Companion Plants for Aphid Pest Management
Abstract
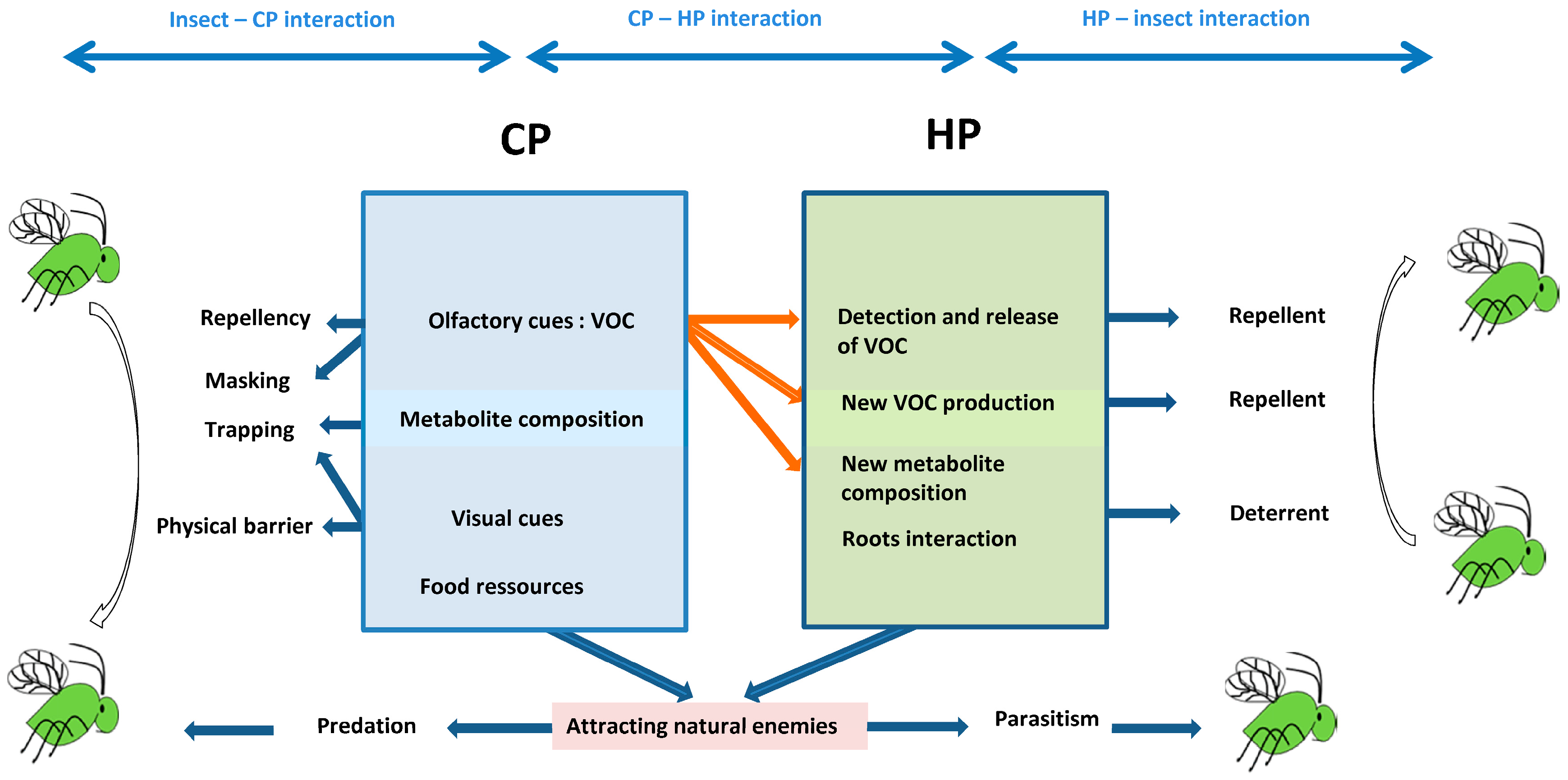
:1. Introduction
2. Trap Plants
3. Companion Plants Altering Host Plant Selection
3.1. Plants Masking Host Plant Odors
3.2. Plants Releasing Repellent Volatiles
3.3. Plants Modifying Host Plant Acceptance
4. Companion Plants Attracting Natural Enemies
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dawson, G.; Griffiths, D.; Merritt, L.; Mudd, A.; Pickett, J.; Wadhams, L.; Woodcock, C. Aphid semiochemicals—A review, and recent advances on the sex pheromone. J. Chem. Ecol. 1990, 16, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A. Aphid ecology: Life cycles, polymorphism, and population regulation. Annu. Rev. Ecol. Syst. 1977, 8, 329–353. [Google Scholar] [CrossRef]
- Dong, M.; Zhang, D.; Du, X. The relationship between aphids and their natural enemies and the ecological management. Acta Phytophylacica Sin. 2011, 38, 327–332. [Google Scholar]
- Blackman, R.; Eastop, V. Aphids on the World’s Crops an Identification and Information Guide; John Wiley & Sons: Oxford, UK, 2000. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Taxonomic issues. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CABI: Wallingford, UK, 2007; pp. 1–30. [Google Scholar]
- Emden, H.; Eastop, V.; Hughes, R.; Way, M. The ecology of Myzus persicae. Annu. Rev. Entomol. 1969, 14, 197–270. [Google Scholar] [CrossRef]
- Dedryver, C.-A.; Le Ralec, A.; Fabre, F. The conflicting relationships between aphids and men: A review of aphid damage and control strategies. C. R. Biol. 2010, 333, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, D.W.; McCornack, B.; Venette, R.; Potter, B.; MacRae, I.V.; Hodgson, E.W.; O’Neal, M.E.; Johnson, K.D.; O’neil, R.; DiFonzo, C. Economic threshold for soybean aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2007, 100, 1258–1267. [Google Scholar] [CrossRef] [PubMed]
- Devonshire, A.L.; Field, L.M.; Foster, S.P.; Moores, G.D.; Williamson, M.S.; Blackman, R.L. The evolution of insecticide resistance in the peach-potato aphid, Myzus persicae. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1998, 353, 1677–1684. [Google Scholar] [CrossRef]
- Foster, S.P.; Harrington, R.; Dewar, A.M.; Denholm, I.; Devonshire, A.L. Temporal and spatial dynamics of insecticide resistance in Myzus persicae (Hemiptera: Aphididae). Pest Manag. Sci. 2002, 58, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Dent, D.R. Priorities in Biopesticide Research and Development in Developing Countries; CABI: Wallingford, UK, 2000. [Google Scholar]
- Yano, E. Ecological considerations for biological control of aphids in protected culture. Popul. Ecol. 2006, 48, 333–339. [Google Scholar] [CrossRef]
- Rousselin, A.; Bevacqua, D.; Sauge, M.-H.; Lescourret, F.; Mody, K.; Jordan, M.-O. Harnessing the aphid life cycle to reduce insecticide reliance in apple and peach orchards. A review. Agron. Sustain. Dev. 2017, 37, 38. [Google Scholar] [CrossRef]
- Andrews, D.; Kassam, A. The Importance of Multiple Cropping in Increasing World Food Supplies; American Society of Agronomy & Crop Science Society of America: Madison, WI, USA; Soil Science Society of America: Fitchburg, WI, USA, 1976. [Google Scholar]
- Lithourgidis, A.; Dordas, C.; Damalas, C.; Vlachostergios, D. Annual intercrops: An alternative pathway for sustainable agriculture. Aust. J. Crop Sci. 2011, 5, 396. [Google Scholar]
- Lopes, T.; Hatt, S.; Xu, Q.; Chen, J.; Liu, Y.; Francis, F. Wheat (Triticum aestivum L.)-based intercropping systems for biological pest control. Pest Manag. Sci. 2016, 72, 2193–2202. [Google Scholar] [CrossRef] [PubMed]
- Vandermeer, J.H. The Ecology of Intercropping; Cambridge University Press: Cambridge, UK, 1992. [Google Scholar]
- Altieri, M.; Nicholls, C. Biodiversity and Pest Management in Agroecosystems; CRC Press: New York, NY, USA, 2004; p. 185. [Google Scholar]
- Parker, J.E.; Snyder, W.E.; Hamilton, G.C.; Rodriguez-Saona, C. Companion planting and insect pest control. In Weed and Pest Control—Conventional and New Challenges; Soloneski, S., Larramendy, M., Eds.; InTech: Rijeka, Croatia, 2013; pp. 1–30. [Google Scholar]
- Cunningham, S.J. Great Garden Companions: A Companion Planting System for a Beautiful, Chemical-Free Vegetable Garden; Rodale Books: Emmaus, PA, USA, 1998. [Google Scholar]
- Sarker, P.; Rahman, M.; Das, B. Effect of intercropping with mustard with onion and garlic on aphid population and yield. J. Bio-Sci. 2009, 15, 35–40. [Google Scholar]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.; Jones, H.G.; Karley, A.J. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malézieux, E.; Crozat, Y.; Dupraz, C.; Laurans, M.; Makowski, D.; Ozier-Lafontaine, H.; Rapidel, B.; De Tourdonnet, S.; Valantin-Morison, M. Mixing plant species in cropping systems: Concepts, tools and models. A review. Agron. Sustain. Dev. 2009, 29, 43–62. [Google Scholar] [CrossRef]
