Morphometric Modifications in Canthon quinquemaculatus Castelnau 1840 (Coleoptera: Scarabaeinae): Sublethal Effects of Transgenic Maize?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A

References
- Burns, J.G.; di Nardo, P.; Rodd, F.H. The role of predation in variation in body shape in guppies Poecilia reticulata: A comparison of field and common garden phenotypes. J. Fish. Biol. 2009, 75, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Dayton, G.H.; Saenz, D.; Baum, K.A.; Langerhans, R.B.; DeWitt, T.J. Body shape, burst speed and escape behavior of larval anurans. Oikos 2005, 111, 582–591. [Google Scholar] [CrossRef]
- Rowe, L.; Arnqvist, G. Sexual selection and the evolution of genital shape and complexity in water striders. Evolution 2012, 66, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.A. Ecological morphology of lacustrine threespine stickleback Gasterosteus aculeatus L. (Gastoresteidae) body shape. Biol. J. Linn. Soc. 1997, 61, 3–50. [Google Scholar] [CrossRef]
- Keast, A.; Webb, D. Mouth and body form relative to feeding ecology in the fish fauna of a small lake, lake Opinicon, Ontario. J. Fish. Res. Board Can. 1966, 23, 1845–1874. [Google Scholar] [CrossRef]
- Forsman, A.; Shine, R. Parallel geographic variation in body shape and reproductive life history within the Australian scincid lizard Lampropholis delicata. Funct. Ecol. 1995, 9, 818–828. [Google Scholar] [CrossRef]
- Vanhooydonck, B.; Damme, R.V. Evolutionary relationships between body shape and habitat use in lacertid lizards. Evol. Ecol. Res. 1999, 1, 785–805. [Google Scholar]
- Hernández, M.I.M.; Monteiro, L.R.; Favila, M.E. The role of body size and shape in understanding competitive interactions within a community of Neotropical dung beetles. J. Insect Sci. 2011, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.H.; Alton, L.A.; Cramp, R.L.; Franklin, C.E. Does simultaneous UV-B exposure