Elliptic Fourier Analysis in the Study of the Male Genitalia to Discriminate Three Macrolophus Species (Hemiptera: Miridae)
Abstract
:1. Introduction
2. Material and Methods
2.1. Samples Collection
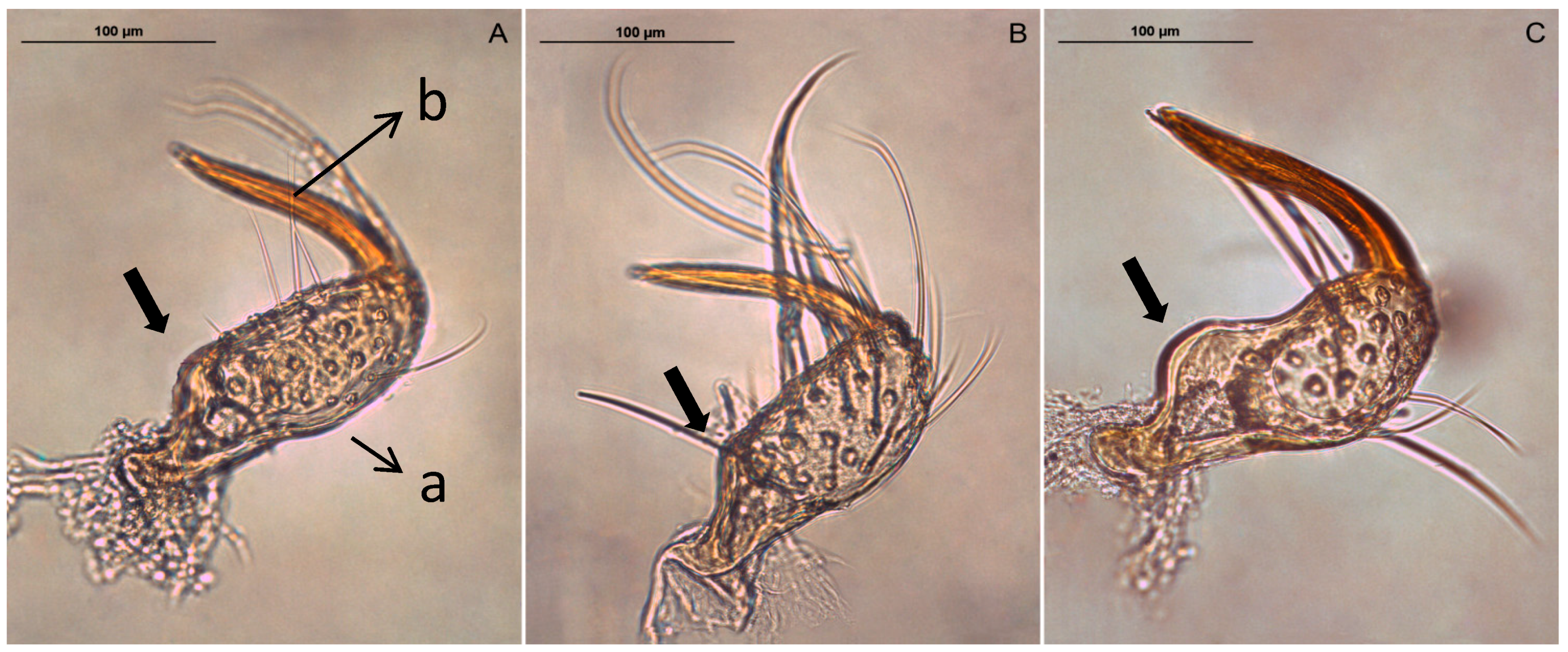
2.2. Preparation of Genitalia
2.3. Elliptic Fourier Analysis
2.4. Statistical Analysis
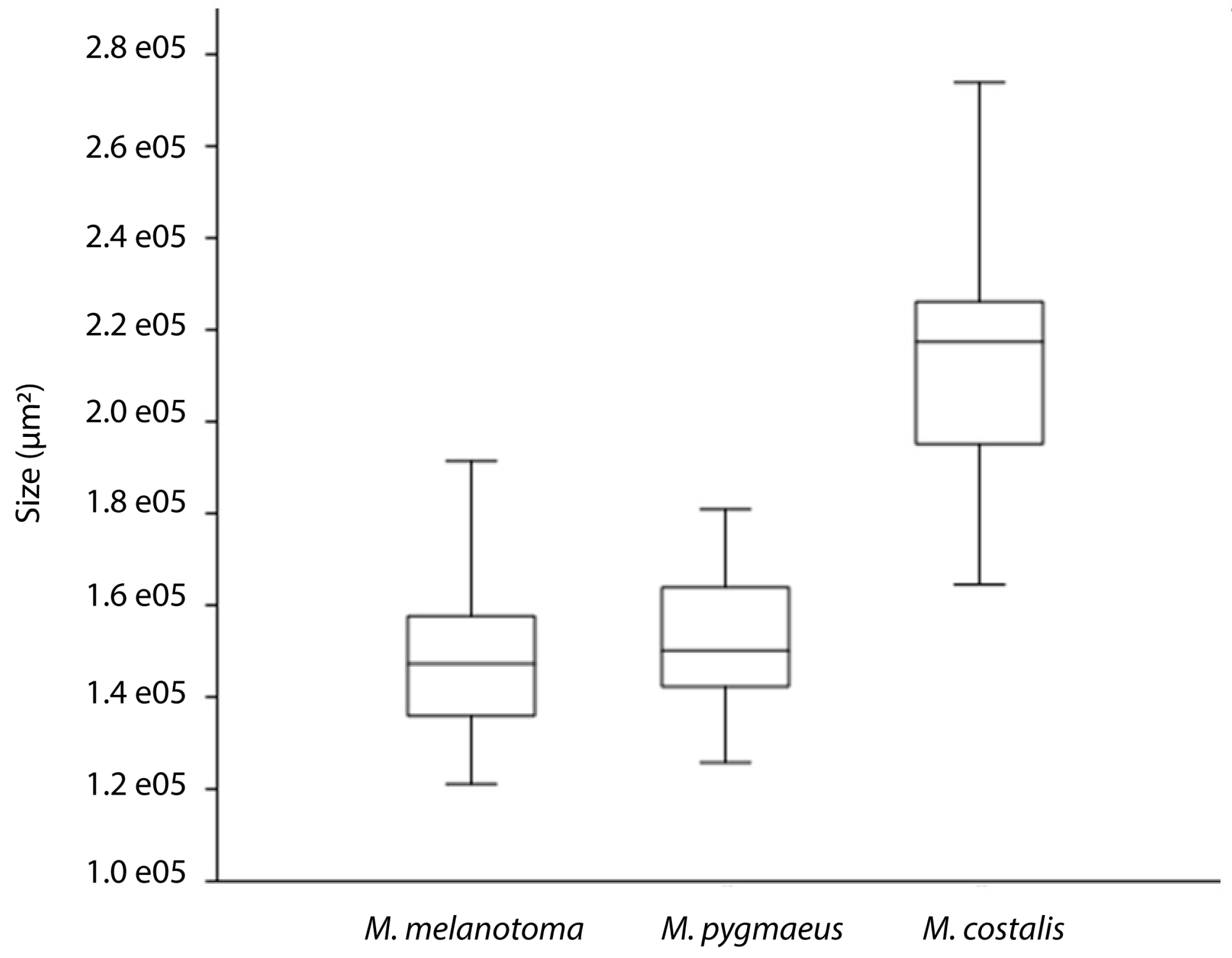
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cassis, G. A Systematic Study of the Subfamily Dicyphinae (Heteroptera: Miridae). Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 1986. [Google Scholar]
- Josifov, M. Zur Taxonomie der paläarktischen Macrolophus-Arten (Insecta, Heteroptera: Miridae). Reichenbachia 1992, 29, 1–4. [Google Scholar]
- Perdikis, D.C.; Margaritopoulos, J.T.; Stamatis, C.; Mamuris, Z.; Lykouressis, D.P.; Tsitsipis, J.A.; Pekas, A. Discrimination of the closely related biocontrol agents Macrolophus melanotoma (Hemiptera: Miridae) and M. pygmaeus using mitochondrial DNA analysis. Bull. Entomol. Res. 2003, 93, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Castañé, C.; Agustí, N.; Arnó, J.; Gabarra, R.; Riudavets, J.; Comas, J.; Alomar, O. Taxonomic identification of Macrolophus pygmaeus and Macrolophus melanotoma based on morphometry and molecular markers. Bull. Entomol. Res. 2013, 103, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Alomar, O.; Goula, M.; Albajes, R. Colonisation of tomato fields by predatory mirid bugs (Hemiptera: Heteroptera) in northern Spain. Agric. Ecosyst. Environ. 2002, 89, 105–115. [Google Scholar] [CrossRef]
- Alomar, O.; Riudavets, J.; Castañe, C. Macrolophus caliginosus in the biological control of Bemisia tabaci on greenhouse melons. Biol. Control 2006, 36, 154–162. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. (Ed.) Commercial availability of biological control agents. In Quality Control and Production of Biological Control Agents, Theory and Testing Procedures; Oxon Publishing Ltd.: London, UK, 2003; pp. 167–179. [Google Scholar]
- Messelink, G.J.; Bloemhard, C.M.J.; Hoogerbrugge, H.; Van Schelt, J.; Ingegno, B.L.; Tavella, L. Evaluation of mirid predatory bugs and release strategy for aphid control in sweet pepper. J. Appl. Entomol. 2014, 139, 333–341. [Google Scholar] [CrossRef]