- Moreno, C.R.; Racelis, A.E. Attraction, repellence, and predation: Role of companion plants in regulating Myzus persicae (Sulzer) (Hemiptera: Aphidae) in organic kale systems of south Texas. Southwest. Entomol. 2015, 40, 1–14. [Google Scholar] [CrossRef]
- Collier, R.H.; Finch, S. The Effect of Increased Crop Diversity on Colonisation by Pest Insects of Brassica Crops; British Crop Protection Council: Farnham, UK, 2003; pp. 439–444. [Google Scholar]
- Parolin, P.; Bresch, C.; Desneux, N.; Brun, R.; Bout, A.; Boll, R.; Poncet, C. Secondary plants used in biological control: A review. Int. J. Pest Manag. 2012, 58, 91–100. [Google Scholar] [CrossRef]
- Gadgil, M.; Berkes, F.; Folke, C. Indigenous knowledge for biodiversity conservation. Ambio 1993, 22, 151–156. [Google Scholar]
- Morales, H.; Perfecto, I. Traditional knowledge and pest management in the guatemalan highlands. Agric. Hum. Values 2000, 17, 49–63. [Google Scholar] [CrossRef]
- Banks, J.E.; Ekbom, B. Modelling herbivore movement and colonization: Pest management potential of intercropping and trap cropping. Agric. For. Entomol. 1999, 1, 165–170. [Google Scholar] [CrossRef]
- Hurej, M. Trap plants and their application in plant protection against pests. Prog. Plant Prot. 2000, 40, 249–253. [Google Scholar]
- Bruce, T.J.A.; Birkett, M.A.; Blande, J.; Hooper, A.M.; Martin, J.L.; Khambay, B.; Prosser, I.; Smart, L.E.; Wadhams, L.J. Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest Manag. Sci. 2005, 61, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T.; Kessler, A.; Halitschke, R. Volatile signaling in plant-plant-herbivore interactions: What is real? Curr. Opin. Plant Biol. 2002, 5, 351–354. [Google Scholar] [CrossRef]
- Balmer, O.; Géneau, C.E.; Belz, E.; Weishaupt, B.; Förderer, G.; Moos, S.; Ditner, N.; Juric, I.; Luka, H. Wildflower companion plants increase pest parasitation and yield in cabbage fields: Experimental demonstration and call for caution. Biol. Control 2014, 76, 19–27. [Google Scholar] [CrossRef]
- Lu, Z.X.; Zhu, P.Y.; Gurr, G.M.; Zheng, X.S.; Read, D.M.; Heong, K.L.; Yang, Y.J.; Xu, H.X. Mechanisms for flowering plants to benefit arthropod natural enemies of insect pests: Prospects for enhanced use in agriculture. Insect Sci. 2014, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Mayse, M.A. Culture control in crop fields: A habitat management technique. Environ. Manag. 1983, 7, 15–22. [Google Scholar] [CrossRef]
- Hokkanen, H.M.T. Trap cropping in pest management. Annu. Rev. Entomol. 1991, 36, 119–138. [Google Scholar] [CrossRef]
- Hooks, C.R.R.; Johnson, M.W. Impact of agricultural diversification on the insect community of cruciferous crops. Crop Prot. 2003, 22, 223–238. [Google Scholar] [CrossRef]
- Shelton, A.; Badenes-Perez, F. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Talekar, N.; Shelton, A. Biology, ecology, and management of the diamondback moth. Annu. Rev. Entomol. 1993, 38, 275–301. [Google Scholar] [CrossRef]
- Foster, S.; Harris, M. Behavioral manipulation methods for insect pest-management. Annu. Rev. Entomol. 1997, 42, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.; Wadhams, L.; Woodcock, C. Developing sustainable pest control from chemical ecology. Agric. Ecosyst. Environ. 1997, 64, 149–156. [Google Scholar] [CrossRef]
- Tillman, P.G.; Khrimian, A.; Cottrell, T.E.; Lou, X.; Mizell, R.F.; Johnson, C.J. Trap cropping systems and a physical barrier for suppression of stink bugs (Hemiptera: Pentatomidae) in cotton. J. Econ. Entomol. 2015, 108, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Hassanali, A.; Herren, H.; Khan, Z.R.; Pickett, J.A.; Woodcock, C.M. Integrated pest management: The push–pull approach for controlling insect pests and weeds of cereals, and its potential for other agricultural systems including animal husbandry. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Atsatt, P.R.; O’Dowd, D.J. Plant defense guilds. Science 1976, 193, 24. [Google Scholar] [CrossRef] [PubMed]
- Shelton, A.; Nault, B. Dead-end trap cropping: A technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 2004, 23, 497–503. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Dively, G.; Pote, J.M.; Zinati, G.; Mathews, C. Identifying a potential trap crop for a novel insect pest, Halyomorpha halys (Hemiptera: Pentatomidae), in organic farms. Environ. Entomol. 2016, 45, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Krishna Moorthy, P. Development and Adoption of Integrated Pest Management for Major Pests of Cabbage Using Indian Mustard as a Trap Crop. In Proceedings of the second international workshop on management of Diamondback moth and other crucifer pests, Tainan, Taiwan, 10–14 December 1992; pp. 511–521. [Google Scholar]