enhance the lethal and sub-lethal effects of aquatic hypoxia on developing anuran embryos and larvae? J. Comp. Physiol. B 2011, 181, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Primost, M.A.; Bigatti, G.; Márquez, F. Shell shape as indicator of pollution in marine gastropods affect by imposex. Mar. Freshw. Res. 2015, 67, 1948–1954. [Google Scholar] [CrossRef]
- Chan, K.Y.K.; Grünbaum, D.; O’Donnell, M.J. Effects of ocean-acidification-induced morphological changes on larval swimming. J. Exp. Biol. 2011, 214, 3857–3867. [Google Scholar] [CrossRef] [PubMed]
- Lõhmus, M.; Susdström, L.F.; Björklund, M.; Devlin, R.H. Genotype-temperature interaction in the regulation of development, growth, and morphometrics in wild-type, and growth-hormone transgenic coho salmon. PLoS ONE 2010, 5, e9980. [Google Scholar] [CrossRef] [PubMed]
- Woodley, S.K.; Matters, B.M.; Yates, E.K.; Relyea, R.A. Exposure to sublethal concentrations of a pesticide or predator cues induces changes in brain architecture in larval amphibians. Oecologia 2015, 179, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on benefical arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef] [PubMed]
- Pecenka, J.R.; Lundegren, J.G. Non-target effects of clothianidin on monarch butterflies. Sci. Nat. 2015, 102, 1–4. [Google Scholar] [CrossRef]
- Dively, G.P.; Embrey, M.S.; Kamel, A.; Hawthorne, D.J.; Pettis, J.S. Assessment of Chronic Sublethal Effects of Imidacloprid on Honey Bee Colony Health. PLoS ONE 2015, 10, e0118748. [Google Scholar] [CrossRef]
- Verdú, J.R.; Cortez, V.; Ortiz, A.J.; González-Rodríguez, E.; Martinez-Pinna, J.; Lumaret, J.P.; Lobo, J.M.; Numa, C.; Sánchez-Piñero, F. Low doses of ivermectin cause sensory and locomotor disorders in dung beetles. Sci. Rep. 2015, 5, 13912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, C.; Nielsen, B.O. Larvae of the dung beetle Onthophagus gazella F. (Col., Scarabaeidae) exposed to lethal and sublethal ivermectin concentrations. J. Appl. Entomol. 1992, 114, 502–509. [Google Scholar] [CrossRef]
