Perennial Grass and Native Wildflowers: A Synergistic Approach to Habitat Management
Abstract
:1. Introduction
2. Materials and Methods
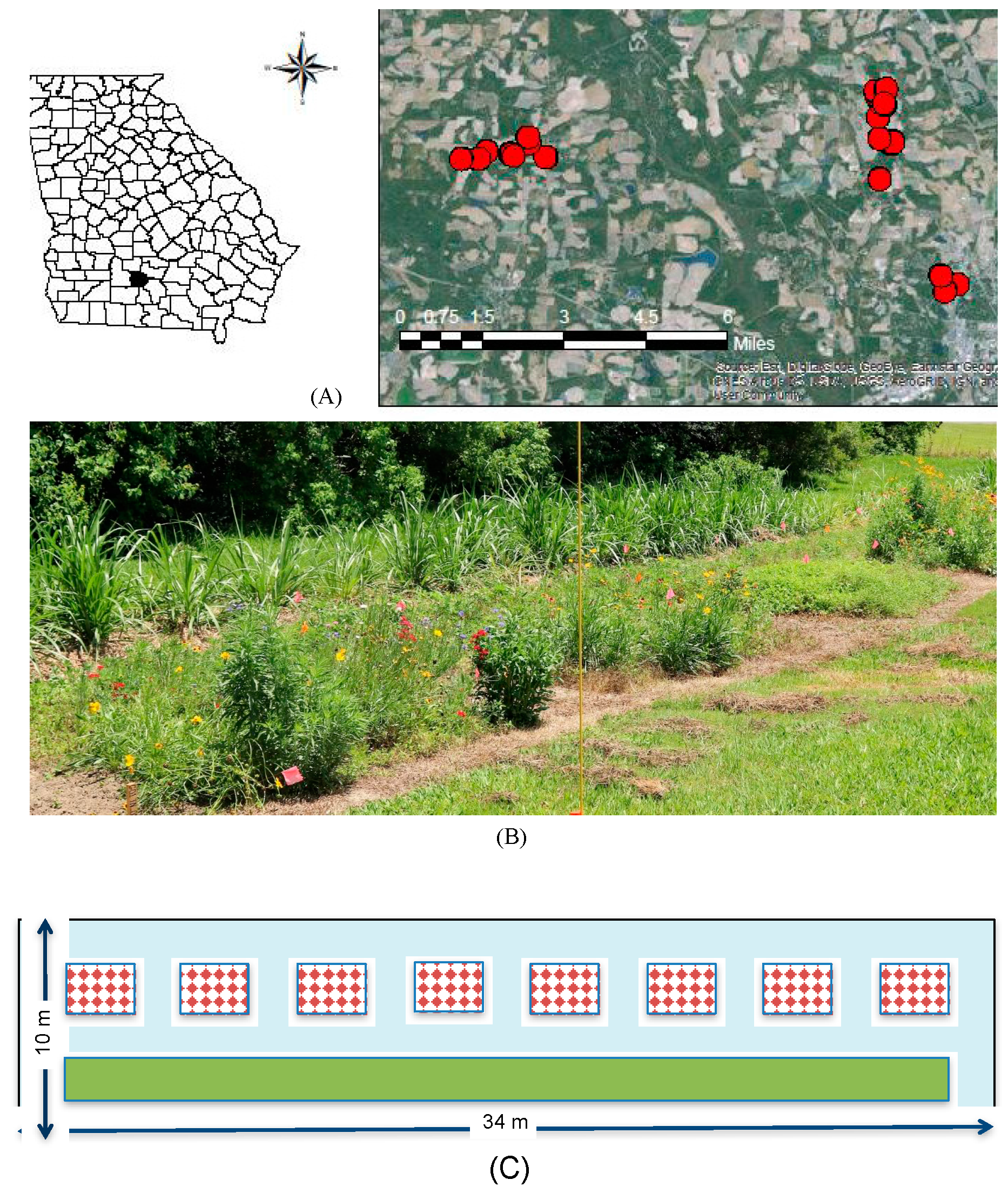
2.1. Study Sites
2.2. Arthropod and Vegetation Sampling
2.3. Bioenergy Production and Soil Nutrients
2.4. Statistical Analyses
3. Results
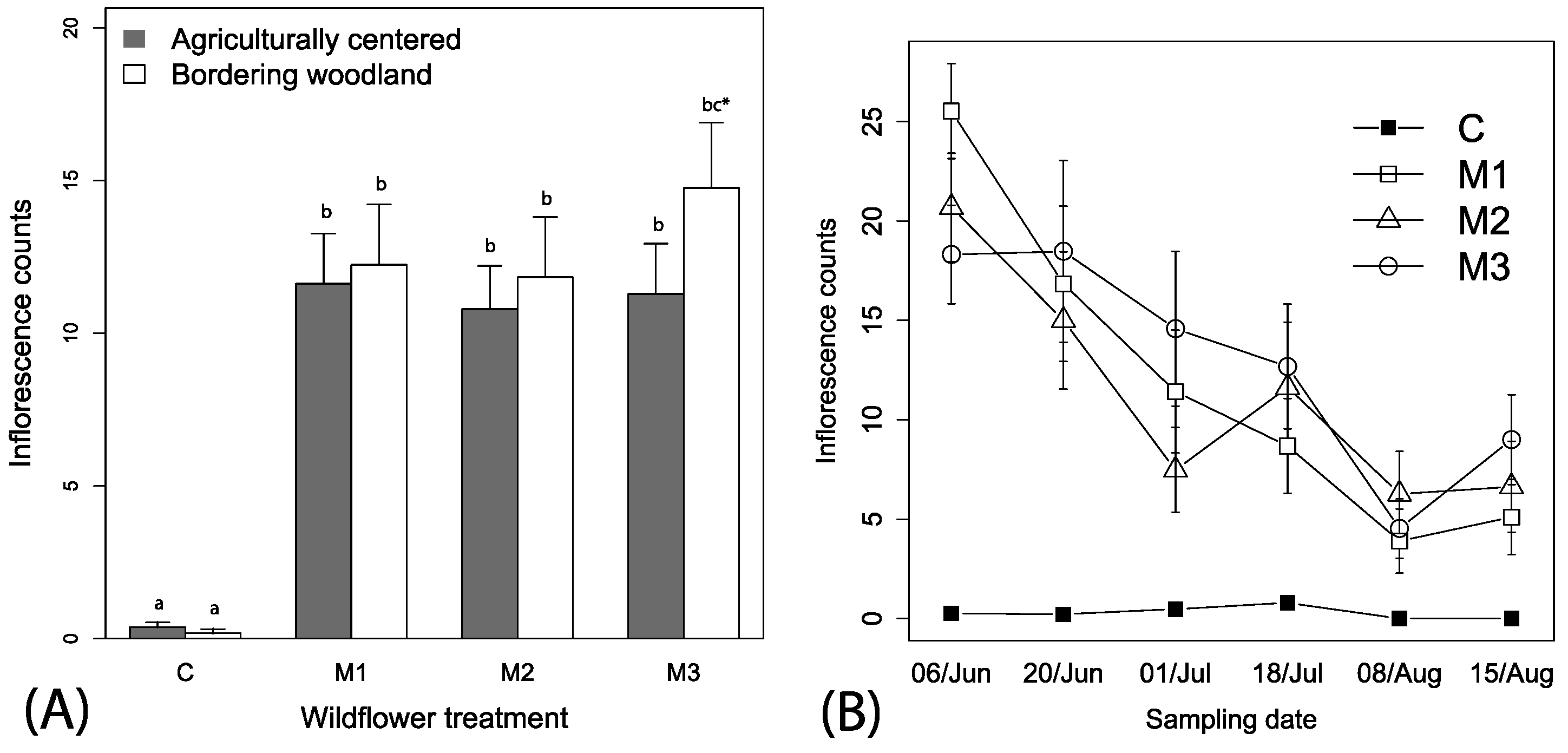
3.1. Wildflowers
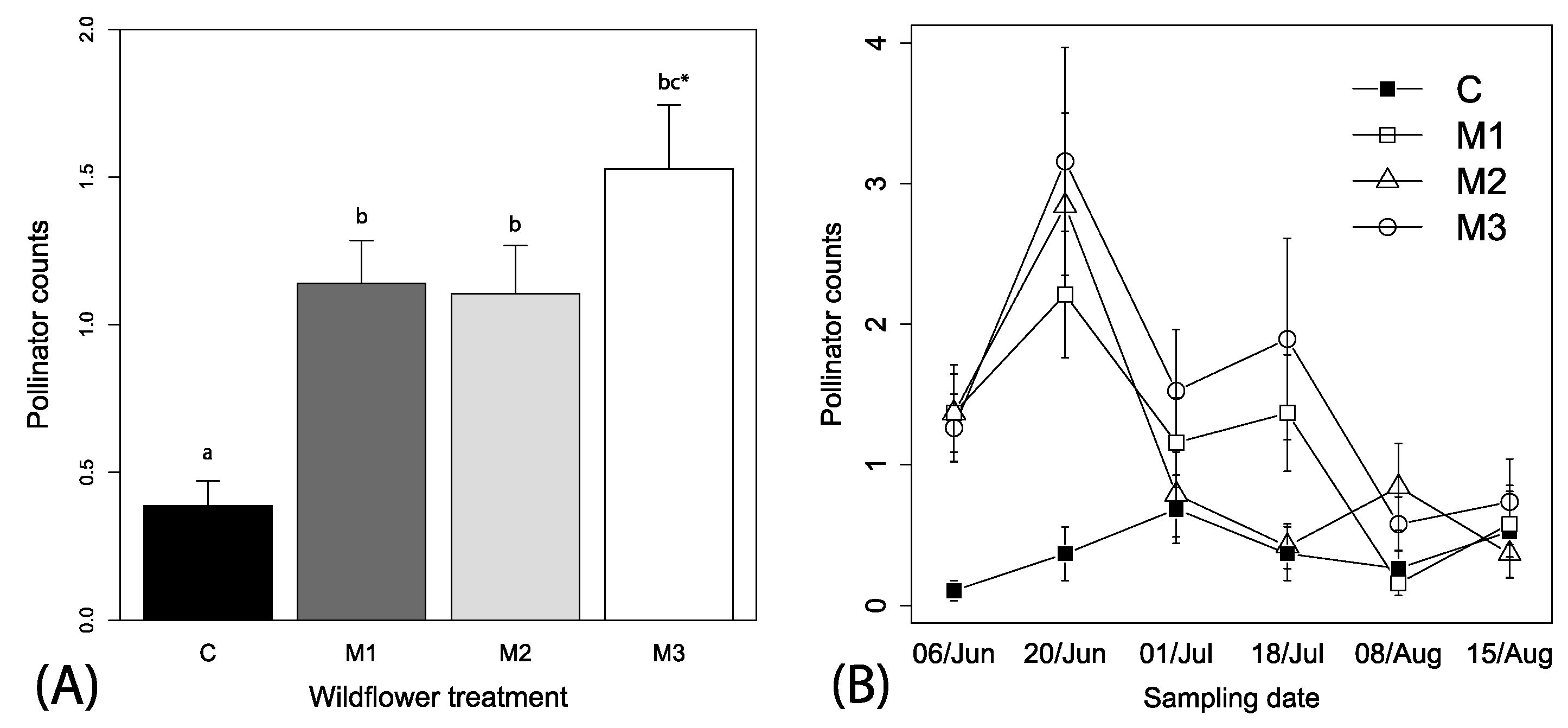
3.2. Pollinators
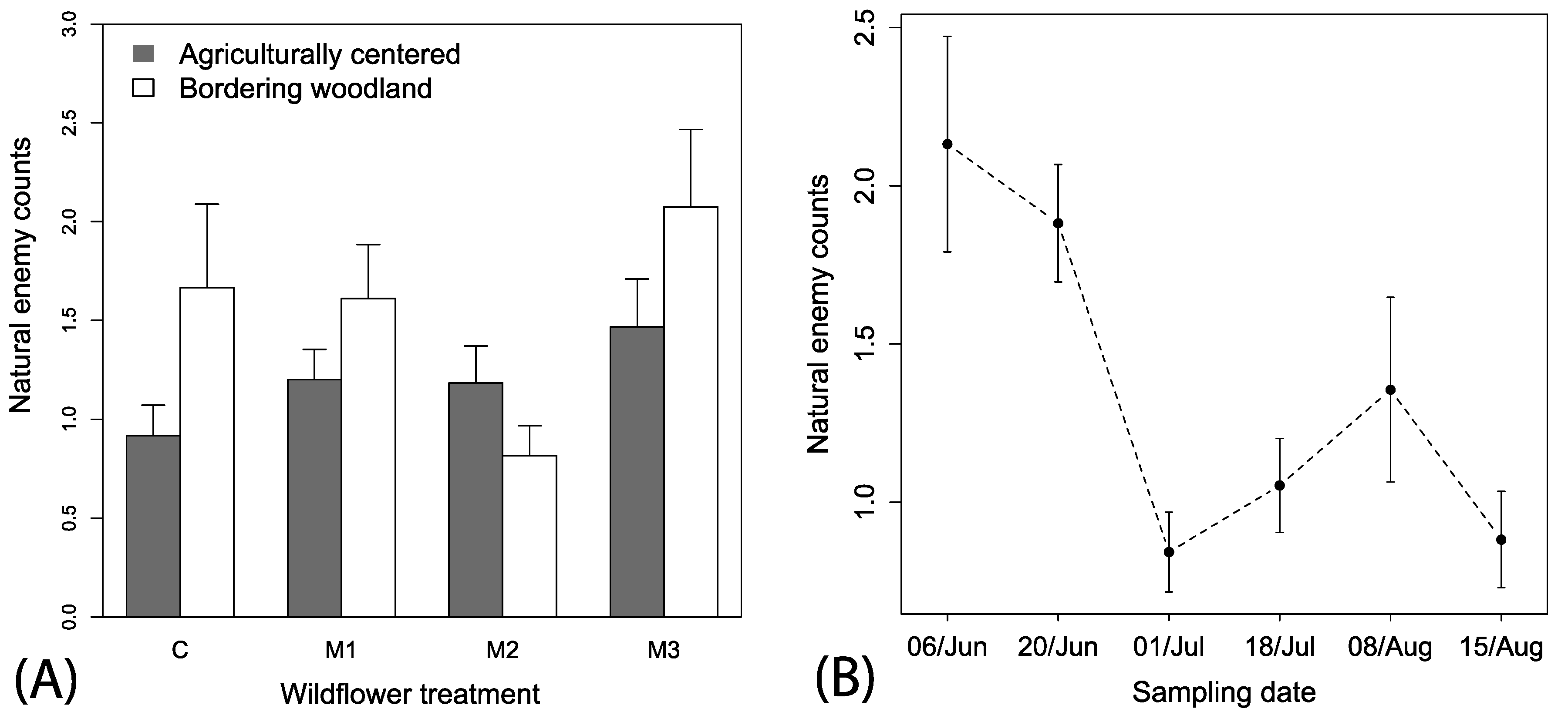
3.3. Natural Enemies
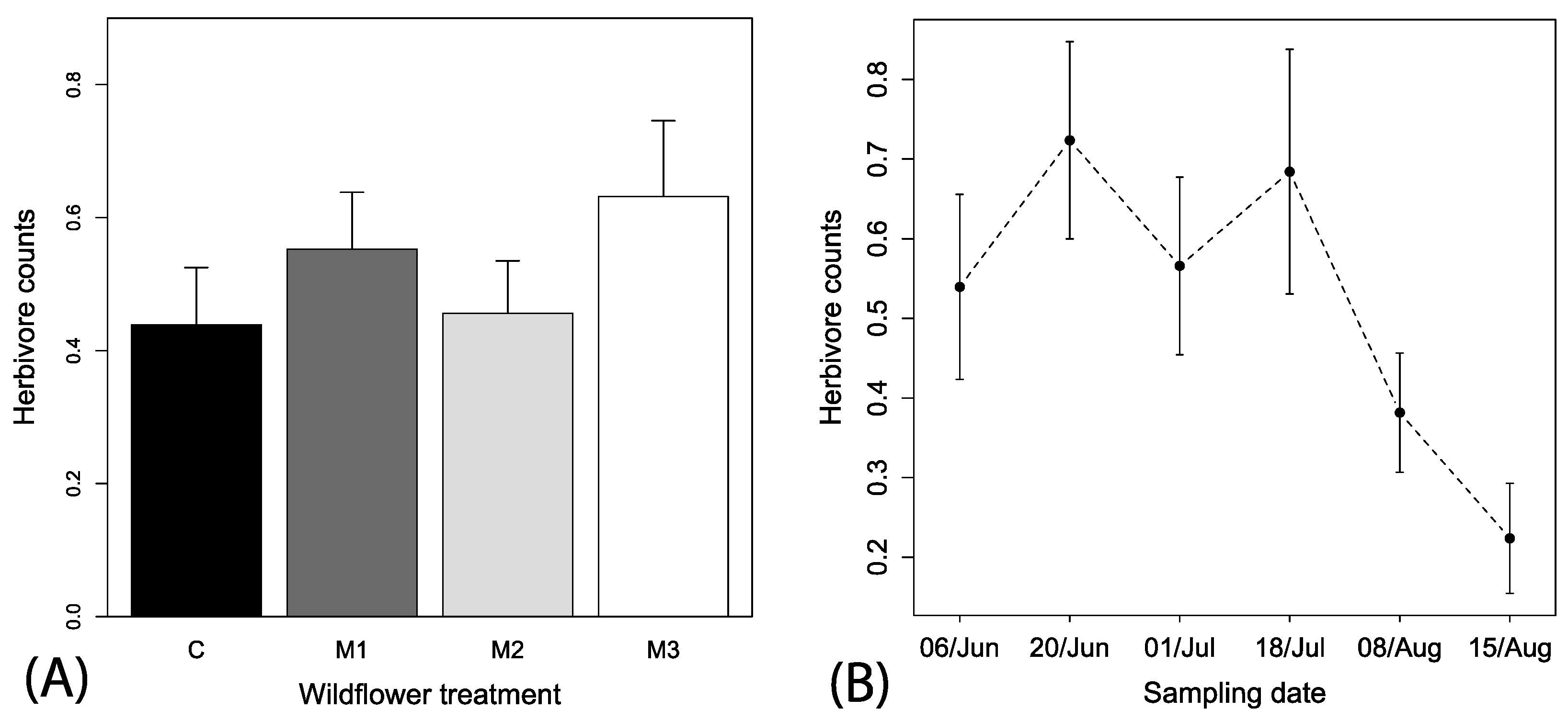
3.4. Herbivores
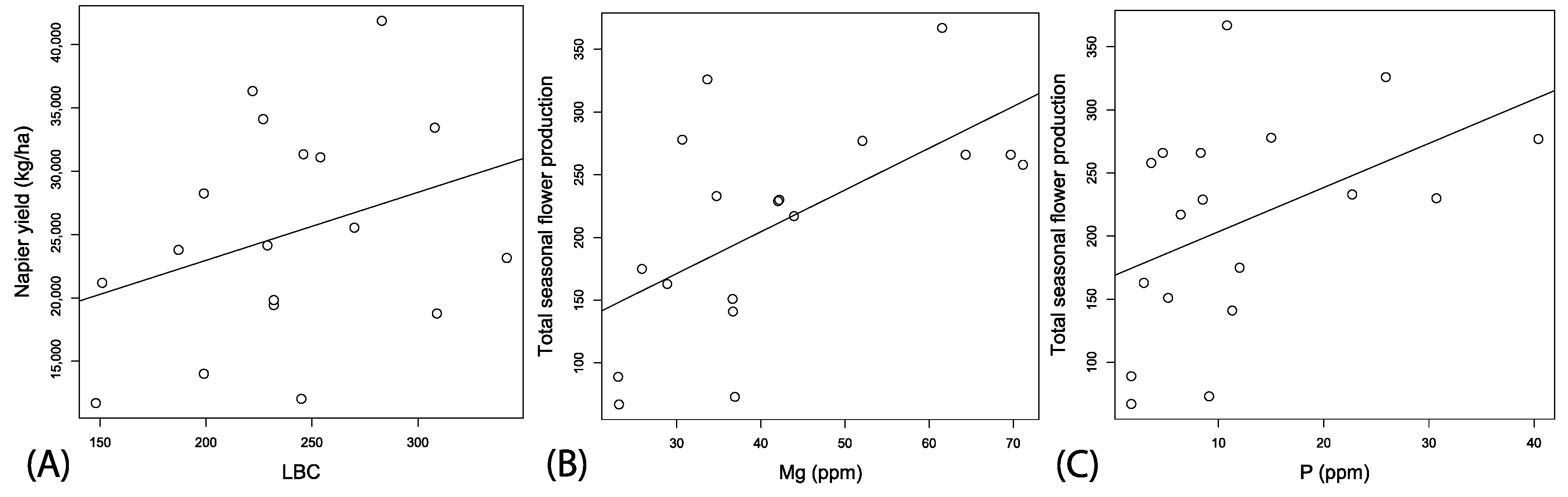
3.5. Bioenergy Production, Widlflower Production and Soil Nutrients
4. Discussion