- Rousse, P.; Fournet, S.; Porteneuve, C.; Brunel, E. Trap cropping to control Delia radicum populations in cruciferous crops: First results and future applications. Entomol. Exp. Appl. 2003, 109, 133–138. [Google Scholar] [CrossRef]
- Potting, R.P.J.; Perry, J.N.; Powell, W. Insect behavioural ecology and other factors affecting the control efficacy of agro-ecosystem diversification strategies. Ecol. Model. 2005, 182, 199–216. [Google Scholar] [CrossRef]
- Vargas, R.R.; Troncoso, A.J.; Tapia, D.H.; Olivares-Donoso, R.; Niemeyer, H.M. Behavioural differences during host selection between alate virginoparae of generalist and tobacco-specialist Myzus persicae. Entomol. Exp. Appl. 2005, 116, 43–53. [Google Scholar] [CrossRef]
- Hussein, M.; Samad, N.A. Intercropping chilli with maize or brinjal to suppress populations of Aphis gossypii Glov., and transmission of chilli viruses. Int. J. Pest Manag. 1993, 39, 216–222. [Google Scholar] [CrossRef]
- DiFonzo, C.; Ragsdale, D.; Radcliffe, E.; Gudmestad, N.; Secor, G. Crop borders reduce potato virus Y incidence in seed potato. Ann. Appl. Biol. 1996, 129, 289–302. [Google Scholar] [CrossRef]
- Finch, S.; Collier, R. Host-plant selection by insects–a theory based on ‘appropriate/inappropriate landings’ by pest insects of cruciferous plants. Entomol. Exp. Appl. 2000, 96, 91–102. [Google Scholar] [CrossRef]
- Andersson, M. The effects of non-host volatiles on habitat location by phytophagous insects. In Introductory Paper at the Faculty of Landscape Planning, Horticulture and Agricultural Science, Swedish University of Agricultural Sciences; Alnap: London, UK, 2007; pp. 1–38. [Google Scholar]
- Döring, T.F. How aphids find their host plants, and how they don’t. Ann. Appl. Biol. 2014, 165, 3–26. [Google Scholar] [CrossRef]
- Döring, T.; Röhrig, K. Behavioural response of winged aphids to visual contrasts in the field. Ann. Appl. Biol. 2016, 168, 421–434. [Google Scholar] [CrossRef]
- Dethier, V.; Browne, B.L. The designation of chemicals in terms of the responses they elicit from insects1. J. Econ. Entomol. 1960, 53, 134–136. [Google Scholar] [CrossRef]
- Månsson, P.E. Host Selection and Antifeedants in Hylobius Abietis Pine Weevils; Alnap: London, UK, 2005; Volume 16. [Google Scholar]
- Amarawardana, L.; Bandara, P.; Kumar, V.; Pettersson, J.; Ninkovic, V.; Glinwood, R. Olfactory response of Myzus persicae (Hemiptera: Aphididae) to volatiles from leek and chive: Potential for intercropping with sweet pepper. Acta Agric. Scand. B 2007, 57, 87–91. [Google Scholar]
- Jankowska, B.; Poniedziaek, M.; Jedrszczyk, E. Effect of intercropping white cabbage with french marigold (Tagetes patula nana L.) and pot marigold (Calendula officinalis l.) on the colonization of plants by pest insects. Folia Hortic. 2009, 21, 95–103. [Google Scholar] [CrossRef]
- Lai, R.; You, M.; Lotz, L.; Vasseur, L. Response of green peach aphids and other arthropods to garlic intercropped with tobacco. Agron. J. 2011, 103, 856–863. [Google Scholar] [CrossRef]
- Potts, M.J.; Gunadi, N. The influence of intercropping with allium on some insect populations in potato (Solatium tuberosum). Ann. Appl. Biol. 1991, 119, 207–213. [Google Scholar] [CrossRef]
- Ben Issa, R.; Gautier, H.; Gomez, L. Influence of neighbouring companion plants on the performance of aphid populations on sweet pepper plants under greenhouse conditions. Agric. For. Entomol. 2017, 19, 181–191. [Google Scholar] [CrossRef]
- Mutiga, S.K.; Gohole, L.S.; Auma, E.O. Effects of integrating companion cropping and nitrogen application on the performance and infestation of collards by Brevicoryne brassicae. Entomol. Exp. Appl. 2010, 134, 234–244. [Google Scholar] [CrossRef]
- Broad, S.T.; Schellhorn, N.A.; Lisson, S.N.; Mendham, N.J.; Corkrey, R. Host location and parasitism of Brevicoryne brassicae in diversified broccoli cropping systems. Entomol. Exp. Appl. 2008, 129, 166–171. [Google Scholar] [CrossRef]
- Basedow, T.; Hua, L.; Aggarwal, N. The infestation of Vicia faba L. (Fabaceae) by Aphis fabae (Scop.) (Hemiptera: Aphididae) under the influence of Lamiaceae (Ocimum basilicum L. And Satureja hortensis L.). J. Pest Sci. 2006, 79, 149–154. [Google Scholar] [CrossRef]
- Beizhou, S.; Jie, Z.; Jinghui, H.; Hongying, W.; Yun, K.; Yuncong, Y. Temporal dynamics of the arthropod community in pear orchards intercropped with aromatic plants. Pest Manag. Sci. 2010, 67, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Yang, B. Effects of Tagetes patula mixed planted with Rosa chinensis on Macrosiphum rosirvorum Zhang. Hubei Agric. Sci. 2011, 8, 25. [Google Scholar]
- Glinwood, R.; Ninkovic, V.; Pettersson, J.; Ahmed, E. Barley exposed to aerial allelopathy from thistles (Cirsium spp.) becomes less acceptable to aphids. Ecol. Entomol. 2004, 29, 188–195. [Google Scholar] [CrossRef]