- Rosales, M.C.; Martínez, M.I.; López-Collado, J.; Vargas-Mendoza, M.; González-Hernández, H.; Fajersson, P. Effect of ivermectin on the survival and fecundity of Euoniticellus intermedius (Coleoptera: Scarabaeidae). Rev. Biol. Trop. 2012, 60, 333–345. [Google Scholar]
- Pérez-Cogollo, L.C.; Rodriguez-Vivas, R.I.; Delfín-González, H.; Reyes-Novelo, E.; Ojeda-Chi, M.M. Lethal and sublethal effects of Ivermectin on Onthophagus landolti (Coleoptera: Scarabaeidae). Environ. Entomol. 2015, 44, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Campos, R.C. Influência do cultivo de milho transgênico em organismos não-alvo (Coleoptera: Scarabaeinae) e da ingestão indireta através da cadeia trófica. Ph.D. Thesis, Federal University of Santa Catarina, Florianópolis, Brazil, July 2016. [Google Scholar]
- Lang, A.; Vojtech, E. The effects of pollen consumption of transgenic Bt maize on the common swallowtail, Papilio machaon L. (Lepidoptera, Papilionidae). Basic Appl. Ecol. 2006, 7, 296–306. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat (accessed on 27 September 2017).
- International Service for the Acquisition of Agri-biotech Applications (ISAAA). Global Status of Commercialized Biotech/GM Crops: 2014. Brief 49–2014: Executive Summary. Available online: http://isaaa.org (accessed on 20 November 2016).
- Ceccarelli, S. GM Crops, Organic Agriculture and Breeding for Sustainability. Sustainability 2014, 6, 4273–4286. [Google Scholar] [CrossRef]
- Zwahlen, C.; Hilbeck, A.; Nentwig, W. Field decomposition of transgenic Bt maize residue and the impact on non-target soil invertebrates. Plant Soil 2007, 300, 245–257. [Google Scholar] [CrossRef]
- Daly, T.; Buntin, G.D. Effect of Bacillus thuringiensis transgenic corn for Lepidopteran control on nontarget arthropods. Environ. Entomol. 2005, 34, 1292–1301. [Google Scholar] [CrossRef]
