The Heartrate Reaction to Acute Stress in Horned Passalus Beetles (Odontotaenius disjunctus) is Negatively Affected by a Naturally-Occurring Nematode Parasite
Abstract
:1. Introduction
2. Methods
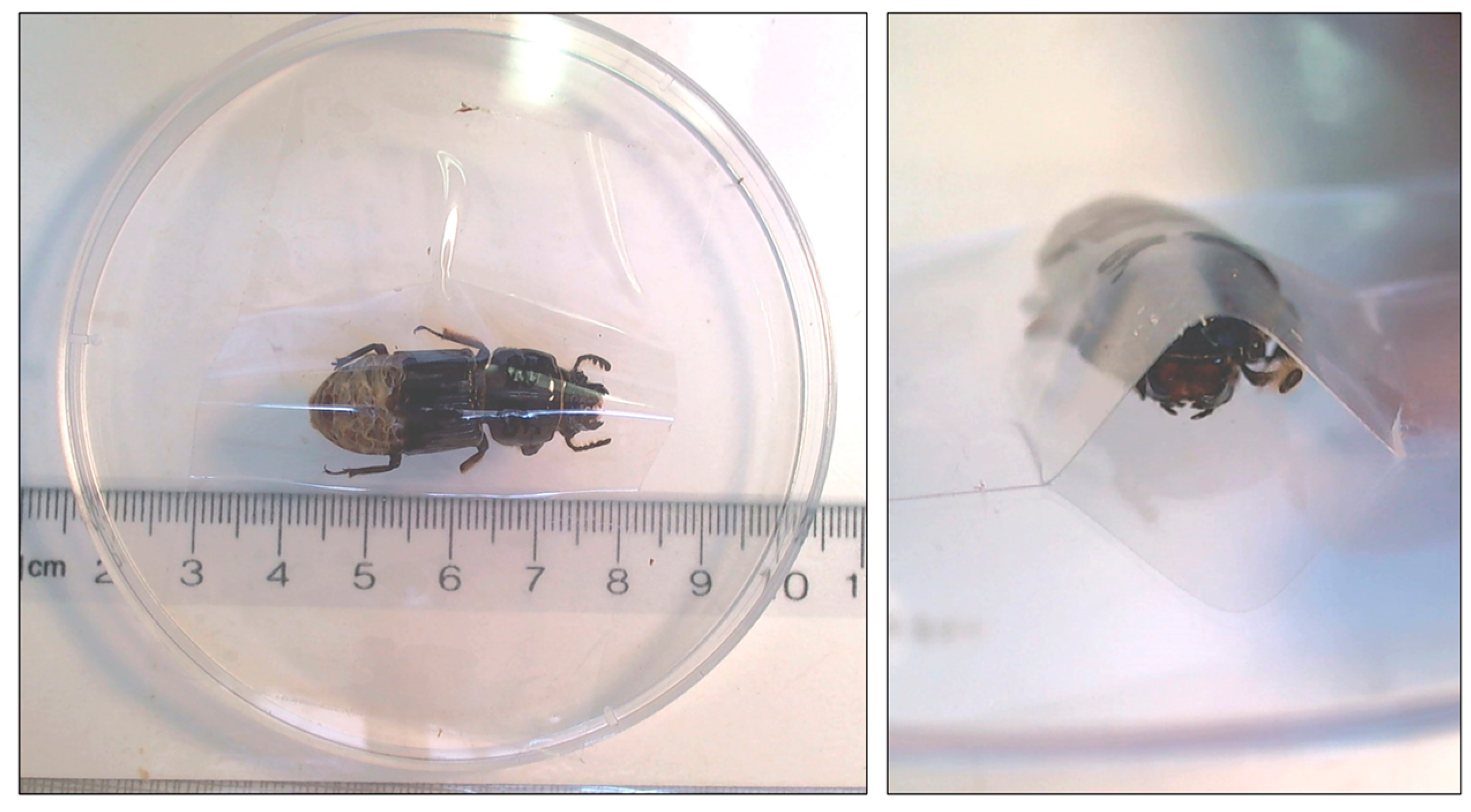
2.1. Beetle Collections
2.2. Measuring Heartrate
2.3. Stress Procedure
2.4. Experiment Goals
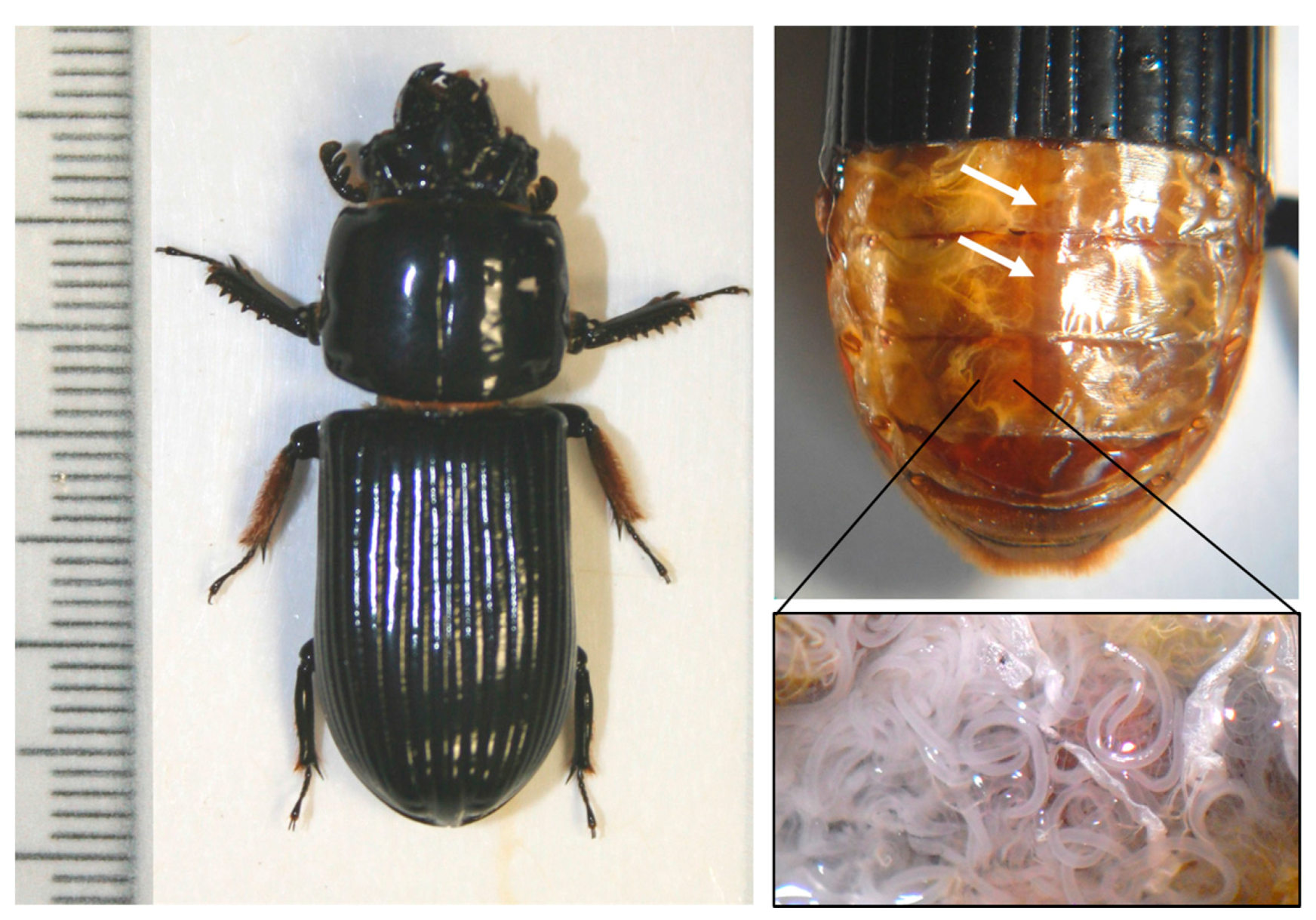
2.5. Parasite Assessment
2.6. Data Analyses
3. Results
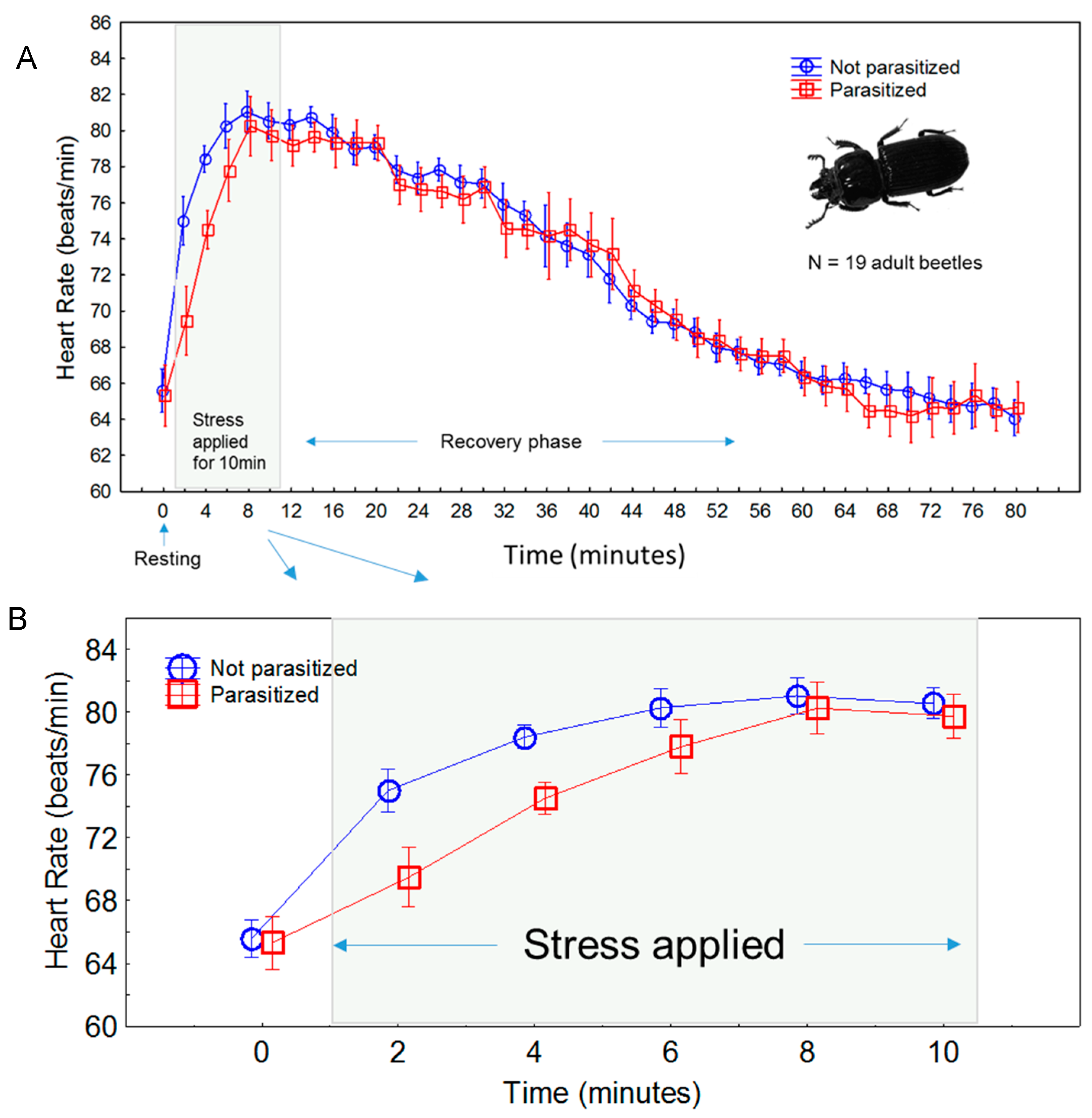
3.1. Comparisons between Experiments
3.2. Time Course of Stress Response
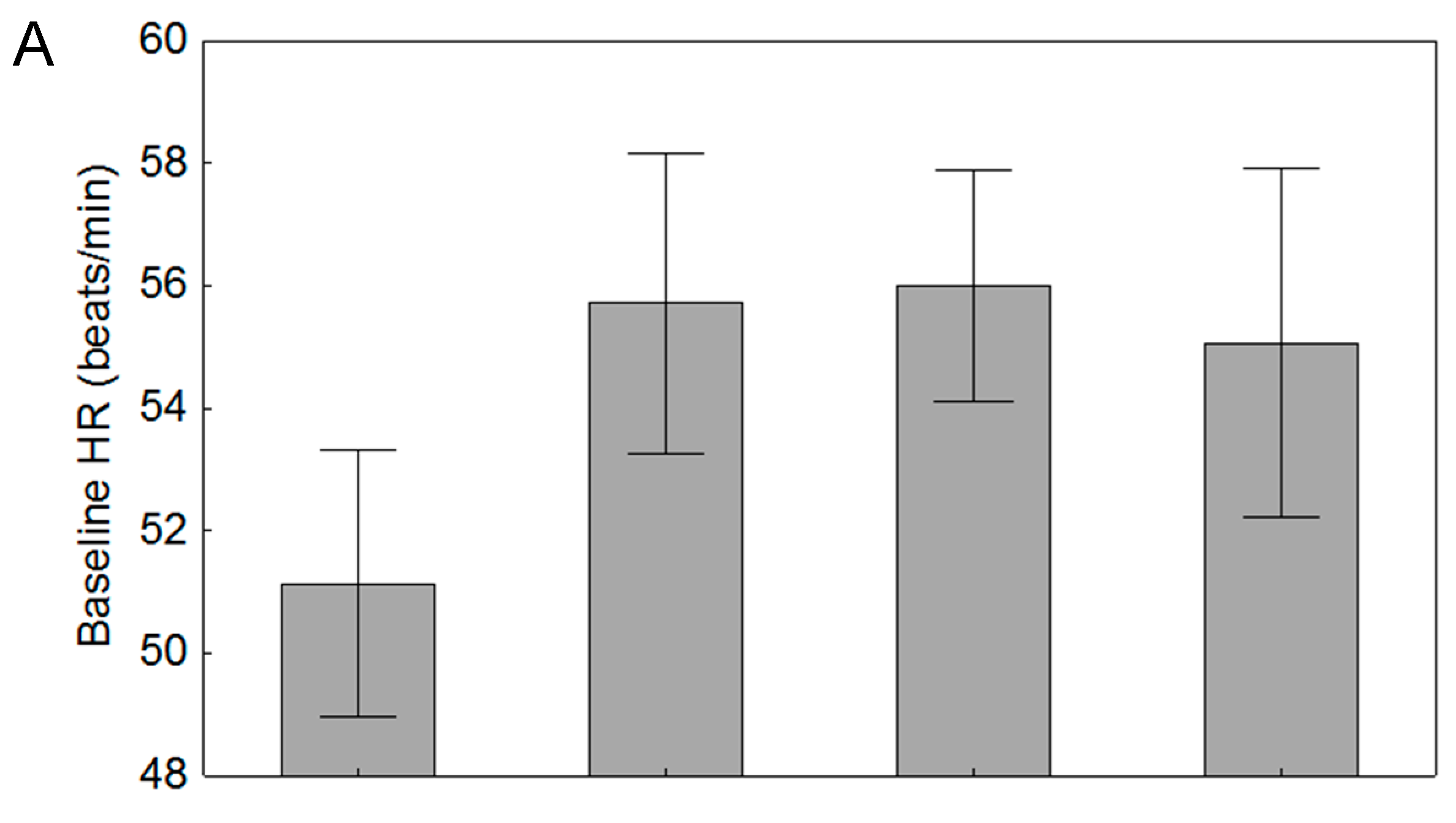
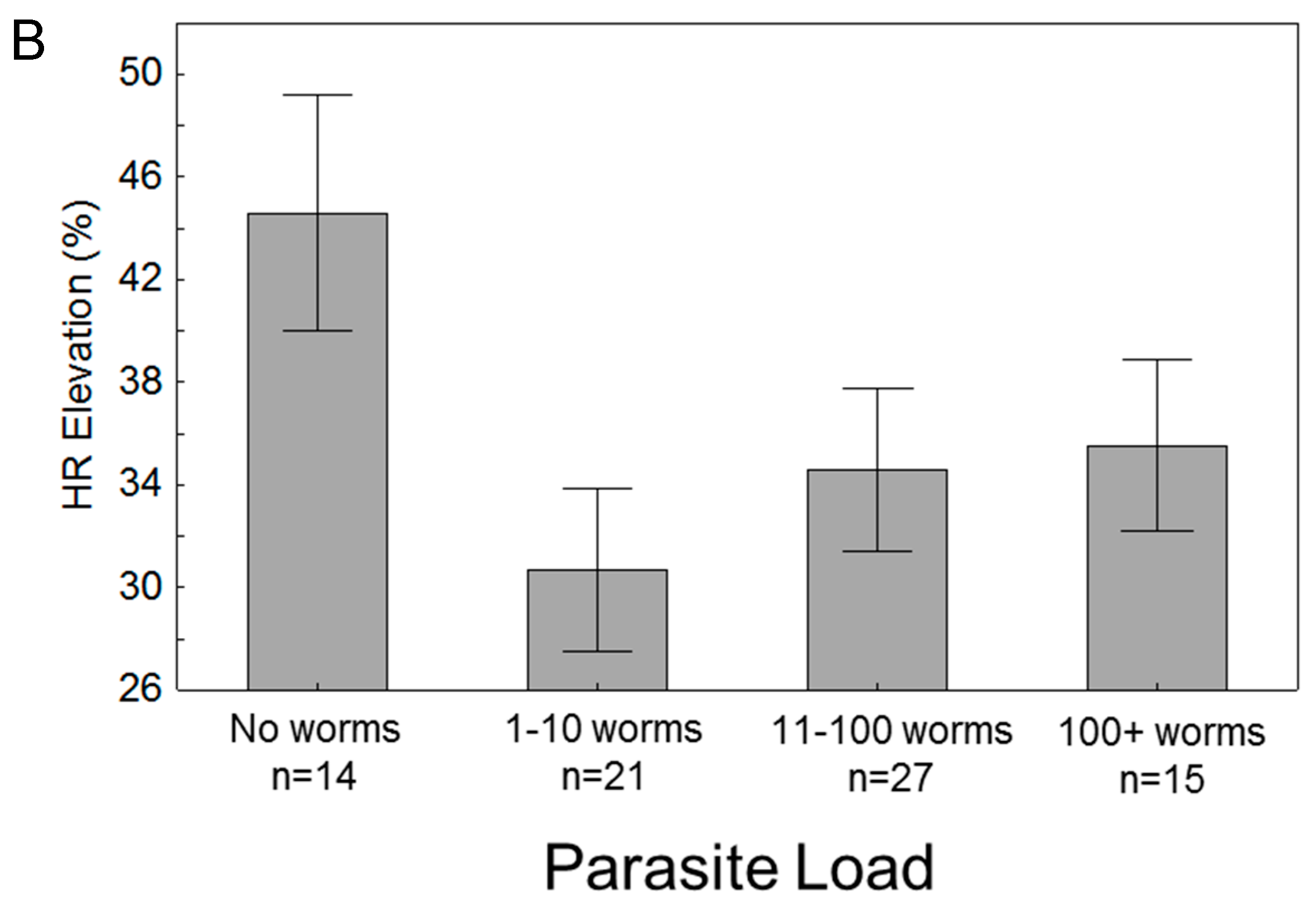
3.3. Effects of Parasitism
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sapolsky, R.M. Why Zebras Don’t Get Ulcers. The Acclaimed Guide to Stress, Stress-Related Diseases, and Coping; Henry Holt and Company: New York, NY, USA, 1994. [Google Scholar]
- Wingfield, J.C.; Romero, L.M. Adrenocortical responses to stress and their modulation in free-living vertebrates. In Handbook of Physiology, Section 7: The Endocrine System, Vol. IV: Coping with the Environment: Neural and Endocrine