- Tahvanainen, J.O.; Root, R.B. The influence of vegetational diversity on the population ecology of a specialized herbivore, Phyllotreta cruciferae (Coleoptera: Chrysomelidae). Oecologia 1972, 10, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Perrin, R.; Phillips, M. Some effects of mixed cropping on the population dynamics of insect pests. Entomol. Exp. Appl. 1978, 24, 585–593. [Google Scholar] [CrossRef]
- Thiery, D.; Visser, J.H. Masking of host plant odour in the olfactory orientation of the colorado potato beetle. Entomol. Exp. Appl. 1986, 41, 165–172. [Google Scholar] [CrossRef]
- Hardie, J.; Isaacs, R.; Pickett, J.; Wadhams, L.; Woodcock, C. Methyl salicylate and (−)-(1r,5s)-myrtenal are plant-derived repellents for black bean aphid, Aphis fabae Scop (Hemiptera: Aphididae). J. Chem. Ecol. 1994, 20, 2847–2855. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.; Pickett, J.; Pye, B.; Quiroz, A.; Smart, L.; Wadhams, L.; Woodcock, C. Winter host component reduces colonization by bird-cherry-oat aphid, Rhopalosiphum padi (L.) (Hemiptera: Aphididae), and other aphids in cereal fields. J. Chem. Ecol. 1994, 20, 2565–2574. [Google Scholar] [CrossRef] [PubMed]
- Hori, M. The effects of rosemary and ginger oils on the alighting behavior of Myzus persicae (Sulzer) (Hemiptera: Aphididae) and on the incidence of yellow spotted streak. Appl. Entomol. Zool. 1999, 34, 351–358. [Google Scholar] [CrossRef]
- Visser, J.; Avé, D. General green leaf volatiles in the olfactory orientation of the colorado beetle, Leptinotarsa decemlineata. Entomol. Exp. Appl. 1978, 24, 738–749. [Google Scholar] [CrossRef]
- Yamasaki, T.; Sato, M.; Sakoguchi, H. (-)-germacrene d: Masking substance of attractants for the cerambycid beetle, Monochamus alternatus (Hope). Appl. Entomol. Zool. 1997, 32, 423–429. [Google Scholar] [CrossRef]
- Schröder, R.; Hilker, M. The relevance of background odor in resource location by insects: A behavioral approach. BioScience 2008, 58, 308–316. [Google Scholar] [CrossRef]
- Hori, M. Repellency of rosemary oil against Myzus persicae in a laboratory and in a screenhouse. J. Chem. Ecol. 1998, 24, 1425–1432. [Google Scholar] [CrossRef]
- Nottingham, S.F.; Hardie, J.; Dawson, G.W.; Hick, A.J.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Behavioral and electrophysiological responses of aphids to host and nonhost plant volatiles. J. Chem. Ecol. 1991, 17, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Zaka, S.M.; Zeng, X.N.; Holford, P.; Beattie, G.A.C. Repellent effect of guava leaf volatiles on settlement of adults of Citrus psylla, Diaphorina citri Kuwayama, on citrus. Insect Sci. 2010, 17, 39–45. [Google Scholar] [CrossRef]
- Ben Issa, R.; Gautier, H.; Costagliola, G.; Gomez, L. Which companion plants affect the performance of green peach aphid on host plants? Testing of 12 candidate plants under laboratory conditions. Entomol. Exp. Appl. 2016, 160, 164–178. [Google Scholar] [CrossRef]
- Duchamp-Viret, P.; Duchamp, A.; Chaput, M.A. Single olfactory sensory neurons simultaneously integrate the components of an odour mixture. Eur. J. Neurosci. 2003, 18, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Wadhams, L.J.; Woodcock, C.M. Insect host location: A volatile situation. Trends Plant Sci. 2005, 10, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.; Collier, R.H. The influence of host and non-host companion plants on the behaviour of pest insects in field crops. Entomol. Exp. Appl. 2012, 142, 84–96. [Google Scholar] [CrossRef]
- Visser, J.H.; Piron, P.G.M.; Hardie, J. The aphids' peripheral perception of plant volatiles. Entomol. Exp. Appl. 1996, 80, 35–38. [Google Scholar] [CrossRef]
- Pickett, J.; Wadhams, L.; Woodcock, C.; Hardie, J. The chemical ecology of aphids. Annu. Rev. Entomol. 1992, 37, 67–90. [Google Scholar] [CrossRef]
- Dancewicz, K.; Gabry, B. Effect of extracts of garlic (Allium sativum L.), wormwood (Artemisia absinthium L.) and tansy (Tanaceum vulgare l.) on the behaviour of the peach potato aphid Myzus persicae (Sulz.) during the settling on plants. Pestycydy 2008, 3–4, 93–99. [Google Scholar]
- Ikeura, H.; Kobayashi, F.; Hayata, Y. Repellent effect of herb extracts on the population of wingless green peach aphid, Myzus persicae Sulzer (Hemiptera: Aphididae). J. Agric. Sci. 2012, 4, 139–144. [Google Scholar] [CrossRef]
- Storer, J.R.; Powell, G.; Hardie, J. Settling responses of aphids in air permeated with non-host plant volatiles. Entomol. Exp. Appl. 1996, 80, 76–78. [Google Scholar] [CrossRef]
- Pettersson, J. Studies on Rhopalosiphum padi (L.). I. Laboratory studies on olfactometric responses to the winter host Prunus padus L. Upps. Lantbrukshogsk Ann. 1970, 36, 381–399. [Google Scholar]
- Pettersson, J. Olfactory reactions of Brevicoryne brassicae (L.) (Hemiptera: Aphididae). Swed. J. Agric. Res. 1973, 3, 95–103. [Google Scholar]