- Gassmann, A.J.; Petzold-Maxwell, J.L.; Keweshan, R.S.; Dunbar, M.W. Field-evolved resistance to Bt maize by Western Corn Rootworm. PLoS ONE 2011, 6, e22629. [Google Scholar] [CrossRef] [PubMed]
- Hilbeck, A.; Moar, W.J.; Pusztai-Carey, M.; Filipini, A.; Bigler, F. Toxicity of Bacillus thuringiensis Cry1Ab toxin to the predator Chrysoperla carnea (Neuroptera, Chrysopidae). Environ. Entomol. 1998, 27, 1255–1263. [Google Scholar] [CrossRef]
- Campos, R.C.; Hernández, M.I.M. The importance of maize management on dung beetle communities in Atlantic Forest fragments. PLoS ONE 2015, 10, e0145000. [Google Scholar] [CrossRef] [PubMed]
- Sandri, C.A.; Tofanelli, M.B.D. Milho crioulo: Uma alternativa para retabilidade no campo. Pesqui. Agropecu. Trop. 2008, 38, 59–61. [Google Scholar]
- Spaner, D.; Brathwaite, R.A.I.; Mather, D.E. Comparison of open-pollinated stress-tolerant and landrace maize for production under stress conditions in Trinidad. Maydica 1995, 40, 331–337. [Google Scholar]
- Ferreira, J.M.; Moreira, R.M.P.; Hidalgo, J.A.F. Capacidade combinatória e heterose em populaçoes de milho crioulo. Cienc. Rural 2008, 39, 332–339. [Google Scholar] [CrossRef]
- Halffter, G.; Matthews, E.G. The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera: Scarabaeidae). Folia Entomol. Mex. 1966, 12–14, 1–312. [Google Scholar]
- Cantil, L.F.; Sánchez, M.V.; Dinghi, P.A.; Genise, J.F. Food relocation behavior, nests, and brood balls of Canthon quinquemaculatus Laporte de Castelnau (Coleoptera: Scarabaeidae: Scarabaeinae). Coleopt. Bull. 2014, 68, 199–208. [Google Scholar] [CrossRef]
- Obrist, L.B.; Dutton, A.; Albajes, R.; Bigler, F. Exposure of arthropod predators to Cry1Ab toxin in Bt maize fields. Ecol. Entomol. 2006, 31, 143–154. [Google Scholar] [CrossRef]