5. Conclusions and Future Research
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Landis, D.A. Designing agricultural landscapes for biodiversity-based ecosystem services. Basic Appl. Ecol. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Coffin, A.W.; Strickland, T.C.; Anderson, W.F.; Lamb, M.C.; Lowrance, R.R.; Smith, C.M. Potential for production of perennial biofuel feedstocks in conservation buffers on the Coastal Plain of Georgia, USA. BioEnergy Res. 2016, 9, 587–600. [Google Scholar] [CrossRef]
- Blaauw, B.R.; Isaacs, R. Larger wildflower plantings increase natural enemy density, diversity, and biological control of sentinel prey, without increasing herbivore density. Ecol. Entomol. 2012, 37, 386–394. [Google Scholar] [CrossRef]
- Harmon, J.P.; Ives, A.R.; Losey, J.E.; Olson, A.C.; Rauwald, K.S. Coleomegilla maculata (Coleoptera: Coccinellidae) predation on pea aphids promoted by proximity to dandelions. Oecologia 2000, 125, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Tschumi, M.; Albrecht, M.; Entling, M.H.; Jacot, K. High effectiveness of tailored flower strips in reducing pests and crop plant damage. Proc. Biol. Sci. 2015, 282, 20151369. [Google Scholar] [CrossRef] [PubMed]
- Tschumi, M.; Albrecht, M.; Bärtschi, C.; Collatz, J.; Entling, M.H.; Jacot, K. Perennial, species-rich wildflower strips enhance pest control and crop yield. Agric. Ecosyst. Environ. 