- Agelopoulos, N.G.; Hooper, A.M.; Maniar, S.P.; Pickett, J.A.; Wadhams, L.J. A novel approach for isolation of volatile chemicals released by individual leaves of a plant in situ. J. Chem. Ecol. 1999, 25, 1411–1425. [Google Scholar] [CrossRef]
- Ben Issa, R.; Gomez, L.; Sauge, M.; Gautier, H. Effects of companion plants on the behavior of the green peach aphid reread on pepper plants. IOBC-WPRS Bull. 2012, 75, 29–33. [Google Scholar]
- Kogel, W.J.; Visser, J.; Tol, R. Repellent odours to protect crops from aphids. Crop Prot. 2000, 6, 42–44. [Google Scholar]
- Dardouri, T.G.H.; Costagliola, G.; Gomez, L. How french marigold (Tagetes patula L.) volatiles can affect the performance of green peach aphid. IOBC-WPRS Bull. 2017, 123, 71–78. [Google Scholar]
- Uvah, I.; Coaker, T. Effect of mixed cropping on some insect pests of carrots and onions. Entomol. Exp. Appl. 1984, 36, 159–167. [Google Scholar] [CrossRef]
- Shirey, R.E. SPME commercial devices and fibre coatings. In Handbook of Solid Phase Microextraction; Pawliszyn, J., Ed.; Chemical Industry Press: Waterloo, ON, Canada, 2009; pp. 87–115. [Google Scholar]
- Visser, J.H.; Piron, P.G.M. Odour response profiles in aphids differentiating for species, clone, form and food. In Proceedings of the 8th Meeting of Experimental and Applied Entomologists, Amsterdam, The Netherlands, 13 December 1996; pp. 115–120. [Google Scholar]
- Nottingham, S.F.; Hardie, J.I.M. Flight behaviour of the black bean aphid, Aphis fabae, and the cabbage aphid, Brevicoryne brassicae, in host and non-host plant odour. Physiol. Entomol. 1993, 18, 389–394. [Google Scholar] [CrossRef]
- Glinwood, R.; Ninkovic, V.; Pettersson, J. Chemical interaction between undamaged plants – effects on herbivores and natural enemies. Phytochemistry 2011, 72, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K. Multiple functions of inducible plant volatiles. Trends Plant Sci. 2004, 9, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Ferrer, J.L.; Ljung, K.; Pojer, F.; Hong, F.; Long, J.A.; Li, L.; Moreno, J.E.; Bowman, M.E.; Ivans, L.J. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 2008, 133, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy; Academic Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Reigosa, M.J.; Sánchez-Moreiras, A.; González, L. Ecophysiological approach in allelopathy. Crit. Rev. Plant Sci. 1999, 18, 577–608. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathy in agroecosystems. J. Crop Prod. 2001, 4, 1–41. [Google Scholar] [CrossRef]
- Ninkovic, V. Volatile communication between barley plants affects biomass allocation. J. Exp. Bot. 2003, 54, 1931. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.; Aarssen, L. Plants helping plants. BioScience 1988, 38, 34–40. [Google Scholar] [CrossRef]
- Chon, S.U.; Nelson, C. Allelopathy in Compositae plants. A review. Agron. Sustain. Dev. 2010, 30, 349–358. [Google Scholar] [CrossRef]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H.M. The role of allelopathy in agricultural pest management. Pest Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Novoplansky, A. Picking battles wisely: Plant behaviour under competition. Plant Cell Environ. 2009, 32, 726–741. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Hilker, M. Induced plant defences: From molecular biology to evolutionary ecology. Basic Appl. Ecol. 2003, 4, 3–14. [Google Scholar] [CrossRef]
- Choh, Y.; Shimoda, T.; Ozawa, R.; Dicke, M.; Takabayashi, J. Exposure of lima bean leaves to volatiles from herbivore-induced conspecific plants results in emission of carnivore attractants: Active or passive process? J. Chem. Ecol. 2004, 30, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.I.; Ozawa, R.; Shimoda, T.; Nishioka, T.; Boland, W.; Takabayashi, J. Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 2000, 406, 512–515. [Google Scholar] [PubMed]
- Tiroesele, B.; Matshela, O. The effect of companion planting on the abundance of cabbage aphid, Brevicoryne brassicae L., on kale (Brassica oleracea var. Acephala). J. Plant Pest. Sci. 2015, 2, 57–65. [Google Scholar]
- Farmer, E.E.; Ryan, C.A. Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. USA 1990, 87, 7713–7716. [Google Scholar] [CrossRef] [PubMed]
- Heil, M.; Silva Bueno, J.C. From the cover: Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Sci. Signal. 2007, 104, 5467. [Google Scholar]
- Khan, Z.R.; Pickett, J.A. The ‘push-pull’ strategy for stemborer management: A case study in exploiting biodiversity and chemical ecology. In Ecological Engineering for Pest Management: Advances in Habitat Manipulations for Arthropods; CSIRO Publishing: Clayton, Australia, 2004; pp. 155–164. [Google Scholar]