- Thomé, V.M.R.; Zampieri, S.; Braga, H.J.; Pandolfo, C.; Silva, V.P.; Bacic, I.; Laus, J.; Soldateli, D.; Gebler, E.; Ore, J.D.; et al. Zoneamento Agroecológico e Socioeconômico de Santa Catarina; Epagri: Florianópolis, Brazil, 1999. Available online: http://ciram.epagri.sc.gov.br (accessed on 3 May 2016).
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology, 1st ed.; Cambridge University Press: Cambridge, UK, 1991; 455p, ISBN 0521585988. [Google Scholar]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes method for optimal superimposition of landmarks. Syst. Zool. 1990, 39, 40–59. [Google Scholar] [CrossRef]
- Rohlf, F.J. TpsDig, Version 2.16. Department of Ecology and Evolution, State University of New York at Stony Brook, 2010. Available online: http://life.bio.sunysb.edu/morph/bibr28 (accessed on 20 May 2016).
- Rohlf, F.J. TpsUtil, File Utility Program. Version 1.53. Department of Ecology and Evolution, State University of New York at Stony Brook, 2012. Available online: http://life.bio.sunysb.edu/morph (accessed on 25 May 2016).
- Klingenberg, C.P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 2011, 11, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.F.; Seiter, N.J.; Krupke, C.H. The impact of Bt maize as a natal host on adult head capsule width in field populations of western corn rootworm. Entomol. Exp. Appl. 2011, 139, 8–16. [Google Scholar] [CrossRef]
- Peters, R.H. The Ecological Implications of Body Size, 1st ed.; Cambridge University Press: Cambridge, UK, 1983; 344p, ISBN 052128886X. [Google Scholar]
- Al-Deeb, M.A.; Wilde, G.E. Effect of bt corn expressing the Cry3Bb1 toxin on western corn rootworm (Coleoptera: Chrysomelidae) Biology. J. Kansas Entomol. Soc. 2005, 78, 142–152. [Google Scholar] [CrossRef]
- Lundgren, J.G.; Wiedenmann, R.N. Coleopteran-specific Cry3Bb toxin from transgenic corn pollen does not affect the fitness of a nontarget Species, Coleomegilla maculata DeGeer (Coleoptera: Coccinellidae). Environ. Entomol. 2002, 31, 1213–1218. [Google Scholar] [CrossRef]