2016, 220, 97–103. [Google Scholar] [CrossRef]
- Tscharntke, T.; Bommarco, R.; Clough, Y.; Crist, T.O.; Kleijn, D.; Rand, T.A.; Tylianakis, J.M.; van Nouhuys, S.; Vidal, S. Conservation biological control and enemy diversity on a landscape scale. Biol. Control 2007, 43, 294–309. [Google Scholar] [CrossRef]
- Walton, N.J.; Isaacs, R. Influence of native flowering plant strips on natural enemies and herbivores in adjacent blueberry fields. Environ. Entomol. 2011, 40, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.X.; Zhu, P.Y.; Gurr, G.M.; Zheng, X.S.; Read, D.M.; Heong, K.; Yang, Y.; Xu, H. Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: Prospects for enhanced use in agriculture. Insect Sci. 2014, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Blaauw, B.R.; Isaacs, R. Flower plantings increase wild bee abundance and the pollination services provided to a pollination-dependent crop. J. Appl. Ecol. 2014, 51, 890–898. [Google Scholar] [CrossRef]
- Feltham, H.; Park, K.; Minderman, J.; Goulson, D. Experimental evidence that wildflower strips increase pollinator visits to crops. Ecol. Evol. 2015, 5, 3523–3530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garibaldi, L.A.; Carvalheiro, L.G.; Leonhardt, S.D.; Aizen, M.A.; Blaauw, B.R.; Isaacs, R.; Kuhlmann, M.; Kleijn, D.; Klein, A.M.; Kremen, C.; et al. From research to action: Enhancing crop yield through wild pollinators. Front. Ecol. Environ. 2014, 12, 439–447. [Google Scholar] [CrossRef]
- Werling, B.P.; Dickson, T.L.; Isaacs, R.; Gaines, H.; Gratton, C.; Gross, K.L.; Liere, H.; Malmstrom, C.M.; Meehan, T.D.; Ruan, L.; et al. Perennial grasslands enhance biodiversity and multiple ecosystem services in bioenergy landscapes. Proc. Nat. Acad. Sci. USA 2014, 111, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. A USDA Regional Roadmap to Meeting the Biofuels Goals of the Renewable Fuels Standard by 2022; U.S. Department of Agriculture: Washington, DC, USA, 2010.
