve de L’écaille à Gembloux. Available online: http://www.gembloux.be/ma-commune/services-communaux/environnement/plan-communal-de-developpement-de-la-nature/le-reseau-ecologique-gembloutois/les-50-fiches-descriptives-du-patrimoine-naturel/site-9.pdf/view (accessed on 2 November 2016).
- Jervis, M.A.; Kidd, N.A.C.; Fitton, M.G.; Huddleston, T.; Dawah, H.A. Flower-visiting by Hymenopteran parasitoids. J. Nat. Hist. 1993, 27, 67–105. [Google Scholar] [CrossRef]
- Lopes, T.; Hatt, S.; Xu, Q.; Chen, J.; Liu, Y.; Francis, F. Wheat (Triticum aestivum L.)-based intercropping systems for biological pest control: A review. Pest Manag. Sci. 2016, 72, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Malézieux, E.; Crozat, Y.; Dupraz, C.; Laurans, M.; Makowski, D.; Ozier-Lafontaine, H.; Rapidel, B.; De Tourdonnet, S.; Valantin-Morison, M. Mixing plant species in cropping systems: Concepts, tools and models. Agron. Sustain. Dev. 2009, 29, 43–62. [Google Scholar] [CrossRef]
- Poveda, K.; Gómez, M.I.; Martínez, E. Diversification practices: Their effect on pest regulation and production. Rev. Colomb. Entomol. 2008, 34, 131–144. [Google Scholar]

| Hoverfly Larvae | df | χ² | p-Value | Effect |
|---|---|---|---|---|
| Habitat | 1 | 5.01 | 0.025 * | |
| Aphid | 1 | 1.13 | 0.288 | |
| Habitat: Aphid | 1 | 0.038 | 0.845 | |
| Ladybeetle adults | ||||
| Habitat | 1 | 0.11 | 0.744 | |
| Aphid | 1 | 11.9 | <0.001 *** | (+) |
| Habitat: Aphid | 1 | 0.026 | 0.871 | |
| Mummified aphids | ||||
| Habitat | 1 | 4.3 | 0.038 * | |
| Aphid | 1 | 8.7 | 0.003 ** | (+) |
| Habitat: Aphid | 1 | 4.47 | 0.034 * | |
| Aphids | ||||
| Habitat | 1 | 0.002 | 0.962 |
| Parasitism Rate | df | F | p-Value | Effect |
|---|---|---|---|---|
| Habitat | 1 | 10.6 | 0.001 ** | |
| Aphid | 1 | 12 | <0.001 *** | (−) |
| Habitat: Aphid | 1 | 4.71 | 0.031 * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatt, S.; Mouchon, P.; Lopes, T.; Francis, F. Effects of Wildflower Strips and an Adjacent Forest on Aphids and Their Natural Enemies in a Pea Field. Insects 2017, 8, 99. https://doi.org/10.3390/insects8030099
Hatt S, Mouchon P, Lopes T, Francis F. Effects of Wildflower Strips and an Adjacent Forest on Aphids and Their Natural Enemies in a Pea Field. Insects. 2017; 8(3):99. https://doi.org/10.3390/insects8030099
Chicago/Turabian StyleHatt, Séverin, Pierre Mouchon, Thomas Lopes, and Frédéric Francis. 2017. "Effects of Wildflower Strips and an Adjacent Forest on Aphids and Their Natural Enemies in a Pea Field" Insects 8, no. 3: 99. https://doi.org/10.3390/insects8030099