- Cavicchi, S.; Giorgi, G.; Mochi, M. Investigation on early divergence between populations of Drosophila melanogaster kept at different temperatures. Genetica 1978, 48, 81–87. [Google Scholar] [CrossRef]
- Marsteller, S.; Adams, D.C.; Collyer, M.L.; Condon, M. Six cryptic species on a single species of host plant: Morphometric evidence for possible reproductive character displacement. Ecol. Entomol. 2009, 34, 66–73. [Google Scholar] [CrossRef]
- Alves, V.M.; Moura, M.O.; de Carvalho, C.J.B. Wing shape is influenced by environmental variability in Polietina orbitalis (Stein) (Diptera: Muscidae). Rev. Bras. Entomol. 2016, 60, 150–156. [Google Scholar] [CrossRef]
- Winans, G.A.; Nishioka, R.S. A multivariate description of change in body shape of coho salmon (Oncorhynchus kisutch) during smoltification. Aquaculture 1987, 66, 235–245. [Google Scholar] [CrossRef]
- McCollum, S.A.; Leimberger, J.D. Predator-induced morphological changes in an amphibian: Predation by dragonflies affects tadpole shape and color. Oecologia 1997, 109, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Mak, R.; Ayad, H.; Smith, A.; Maclean, N. Expression of a novel piscine growth hormone gene results in growth enhancement in transgenic tilapia (Oreochromis niloticus). Transgenic Res. 1998, 7, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Ostenfeld, T.H.; McLean, E.; Devlin, R.H. Transgenesis change body and head shape in Pacific salmon. J. Fish Biol. 1998, 52, 850–854. [Google Scholar] [CrossRef]
- Dunham, R.A.; Chatakondi, N.; Nichols, A.J.; Kucuktas, H.; Chen, T.T.; Powers, D.A.; Weetle, J.D.; Cummins, K.; Lovell, R.T. Effect of rainbow trout growth hormone complementary DNA on body shape, carcass yeld, and carcass composition of F1 and F2 transgenic common carp (Cyprinus carpio). Mar. Biotechnol. 2002, 4, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Fares, N.H.; El-Sayed, A.K. Fine structural changes in the ileum of mice fed on endotoxin-treated potatoes and transgenic potatoes. Nat. Toxins 1998, 6, 219–233. [Google Scholar] [CrossRef]