- Lowrance, R.; Anderson, W.F.; Miguez, F.; Strickland, T.; Knoll, J.; Sauer, T. Landscape management and sustainable feedstock production: Enhancing net regional primary productivity while minimizing externalities. In Sustainable Feedstocks for Advanced Biofuels: Sustainable Alternative Fuel Feedstock Opportunities, Challenges and Roadmaps for Six U.S. Regions; Braun, R., Karlen, D.L., Johnson, D., Eds.; Soil and Water Conservation Society: Ankeny, IA, USA, 2011; pp. 1–19. [Google Scholar]
- Knoll, J.E.; Anderson, W.F. Low-input production of biomass from perennial grasses in the Coastal Plain of Georgia, USA. BioEnergy Res. 2012, 5, 206–214. [Google Scholar] [CrossRef]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.A.; Porter, C.; Landis, D.; Schemske, D. Agroenergy crops influence the diversity, biomass, and guild structure of terrestrial arthropod communites. BioEnergy Res. 2012, 5, 179–188. [Google Scholar] [CrossRef]
- Gardiner, M.; Wilson, J.K.; Isaacs, R.; Landis, D. Implications of three biofuel crops for benefical arthropods in agricultural landscapes. BioEnergy Res. 2010, 3, 6–19. [Google Scholar] [CrossRef]
- Werling, B.P.; Meehan, T.D.; Robertson, B.A.; Gratton, C. Biocontrol potential varies with changes in biofuel-crop plant communities and landscape perenniality. Glob. Change Biol. Bioenergy 2011, 3, 347–359. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity-ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Johnson, M.V.V.; Bruckerhoff, S.B.; Kaiser, J.U.; Cordsiemon, R.L.; Harmel, R.D. Clash of the Titans: Comparing productivity via radiation use efficiency for two grass giants of the biofuel field. BioEnergy Res. 2012, 5, 41–48. [Google Scholar] [CrossRef]
- Weijde, T.; Huxley, L.M.; Hawkins, S.; Semviring, E.H.; Farrar, K.; Dolstra, O.; Visser, R.G.F.; Trindade, L.M. Impact of drought stress on growth and quality of miscanthus for biofuel production. Glob. Chang. Biol. Bioenergy 2017, 9, 770–782. [Google Scholar] [CrossRef] [Green Version]
- Ingrao, A.J.; Schmidt, J.; Jubenville, J.; Grode, A.; Komondy, L.; VanderZee, D.; Szendrei, Z. Biocontrol on the edge: Field margin habitats in asparagus fields influence natural enemy-pest interactions. Agric. Ecosyst. Environ. 2017, 243, 47–54. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- R CoreTeam. R: A Language and Environment for Statistical Computing [Computer Software]; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Liere, H.; Kim, T.N.; Werling, B.P.; Meehan, T.D.; Landis, D.A.; Gratton, C. Trophic cascades in agricultural landscapes: Indirect effects of landscape composition on crop yield. Ecol. Appl. 2015, 25, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Garratt, M.P.; Senapathi, D.; Coston, D.J.; Mortimer, S.R.; Potts, S.G. The benefits of hedgerows for pollinators and natural enemies depends on hedge quality and landscape context. Agric. Ecosyst. Environ. 2017, 247, 363–370. [Google Scholar] [CrossRef]
- Torres, C.; Galetto, L. Are nectar sugar composition and corolla tube length related to the diversity of insects that visit Asteraceae flowers? Plant Biol. 2002, 4, 360–366. [Google Scholar] [CrossRef]
- Carvell, C.; Westrich, P.; Meek, W.R.; Pywell, R.F.; Nowakowski, M. Assessing the value of annual and perennial forage mixtures for bumblebees by direct observation and pollen analysis. Apidologie 2006, 37, 326–340. [Google Scholar] [CrossRef]