- Heil, M.; Karban, R. Explaining evolution of plant communication by airborne signals. Trends Ecol. Evol. 2010, 25, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Renwick, J. Chemical ecology of oviposition in phytophagous insects. Cell. Mol. Life Sci. 1989, 45, 223–228. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.R.; Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-H.; Schlyter, F. Redundancy, synergism, and active inhibitory range of non-host volatiles in reducing pheromone attraction in european spruce bark beetle Ips typographus. Oikos 2003, 101, 299–310. [Google Scholar] [CrossRef]
- Karban, R.; Baldwin, I.; Baxter, K.; Laue, G.; Felton, G. Communication between plants: Induced resistance in wild tobacco plants following clipping of neighboring sagebrush. Oecologia 2000, 125, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, S.Y.; Birkett, M.A.; Loza-Reyes, E.; Martin, J.L.; Pye, B.J.; Smart, L.E.; Hardie, J.; Pickett, J.A. Activation of defence in sweet pepper, Capsicum annum, by cis-jasmone, and its impact on aphid and aphid parasitoid behaviour. Pest Manag. Sci. 2012, 68, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.A.; Campbell, C.A.M.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef] [PubMed]
- Slesak, E.; Slesak, M.; Gabrys, B. Effect of methyl jasmonate on hydroxamic acid content, protease activity, and bird cherry–oat aphid Rhopalosiphum padi (L.) probing behavior. J. Chem. Ecol. 2001, 27, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Karban, R.; Maron, J.; Felton, G.W.; Ervin, G.; Eichenseer, H. Herbivore damage to sagebrush induces resistance in wild tobacco: Evidence for eavesdropping between plants. Oikos 2003, 100, 325–332. [Google Scholar] [CrossRef]
- Glinwood, R.; Pettersson, J.; Ahmed, E.; Ninkovic, V.; Birkett, M.; Pickett, J. Change in acceptability of barley plants to aphids after exposure to allelochemicals from couch-grass (Elytrigia repens). J. Chem. Ecol. 2003, 29, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Ninkovic, V.; Glinwood, R.; Dahlin, I. Weed–barley interactions affect plant acceptance by aphids in laboratory and field experiments. Entomol. Exp. Appl. 2009, 133, 38–45. [Google Scholar] [CrossRef]
- Himanen, S.J.; Blande, J.D.; Klemola, T.; Pulkkinen, J.; Heijari, J.; Holopainen, J.K. Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants—A mechanism for associational herbivore resistance? New Phytol. 2010, 186, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Tosh, C.R.; Powell, G.; Hardie, J. Maternal reproductive decisions are independent of feeding in the black bean aphid, Aphis fabae. J. Insect Physiol. 2002, 48, 619–629. [Google Scholar] [CrossRef]
- Wilby, A.; Thomas, M.B. Natural enemy diversity and pest control: Patterns of pest emergence with agricultural intensification. Ecol. Lett. 2002, 5, 353–360. [Google Scholar] [CrossRef]
- Frank, S.D. Biological control of arthropod pests using banker plant systems: Past progress and future directions. Biol. Control 2010, 52, 8–16. [Google Scholar] [CrossRef]
- Song, B.; Wu, H.; Kong, Y.; Zhang, J.; Du, Y.; Hu, J.; Yao, Y. Effects of intercropping with aromatic plants on the diversity and structure of an arthropod community in a pear orchard. BioControl 2010, 55, 741–751. [Google Scholar] [CrossRef]
- Miñarro, M.; Hemptinne, J.-L.; Dapena, E. Colonization of apple orchards by predators of Dysaphis plantaginea: Sequential arrival, response to prey abundance and consequences for biological control. BioControl 2005, 50, 403–414. [Google Scholar] [CrossRef]
- Dib, H.; Ben-Issa, R.; Sauphanor, B.; Capowiez, Y. Feasibility and efficacy of a new approach for controlling populations of the rosy apple aphid, Dysaphis plantaginea Passerini (Hemiptera: Aphididae) in south-eastern France. Int. J. Pest Manag. 2017, 63, 128–137. [Google Scholar] [CrossRef]
- Ferguson, K.I.; Stiling, P. Non-additive effects of multiple natural enemies on aphid populations. Oecologia 1996, 108, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.-C.; Stoeckel, S.; Tagu, D. Evolutionary and functional insights into reproductive strategies of aphids. C. R. Biol. 2010, 333, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ehler, L. Conservation biological control: Past, present, and future. In Conservation Biological Control; Academic Press: Cambridge, MA, USA, 1998; pp. 1–8. [Google Scholar]
- Van Lenteren, J.C. Implementation of biological control. Am. J. Altern. Agric. 1988, 3, 102–109. [Google Scholar] [CrossRef]
- Begg, G.S.; Cook, S.M.; Dye, R.; Ferrante, M.; Franck, P.; Lavigne, C.; Lövei, G.L.; Mansion-Vaquie, A.; Pell, J.K.; Petit, S. A functional overview of conservation biological control. Crop Prot. 2017, 97, 145–158. [Google Scholar] [CrossRef]
- Andow, D.A. Vegetational diversity and arthropod population response. Annu. Rev. Entomol. 1991, 36, 561–586. [Google Scholar] [CrossRef]