Mechanisms; McEwen, B.S., Goodman, H.M., Eds.; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Dhabhar, F.S. A hassle a day may keep the doctor away: Stress and the augmentation of immune function. Integr. Comp. Biol. 2002, 42, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Mowlds, P.; Barron, A.; Kavanagh, K. Physical stress primes the immune response of Galleria mellonella larvae to infection by Candida albicans. Microbes Infect. 2008, 10, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Adamo, S.A. Stress responses sculpt the insect immune system, optimizing defense in an ever-changing world. Dev. Comp. Immunol. 2017, 66, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Browne, N.; Surlis, C.; Kavanagh, K. Thermal and physical stresses induce a short-term immune priming effect in Galleria mellonella larvae. J. Insect Physiol. 2014, 63, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Adamo, S.A.; Kovalko, I.; Mosher, B. The behavioural effects of predator-induced stress responses in the cricket (Gryllus texensis): The upside of the stress response. J. Exp. Biol. 2013, 216, 4608–4614. [Google Scholar] [CrossRef] [PubMed]
- Newport, G. On the temperature of insects, and its connection with the functions of respiration and circulation in this class of invertebrate animals. Philos. Trans. R. Soc. Lond. 1837, 127, 259–339. [Google Scholar] [CrossRef]
- Adamo, S.A.; Linn, C.E.; Hoy, R.R. The role of neurohormonal octopamine during fight or flight behavior in the field cricket Gryllus bimaculatus. J. Exp. Biol. 1995, 198, 1691–1700. [Google Scholar] [PubMed]
- Davenport, A.P.; Evans, P.D. Stress-induced changes in the octopamine levels of insect hemolymph. Insect Biochem. 1984, 14, 135–143. [Google Scholar] [CrossRef]
- Hirashima, A.; Ueno, R.; Eto, M. Effects of various stressors on larval growth and whole-body octopamine levels of Tribolium castaneum. Pestic. Biochem. Physiol. 1992, 44, 217–225. [Google Scholar] [CrossRef]
- Harris, J.W.; Woodring, J. Effects of stress, age, season, and source colony on levels of octopamine, dopamine and serotonin in the honey-bee (Apis mellifera L.) brain. J. Insect Physiol. 1992, 38, 29–35. [Google Scholar] [CrossRef]