- Martinez-Cascales, J.I.; Cenis, J.L.; Cassis, G.; Sánchez, J.A. Species identity of Macrolophus melanotoma (Costa 1853) and Macrolophus pygmaeus (Rambur 1839) (Insecta: Heteroptera: Miridae) based on morphological and molecular data and bionomic implications. Insect Syst. Evol. 2006, 37, 385–404. [Google Scholar] [CrossRef]
- Gemeno, C.; Laserna, N.; Riba, M.; Valls, J.; Castañé, C.; Alomar, O. Cuticular hydrocarbons discriminate cryptic Macrolophus species (Hemiptera: Miridae). Bull. Entomol. Res. 2012, 102, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Jauset, A.M.; Edo-Tena, E.; Castañé, C.; Agustí, N.; Alomar, O.; Grozeva, S. Comparative cytogenetic study of three Macrolophus species (Heteroptera, Miridae). Comp. Cytogenet. 2015, 9, 613–623. [Google Scholar] [PubMed]
- Eberhard, W.G. Sexual Selection and Animal Genitalia; Harvard University Press: Cambridge, MA, USA, 1985. [Google Scholar]
- Rohlf, F.J.; Marcus, L.F. A revolution in morphometrics. Trends Ecol. Evol. 1993, 8, 129–132. [Google Scholar] [CrossRef]
- Shen, L.; Farid, H.; McPeek, M.A. Modelling three-dimensional morphological structures using spherical harmonics. Evolution 2009, 63, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, F.V. Male genitalia in Miridae (Heteroptera) and their significance for suprageneric classification of the family. Part I: General review, Isometopinae and Psallopinae. Belg. J. Entomol. 2003, 5, 3–36. [Google Scholar]
- Wagner, E. Contributo alla conoscenza della fauna emitterologica italiana. II. Zwei neue Miriden-Arten und eine bisher übersehene Art aus Italien (Hem. Het.). B. Macrolophus caliginosus n. sp., eine neue Miridenart aus Italien (Hem. Het.). Boll. Assoc. Romana Entomol. 1951, 5, 29–31. [Google Scholar]
- Carapezza, A. The specific identities of Macrolophus melanotoma (A. Costa, 1853) and Stenodema curticolle (A. Costa, 1853) (Insecta Heteroptera, Miridae). Nat. Siciliano 1995, 19, 295–298. [Google Scholar]
- Wagner, E.; Weber, H. Héteroptères Miridae. Faune de France; Office Céntrale de Faunistique: Paris, France, 1964. [Google Scholar]
- Tamanini, L. Gli eterotteri della Basilicata edella Calabria (Italia meridionale) (Hemiptera Heteroptera). Mem. Mus. Civ. St. Nat. Verona (II Ser.) Sez. Sc. Vita (A Biol.) 1981, 3, 1–164. [Google Scholar]