- Bugg, R.L.; Dutcher, J.D.; McNeill, P.J. Cool-season cover crops in the pecan orchard understory: Effects on Coccinellidae (Coleoptera) and pecan aphids (Hemiptera: Aphididae). Biol. Control 1991, 1, 8–15. [Google Scholar] [CrossRef]
- Géneau, C.E.; Wäckers, F.L.; Luka, H.; Daniel, C.; Balmer, O. Selective flowers to enhance biological control of cabbage pests by parasitoids. Basic Appl. Ecol. 2012, 13, 85–93. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Van Rijn, P.C. Pick and mix: Selecting flowering plants to meet the requirements of target biological control insects. Biodivers. Insect Pests 2012, 9, 139–165. [Google Scholar]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Gurr, G.M.; Lu, Z.; Heong, K.; Chen, G.; Zheng, X.; Xu, H.; Yang, Y. Laboratory screening supports the selection of sesame (Sesamum indicum) to enhance Anagrus spp. parasitoids (Hymenoptera: Mymaridae) of rice planthoppers. Biol. Control 2013, 64, 83–89. [Google Scholar] [CrossRef]
- White, A.J.; Wratten, S.D.; Berry, N.A.; Weigmann, U. Habitat manipulation to enhance biological control of Brassica pests by hover flies (Diptera: Syrphidae). J. Econ. Entomol. 1995, 88, 1171–1176. [Google Scholar] [CrossRef]
- Colley, M.; Luna, J. Relative attractiveness of potential beneficial insectary plants to aphidophagous hoverflies (Diptera: Syrphidae). Environ. Entomol. 2000, 29, 1054–1059. [Google Scholar] [CrossRef]
- Costello, M.J.; Altieri, M.A. Abundance, growth rate and parasitism of Brevicoryne brassicae and Myzus persicae (Hemiptera: Aphididae) on broccoli grown in living mulches. Agric. Ecosyst. Environ. 1995, 52, 187–196. [Google Scholar] [CrossRef]
- Bowie, M.; Wratten, S.; White, A. Agronomy and phenology of companion plants of potential for enhancement of insect biological control. N. Z. J. Crop Hortic. Sci. 1995, 23, 423–427. [Google Scholar] [CrossRef]
- Morris, M.C.; Li, F.Y. Coriander (Coriandrum sativum) companion plants can attract hoverflies, and may reduce pest infestation in cabbages. N. Z. J. Crop Hortic. Sci. 2000, 28, 213–217. [Google Scholar] [CrossRef]
- Martínez-Uña, A.; Martín, J.; Fernández-Quintanilla, C.; Dorado, J. Provisioning floral resources to attract aphidophagous hoverflies (Diptera: Syrphidae) useful for pest management in central spain. J. Econ. Entomol. 2013, 106, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Haslett, J. Interpreting patterns of resource utilization: Randomness and selectivity in pollen feeding by adult hoverflies. Oecologia 1989, 78, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Wäckers, F. The effect of food deprivation on the innate visual and olfactory preferences in the parasitoid Cotesia rubecula. J. Insect Physiol. 1994, 40, 641–649. [Google Scholar] [CrossRef]
- Adedipe, F.; Park, Y.-L. Visual and olfactory preference of Harmonia axyridis (Coleoptera: Coccinellidae) adults to various companion plants. J. Asia Pac. Entomol. 2010, 13, 319–323. [Google Scholar] [CrossRef]
- Turlings, T.; Tumlinson, J.; Eller, F.; Lewis, W. Larval-damaged plants: Source of volatile synomones that guide the parasitoid Cotesia marginiventris to the micro-habitat of its hosts. Entomol. Exp. Appl. 1991, 58, 75–82. [Google Scholar] [CrossRef]
- Vet, L.E.; Dicke, M. Ecology of infochemical use by natural enemies in a tritrophic context. Ann. Rev. Entomol. 1992, 37, 141–172. [Google Scholar] [CrossRef]
- Bottrell, D.G.; Barbosa, P.; Gould, F. Manipulating natural enemies by plant variety selection and modification: A realistic strategy? Annu. Rev. Entomol. 1998, 43, 347–367. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Nentwig, W. Olfactory orientation of the seven-spot ladybird beetle, Coccinella septempunctata (Coleoptera: Coccinellidae): Attraction of adults to plants and conspecific females. Eur. J. Entomol. 2000, 97, 155–160. [Google Scholar] [CrossRef]
- Beizhou, S.; Jie, Z.; Wiggins, N.L.; Yuncong, Y.; Guangbo, T.; Xusheng, S. Intercropping with aromatic plants decreases herbivore abundance, species richness, and shifts arthropod community trophic structure. Environ. Entomol. 2012, 41, 872–879. [Google Scholar] [CrossRef]
- Mizutani, F.; Sugaya, K.; Yamauchi, Y. Intercropping of buckwheat as an insectary plant for hover flies of aphid enemy in non-pesticide peach orchard. Bull. Exp. Farm Fac. Agric. Ehime Univ. 2010, 32, 1–6. [Google Scholar]
- Lin, R.; Liang, H.; Zhang, R.; Tian, C.; Ma, Y. Impact of alfalfa/cotton intercropping and management on some aphid predators in China. J. Appl. Entomol. 2003, 127, 33–36. [Google Scholar] [CrossRef]