- Perović, D.J.; Gámez-Virués, S.; Landis, D.A.; Wäckers, F.; Gurr, G.M.; Wratten, S.D.; You, M.; Desneux, N. Managing biological control services through multi-trophic trait interactions: Review and guidelines for implementation at local and landscape scales. Biol. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, T.H.; Regetz, J.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Bogdanski, A.; Morandin, L.A. Landscape effects on crop pollination services: Are there general patterns? Ecol. Lett. 2008, 11, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.D.; Shewsbury, P.M.; Esiekpe, O. Spatial and temporal variation in natural enemy assemblages on Maryland native plant species. Environ. Entomol. 2008, 37, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.S.; Hugo, J.; Schmidt, J.M.; Harwood, J.D.; Madelaine, V. Non-crop plant communities conserve spider populations in chili pepper agroecosystems. Biol. Control 2016, 103, 69–77. [Google Scholar] [CrossRef]
- McCabe, E.; Loeb, G.; Grab, H. Responses of Crop Pests and Natural Enemies to Wildflower Borders Depends on Functional Group. Insects 2017, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Fielder, A.K.; Landis, D.A. Attractiveness of Michigan native plants to arthropod natural enemies and herbivores. Environ. Entoml. 2007, 36, 751–765. [Google Scholar]
- Richard, E.P.; Anderson, W.F. Sugarcane, energy cane and napier grass. Cellul. Energy Crop. Syst. 2014, 91–108. [Google Scholar] [CrossRef]
| Wildflower Species | Presence in Mix | 6-Jun | 20-Jun | 1-Jul | 18-Jul | 8-Aug | 15-Aug | Total Flowers |
|---|---|---|---|---|---|---|---|---|
| Cynoglossum amabile | ∆○ | 8 | 2 | 10 | ||||
| Eschscholzia californica | □∆○ | 3 | 3 | |||||
| Achillea millefolium | □∆○ | 6 | 6 | |||||
| Salvia coccinea | □∆○ | 4 | 6 | 10 | ||||
| Cosmos bipinnatus | □∆○ | 7 | 7 | |||||
| Echinacea purpurea | □∆○ | 3 | 3 | |||||
| Nemophilia maculata | □ | 2 | 2 | |||||
| Coreopsis tinctoria | □∆○ | 490 | 289 | 210 | 4 | 993 | ||
| Phlox drummondi | □∆○ | 57 | 12 | 69 | ||||
| Rudbeckia amplexicaulis | ∆○ | 29 | 13 | 42 | ||||
| Linum grandiflorum r. | □∆○ | 9 | 1 | 13 | 23 | |||
| Centaurea cyanus | □ | 91 | 25 | 27 | 16 | 159 | ||
| Rudbeckia gloriosa | □∆○ | 3 | 12 | 7 | 6 | 28 | ||
| Oenothera lamarckiana | ∆○ | 5 | 6 | 3 | 5 | 19 | ||
| Cosmos sulphureus | □ | 8 | 15 | 21 | 15 | 2 | 4 | 101 |
| Monarda citriodora | □ | 75 | 61 | 12 | 11 | 4 | 163 | |
| Coreopsis lanceolata | □∆○ | 63 | 93 | 19 | 32 | 12 | 2 | 221 |
| Gaillardia pulchella | □∆○ | 161 | 177 | 103 | 405 | 218 | 325 | 1389 |
| Rudbeckia hirta | □∆○ | 278 | 239 | 198 | 148 | 19 | 21 | 903 |
| Total flowers | 1231 | 960 | 645 | 642 | 279 | 394 | 4151 |
| Local Spatial Context Treatments | Napier Yield kg/ha | Mehlich 1 mg/kg (ppm) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| LBC | pH | Ca | K | Mg | Mn | P | Zn | ||
| AI | 27,462 (4527) | 278 (22) | 4.87 (0.09) | 339 (37) | 53.66 (10.23) | 55.29 (6.30) | 8.08 (2.19) | 7.86 (1.06) | 4.93 (2.05) |
| AN | 22,590 (4797) | 211 (18) | 4.71 (0.09) | 205 (21) | 34.96 (8.21) | 33.08 (3.27) | 4.34 (0.92) | 9.42 (3.76) | 1.48 (0.31) |
| TI | 26,661 (2641) | 211 (33) | 5.07 (0.20) | 330 (61) | 30.46 (8.78) | 45.16 (8.98) | 7.37 (2.53) | 13.63 (6.22) | 2.31 (0.93) |
| TN | 25,688 (3966) | 271 (25) | 4.74 (0.16) | 487 (202) | 32.93 (6.55) | 38.28 (6.83) | 7.61 (1.80) | 90.78 (71.08) | 4.97 (1.96) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, S.S.; Olson, D.M.; Coffin, A.W.; Strickland, T.C.; Schmidt, J.M. Perennial Grass and Native Wildflowers: A Synergistic Approach to Habitat Management. Insects 2017, 8, 104. https://doi.org/10.3390/insects8040104
Xavier SS, Olson DM, Coffin AW, Strickland TC, Schmidt JM. Perennial Grass and Native Wildflowers: A Synergistic Approach to Habitat Management. Insects. 2017; 8(4):104. https://doi.org/10.3390/insects8040104
Chicago/Turabian StyleXavier, Shereen S., Dawn M. Olson, Alisa W. Coffin, Timothy C. Strickland, and Jason M. Schmidt. 2017. "Perennial Grass and Native Wildflowers: A Synergistic Approach to Habitat Management" Insects 8, no. 4: 104. https://doi.org/10.3390/insects8040104