- Papaefthimiou, C.; Theophilidis, G. Octopamine-A single modulator with double action on the heart of two insect species (Apis mellifera macedonica and Bactrocera oleae): Acceleration vs. inhibition. J. Insect Physiol. 2011, 57, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-P.; Tung, L.-C.; Ming-Chung, L.; Lin, J.-T. The effects of octopamine on the cardiac output of cockroach by using computer-based video analysis on measuring stroke volume. Taiwania 2004, 49, 7–15. [Google Scholar]
- Miller, T. Nervous versus neurohormonal control of insect heartbeat. Am. Zool. 1979, 19, 77–86. [Google Scholar] [CrossRef]
- Pearse, A.S.; Patterson, M.; Rankin, J.S.; Wharton, G.W. The ecology of Passalus cornutus Fabricius, a beetle which lives in rotting logs. Ecol. Monogr. 1936, 6, 456–490. [Google Scholar] [CrossRef]
- Gray, I.E. Observations on the life history of the horned passalus. Am. Midl. Nat. 1946, 35, 728–746. [Google Scholar] [CrossRef]
- Schuster, J.C. Comparative Behavior, Acoustic Signals, and Ecology of New World Passalidae (Coleoptera). Doctoral Thesis, University of Florida, Gainesville, FL, USA, 1975; 127p. [Google Scholar]
- Reinert, J.A. Parasites associated with Popilius disjunctus in South Carolina (Coleoptera: Passalidae). Fla. Entomol. 1973, 56, 273–276. [Google Scholar] [CrossRef]