- Qureshi, S.; Midmore, D.; Syeda, S.; Reid, D. A comparison of alternative plant mixes for conservation bio-control by native beneficial arthropods in vegetable cropping systems in Queensland Australia. Bull. Entomol. Res. 2010, 100, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Haaland, C.; Naisbit, R.E.; Bersier, L.F. Sown wildflower strips for insect conservation: A review. Insect Conserv. Divers. 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Tschumi, M.; Albrecht, M.; Entling, M.H.; Jacot, K. High effectiveness of tailored flower strips in reducing pests and crop plant damage. Proc. R. Soc. Lond. A 2015, 282, 1369. [Google Scholar] [CrossRef] [PubMed]
- Hatt, S.; Lopes, T.; Boeraeve, F.; Chen, J.; Francis, F. Pest regulation and support of natural enemies in agriculture: Experimental evidence of within field wildflower strips. Ecol. Eng. 2017, 98, 240–245. [Google Scholar] [CrossRef]
- Hatt, S.; Uyttenbroeck, R.; Lopes, T.; Mouchon, P.; Chen, J.; Piqueray, J.; Monty, A.; Francis, F. Do flower mixtures with high functional diversity enhance aphid predators in wildflower strips? Eur. J. Entomol. 2017, 114, 66. [Google Scholar] [CrossRef]
- Delfine, S.; Loreto, F.; Pinelli, P.; Tognetti, R.; Alvino, A. Isoprenoids content and photosynthetic limitations in rosemary and spearmint plants under water stress. Agric. Ecosyst. Environ. 2005, 106, 243–252. [Google Scholar] [CrossRef]
- Filella, I.; Penuelas, J.; Seco, R. Short-chained oxygenated voc emissions in Pinus halepensis in response to changes in water availability. Acta Physiol. Plant. 2009, 31, 311–318. [Google Scholar] [CrossRef]
- Ormeno, E.; Mevy, J.P.; Vila, B.; Bousquet-Melou, A.; Greff, S.; Bonin, G.; Fernandez, C. Water deficit stress induces different monoterpene and sesquiterpene emission changes in mediterranean species. Relationship between terpene emissions and plant water potential. Chemosphere 2007, 67, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.A.; Laue, G.; Baldwin, I.T. Plant–plant signaling: Application of trans-or cis-methyl jasmonate equivalent to sagebrush releases does not elicit direct defenses in native tobacco. J. Chem. Ecol. 2004, 30, 2193–2214. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, K.; Singh, V. Effect of planting date on growth, development, aerial biomass partitioning and essential oil productivity of wild marigold (Tagetes minuta) in mid hills of Indian western Himalaya. Ind. Crops Prod. 2008, 27, 380–384. [Google Scholar] [CrossRef]
| Definition | Response Elicited from Insect | Type of Receptors Involved |
|---|---|---|
| Repellent | A chemical which causes insects to make oriented movements away from its source (without contact with the HP) | Olfactory receptors |
| Arrestant | A chemical which causes insects to aggregate because it reduces their tendencies to move away from its source (without contact with the HP) | Olfactory receptors |
| Deterrent or feeding deterrent | A chemical which inhibits feeding, mating, or oviposition behavior after landing on the HP | Gustatory receptors |
| Species of Aphid | Host Plant | Companion Plants | Proposed Mechanisms | Reference |
|---|---|---|---|---|
| Myzus persicae | Capsicum annuum | Allium schoenoprasum | Masking odors Repellency Reduces the attractiveness of hosts | [60] |
| Brevicoryne brassicae | Brassica oleracea Bently F1 | Tagetes patula nana Calendula officinalis | Repellency | [61] |
| Myzus persicae | Nicotiana tabacum | Allium sativum | Repellency Deterrent effect | [62] |
| Myzus persicae Aphis gossypii | Solanum tuberosum | Allium sativum | Repellency Reduces the attractiveness of hosts | [63] |
| Myzus persicae | Capsicum annuum | Ocimum basilicum Rosmarinus officinalis Lavandula latifolia | Repellency Reduces the attractiveness of hosts | [64] |
| Brevicoryne brassicae | Brassica oleracea | Allium cepa | Reduces host-finding ability | [65] |
| Brevicoryne brassicae | Brassica oleracea | Secale cereal | Reduces host-finding ability | [66] |
| Aphis fabae | Vicia faba | Satureja hortensis Ocimum basilicum | Deterrent effect Repellency | [67] |
| Aphis citricola | Pyrus communis | Satureja hortensis Ocimum basilicum | Repellency | [68] |
| Lipaphis erysimi | Brassica napus | Allium cepa Allium sativum | Repellency Deterrent effect Disrupts behavior | [21] |
| Macrosiphum rosivorum | Rosa chinensis | Tagetes patula | Repellency Allelopaty change | [69] |
| Rhopalosiphuim padi | Hordeum vulgare | Cirsium vulgare | Allelopaty change Reduces the attractiveness of hosts | [70] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Issa, R.; Gomez, L.; Gautier, H. Companion Plants for Aphid Pest Management. Insects 2017, 8, 112. https://doi.org/10.3390/insects8040112
Ben-Issa R, Gomez L, Gautier H. Companion Plants for Aphid Pest Management. Insects. 2017; 8(4):112. https://doi.org/10.3390/insects8040112
Chicago/Turabian StyleBen-Issa, Refka, Laurent Gomez, and Hélène Gautier. 2017. "Companion Plants for Aphid Pest Management" Insects 8, no. 4: 112. https://doi.org/10.3390/insects8040112