- Armon, S.; Yanai, O.; Sharon, E. Quantitative phenotyping of leaf margins in three dimensions, demonstrated on KNOTTED and TCP trangenics in Arabidopsis. J. Exp. Bot. 2014, 65, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Habes, D.; Messiad, R.; Gouasmia, S.; Grib, L. Effects of an inorganic insecticide (boric acid) against Blatella germanica: Morphometric measurements and biochemical composition of ovaries. Afr. J. Biotechnol. 2013, 12, 2492–2497. [Google Scholar]
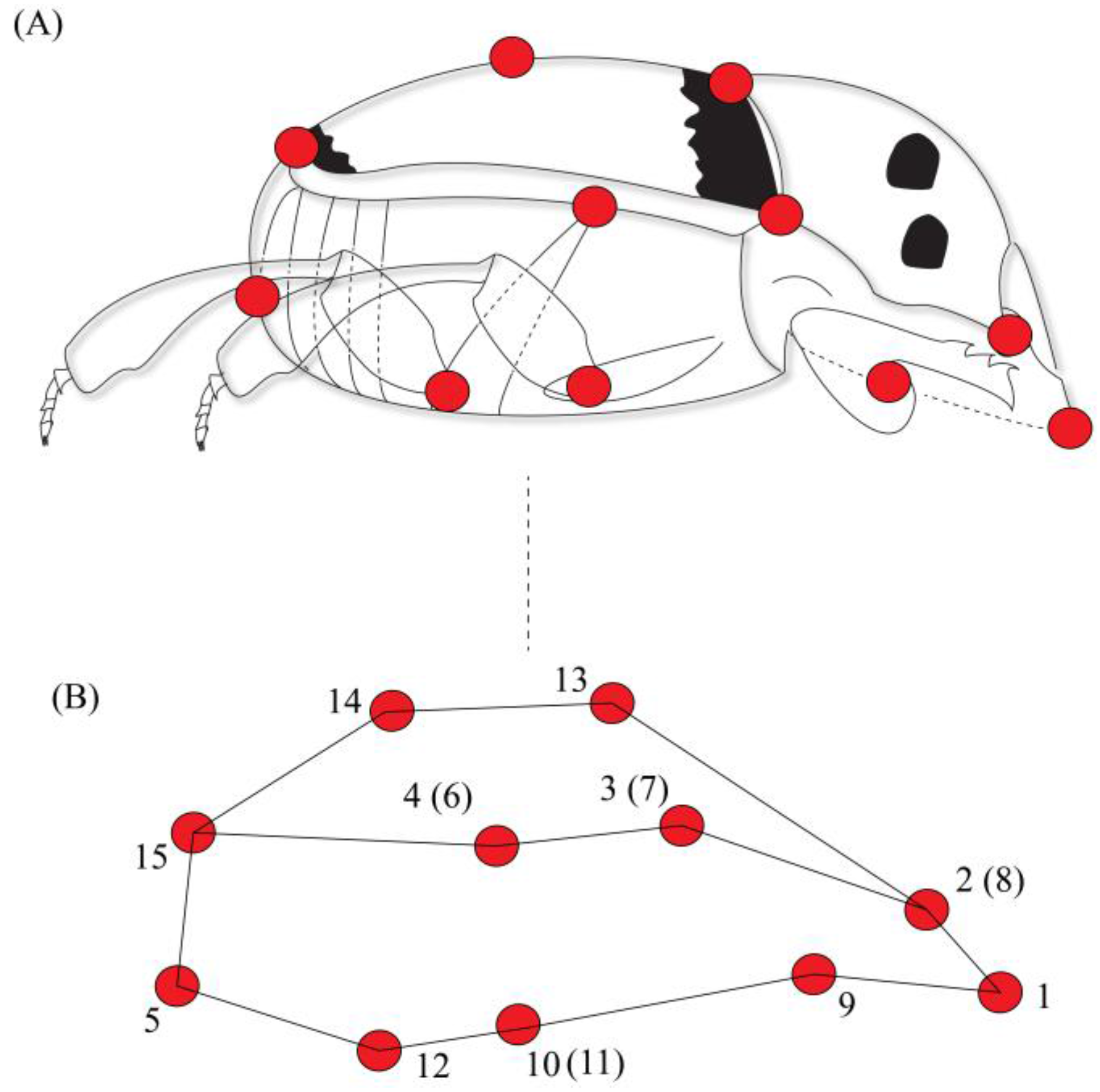
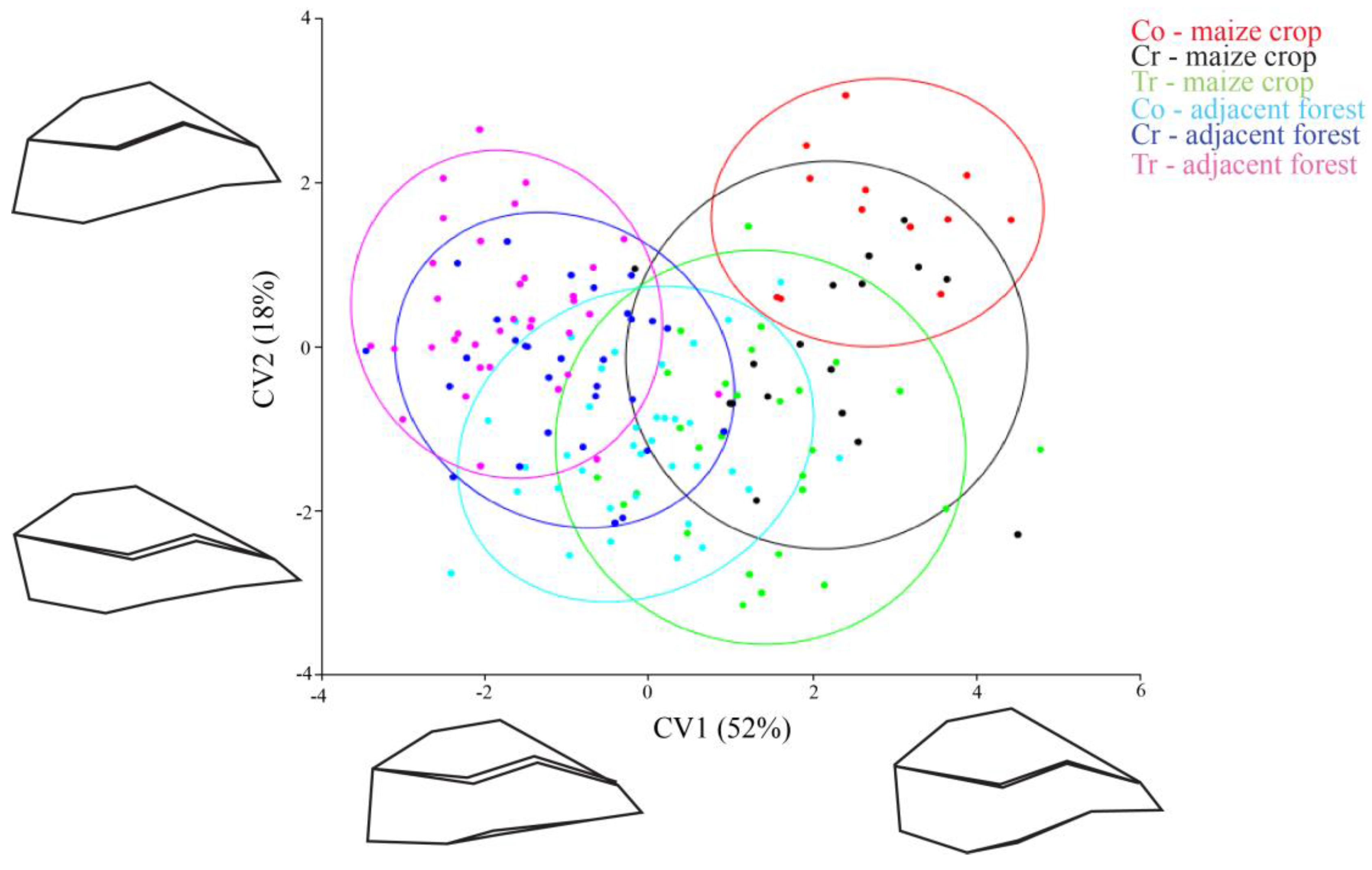
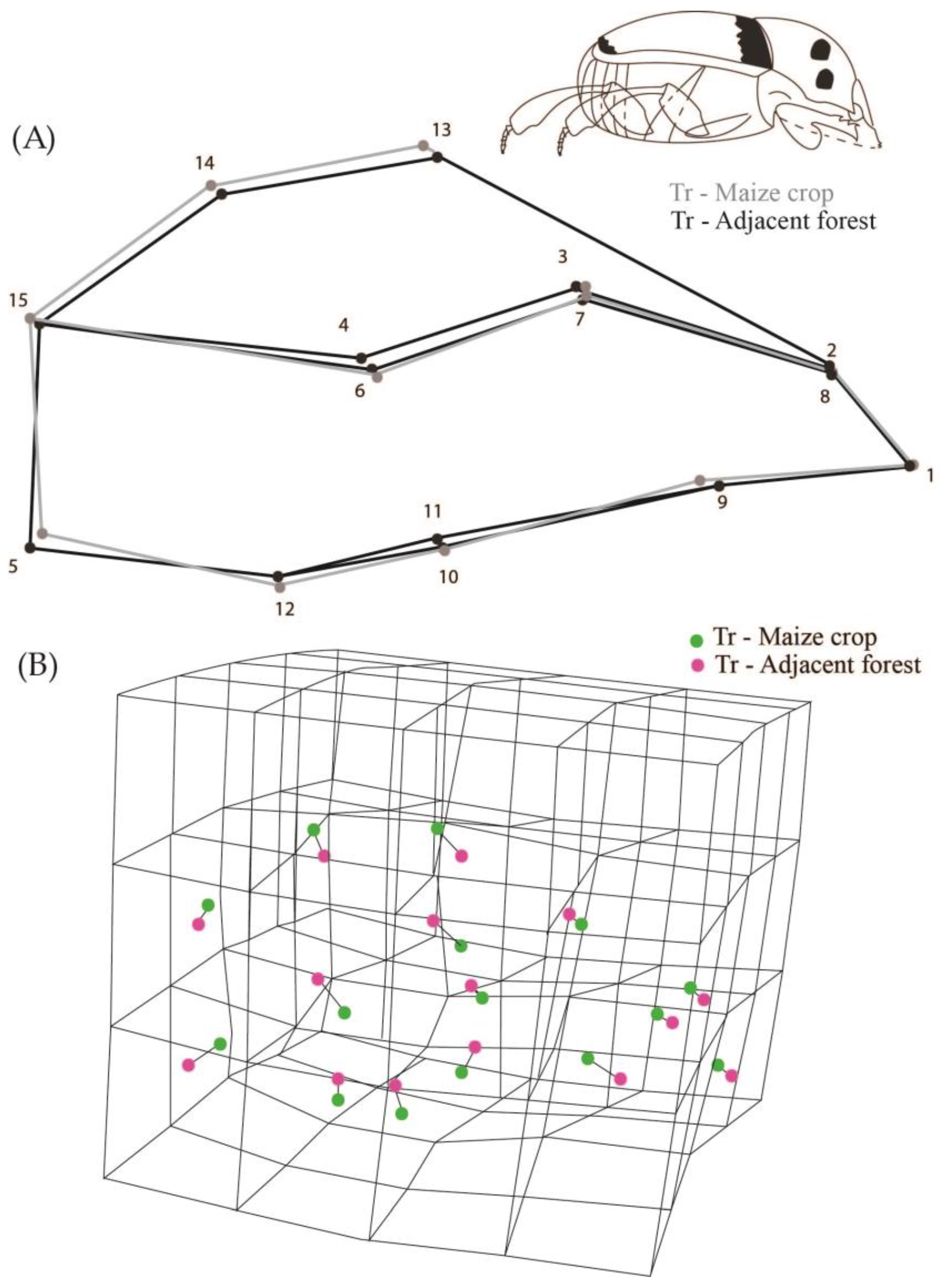
| Population | Allocation Value (%) |
|---|---|
| Conventional—Maize crop | 90.66 |
| Creole—Maize crop | 64.70 |
| Transgenic—Maize crop | 82.14 |
| Conventional—Adjacent forest | 58.33 |
| Creole—Adjacent forest | 73.33 |
| Transgenic—Adjacent forest | 82.85 |
| Overall classification accuracy | 75.33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, V.M.; Hernández, M.I.M. Morphometric Modifications in Canthon quinquemaculatus Castelnau 1840 (Coleoptera: Scarabaeinae): Sublethal Effects of Transgenic Maize? Insects 2017, 8, 115. https://doi.org/10.3390/insects8040115
Alves VM, Hernández MIM. Morphometric Modifications in Canthon quinquemaculatus Castelnau 1840 (Coleoptera: Scarabaeinae): Sublethal Effects of Transgenic Maize? Insects. 2017; 8(4):115. https://doi.org/10.3390/insects8040115
Chicago/Turabian StyleAlves, Victor Michelon, and Malva Isabel Medina Hernández. 2017. "Morphometric Modifications in Canthon quinquemaculatus Castelnau 1840 (Coleoptera: Scarabaeinae): Sublethal Effects of Transgenic Maize?" Insects 8, no. 4: 115. https://doi.org/10.3390/insects8040115