- Christie, J.R.; Chitwood, B.G. Chondronema passali (Leidy, 1852) n.g. (Nematoda), with notes on its life history. J. Wash. Acad. Sci. 1931, 21, 356–364. [Google Scholar]
- Vasquez, D., Jr.; Willoughby, A.; Davis, A.K. Fighting while parasitized: Can nematode infections affect the outcome of staged combat in beetles? PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Davis, A.K.; Vasquez, D.; LeFeuvre, J.; Sims, S.; Craft, M.; Vizurraga, A. Parasite manipulation of its host’s physiological reaction to acute stress: Experimental results from a natural beetle-nematode system. Physiol. Biochem. Zool. 2017, 90, 273–280. [Google Scholar] [CrossRef] [PubMed]
- LeFeuvre, J.; Davis, A.K. Effects of a naturally-occurring nematode parasite on lifting strength and captivity-related body mass patterns in horned passalus beetles, Odontotaenius disjunctus. Coleopt. Bull. 2015, 69, 1–7. [Google Scholar] [CrossRef]
- Sláma, K. Recording of haemolymph pressure pulsations from the insect body surface. J. Comp. Physiol. 1984, 154, 635–643. [Google Scholar] [CrossRef]
- Tartes, U.; Kuusik, A.; Hiiesaar, K.; Metspalu, L.; Vanatoa, A. Abdominal movements, heartbeats and gas exchange in pupae of the Colorado potato beetle, Leptinotarsa decemlineata. Physiol. Entomol. 2000, 25, 151–158. [Google Scholar] [CrossRef]
- Smits, A.W.; Burggren, W.W.; Oliveras, D. Developmental changes in in vivo cardiac performance in the moth Manduca sexta. J. Exp. Biol. 2000, 203, 369–378. [Google Scholar] [PubMed]
- Chiang, R.G.; Chiang, J.A.; Davey, K.G. A sensory input inhibiting heart-rate in an insect, Rhodnius prolixus. Experientia 1992, 48, 1122–1125. [Google Scholar] [CrossRef]
- Calderon, L.; Davis, A.K. Observations of Steinernema nematode and Tachinid fly parasites in horned passalus beetles, Odontotaenius disjunctus, from Georgia, U.S.A. Comp. Parasitol. 2016, 83, 265–268. [Google Scholar] [CrossRef]
- Burnett, N.P.; Seabra, R.; de Pirro, M.; Wethey, D.S.; Woodin, S.A.; Helmuth, B.; Zippay, M.L.; Sara, G.; Monaco, C.; Lima, F.P. An improved noninvasive method for measuring heartbeat of intertidal animals. Limnol. Oceanogr. Methods 2013, 11, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.K. University of Georgia: Athens, GA, USA, Unpublished work. 2017.
- Adamo, S.A. Why should an immune response activate the stress response? Insights from the insects (the cricket Gryllus texensis). Brain Behav. Immun. 2010, 24, 194–200. [Google Scholar] [PubMed]
- Shutt, D.A.; Smith, A.I.; Wallace, C.A.; Connell, R.; Fell, L.R. Effect of myiasis and acute restraint stress on plasma-levels of immunoreactive beta-endorphin, adrenocorticotropin (ACTH) and cortisol in the sheep. Aust. J. Biol. Sci. 1988, 41, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Guimont, F.O.S.; Wynne-Edwards, K.E. Cortisol responses to acute restraint stress in dwarf hamsters (Phodopus campbelli): Defining baseline concentration and quantifying individual variability. Horm. Behav. 2005, 48, 104. [Google Scholar]
- Olayaki, L.A.; Suleiman, S.O. Repeated acute restraint-induced stress: Effects on body weight, cortisol and reproductive hormones in female wistar rats. Faseb J. 2013, 27, 1. [Google Scholar]
- DuRant, S.E.; Romero, L.M.; Davis, A.K.; Hopkins, W.A. Evidence of ectoparasite-induced endocrine disruption in an imperiled giant salamander, the eastern hellbender (Cryptobranchus alleganiensis). J. Exp. Biol. 2015, 218, 2297–2304. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.D.; Schall, J.J. Hormonal alterations and reproductive inhibition in male fence lizards (Sceloporus occidentalis) infected with the malarial parasite Plasmodium mexicanum. Physiol. Zool. 1995, 68, 608–621. [Google Scholar] [CrossRef]
- Graham, S.P.; Kelehear, C.; Brown, G.P.; Shine, R. Corticosterone-immune interactions during captive stress in invading Australian cane toads (Rhinella marina). Horm. Behav. 2012, 62, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Boughton, R.K.; Atwell, J.W.; Schoech, S.J. An introduced generalist parasite, the sticktight flea (Echidnophaga gallinacea), and its pathology in the threatened Florida scrub-jay (Aphelocoma coerulescens). J. Parasitol. 2006, 92, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Cyr, N.E.; Wikelski, M.; Romero, L.M. Increased energy expenditure but decreased stress responsiveness during molt. Physiol. Biochem. Zool. 2008, 81, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Dickens, M.J.; Delehanty, D.J.; Romero, L.M. Stress and translocation: Alterations in the stress physiology of translocated birds. Proc. R. Soc. B Biol. Sci. 2009, 276, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Gonzalez, J. Territorial behavior of the subsocial beetle Heliscus tropicus under laboratory conditions (Coleoptera, Passalidae). Folia Entomol. Mex. 1986, 70, 53–63. [Google Scholar]
- King, A.; Fashing, N. Infanticidal behavior in the subsocial beetle Odontotaenius disjunctus (Illiger) (Coleoptera: Passalidae). J. Insect Behav. 2007, 20, 527–536. [Google Scholar] [CrossRef]
- Wicknick, J.A.; Miskelly, S.A. Behavioral interactions between non-cohabiting bess beetles, Odontotaenius disjunctus (Illiger) (Coleoptera: Passalidae). Coleopt. Bull. 2009, 63, 108–116. [Google Scholar] [CrossRef]
- Carnevali, L.; Sgoifo, A. Vagal modulation of resting heart rate in rats: The role of stress, psychosocial factors, and physical exercise. Front. Physiol. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cyr, N.E.; Dickens, M.J.; Romero, L.M. Heart rate and heart-rate variability responses to acute and chronic stress in a wild-caught passerine bird. Physiol. Biochem. Zool. 2009, 82, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.; Davis, A.K. Effect of a parasitic nematode, Chondronema passali Leidy (Incertae sedis), on the size and strength of the horned passalus, Odontotaenius disjunctus Illiger (Coleoptera: Passalidae). Coleopt. Bull. 2013, 67, 1–7. [Google Scholar] [CrossRef]
| Heartrate Measurement | First Expt. (19 Reared) | Second Expt. (77 Wild) | Z | p |
|---|---|---|---|---|
| Initial HR (beats/min) | 65.5 (1.7) | 54.9 (10.2) | 4.8 | <0.0001 |
| Max Stressed HR (beats/min) | 81.5 (2.0) | 74.2 (16.1) | 3.3 | 0.001 |
| Max Stressed HR (%) | 24.5 (4.6) | 35.5 (15.9) | −3.4 | <0.001 |
| Baseline Heartrate | ||||
| Predictor | df | MS | F | p |
| Mass | 1 | 0.63 | 0.18 | 0.6815 |
| Parasite | 1 | 0.30 | 0.08 | 0.7776 |
| Error | 15 | 3.57 | ||
| Stress Heartrate | ||||
| Predictor | df | MS | F | p |
| Mass | 1 | 25.45 | 3.97 | 0.0648 |
| Parasite | 1 | 145.00 | 22.64 | 0.0003 |
| Error | 15 | 6.41 | ||
| Time | 4 | 4.16 | 1.78 | 0.1445 |
| Time*Mass | 4 | 5.14 | 2.20 | 0.0796 |
| Time*Parasite | 4 | 16.70 | 7.15 | 0.0001 |
| Error | 60 | 2.33 | ||
| Recovery Heartrate | ||||
| Predictor | df | MS | F | p |
| Mass | 1 | 2.09 | 0.10 | 0.7541 |
| Parasite | 1 | 7.77 | 0.38 | 0.5476 |
| Error | 15 | 20.53 | ||
| Time | 34 | 24.25 | 14.54 | 0.0000 |
| Time*Mass | 34 | 1.82 | 1.09 | 0.3335 |
| Time*Parasite | 34 | 2.31 | 1.38 | 0.0764 |
| Error | 510 | 1.67 | ||
| Heartrate Measurement | Non-Parasitized Beetles (n = 14) | Parasitized Beetles (n = 63) |
|---|---|---|
| Baseline HR (beats/min) | 51.1 (8.1) | 55.7 (10.4) |
| Maximum Stressed HR (beats/min) | 74.3 (16.6) | 74.2 (16.1) |
| Maximum Stressed HR (%) | 44.6 (17.1) | 33.5 (15.0) |
| Baseline Heartrate | ||||
| Predictor | df | MS | F | p |
| Mass (g) | 1 | 25.63 | 0.25 | 0.6185 |
| Parasite | 1 | 205.89 | 2.01 | 0.1605 |
| Error | 74 | 102.46 | ||
| Stress Heartrate | ||||
| Predictor | df | MS | F | p |
| Mass (g) | 1 | 395.66 | 1.69 | 0.1975 |
| Parasite | 1 | 1620.75 | 6.93 | 0.0103 |
| Error | 74 | 233.94 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, A.K.; Coogler, B.; Johnson, I. The Heartrate Reaction to Acute Stress in Horned Passalus Beetles (Odontotaenius disjunctus) is Negatively Affected by a Naturally-Occurring Nematode Parasite. Insects 2017, 8, 110. https://doi.org/10.3390/insects8040110
Davis AK, Coogler B, Johnson I. The Heartrate Reaction to Acute Stress in Horned Passalus Beetles (Odontotaenius disjunctus) is Negatively Affected by a Naturally-Occurring Nematode Parasite. Insects. 2017; 8(4):110. https://doi.org/10.3390/insects8040110
Chicago/Turabian StyleDavis, Andrew K., Brandon Coogler, and Isaac Johnson. 2017. "The Heartrate Reaction to Acute Stress in Horned Passalus Beetles (Odontotaenius disjunctus) is Negatively Affected by a Naturally-Occurring Nematode Parasite" Insects 8, no. 4: 110. https://doi.org/10.3390/insects8040110