- Klaus, A.V.; Kulasekera, V.L.; Schawaroch, V. Three-dimensional visualization of insect morphology using confocal laser scanning microscopy. J. Microsc. 2003, 212, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Rohlf, F.J.; Slice, D. Geometric morphometrics: ten years of progress following the “revolution”. Ital. J. Zool. 2004, 71, 5–16. [Google Scholar] [CrossRef]
- Monti, L.; Baylac, M.; Lalanne-Cassou, B. Elliptic Fourier analysis of the form of genitalia in two Spodoptera species and their hybrids (Lepidoptera: Noctuidae). Biol. J. Linn. Soc. 2001, 72, 391–400. [Google Scholar] [CrossRef]
- Kuhl, F.P.; Giardina, C.R. Elliptic Fourier Features of a Closed Contour. Comput. Vis. Graph. 1982, 18, 236–258. [Google Scholar] [CrossRef]
- Arnqvist, G. Comparative evidence for the evolution of genitalia by sexual selection. Nature 1998, 393, 784–786. [Google Scholar] [CrossRef]
- Arnqvist, G.; Danielsson, I. Copulatory behavior, genital morphology and male fertilization success in water striders. Evolution 1999, 53, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.R.; Lewis, T.M. Size and shape differences in genitalia of males from sympatric and reproductively isolated populations of Anthocoris antevolens White (Heteroptera: Anthocoridae) in the Yakima Valley, Washington. Ann. Entomol. Soc. Am. 2005, 98, 527–535. [Google Scholar] [CrossRef]
- Holwell, G.I. Geographic variation in genital morphology of Ciulfina praying mantids. J. Zool. (Lond.) 2008, 276, 108–114. [Google Scholar] [CrossRef]
- Tabugo, S.R.; Torres, M.; Torres, M.A.J.; Demayo, C.G. Elliptic Fourier Analysis in describing shape of male appendages in Neurothemis species found in Iligan City, Philippines. Nat. J. 2014, 18, 2–18. [Google Scholar]
- Özenirler, Ç.; Aytekin, A.M. Elliptic Fourier Analysis of Male Genitalia in Bombus (S. Lato) Latreille. Pak. J. Zool. 2015, 47, 1525–1532. [Google Scholar]
- Agustí, N.; Gabarra, R. Puesta a punto de una cría masiva del depredador polífago Dicyphus tamaninii Wagner (Heteroptera: Miridae). Bol. Sanid. Veg. Plagas. 2009, 35, 205–218. [Google Scholar]
- Agustí, N.; Gabarra, R. Effect of adult age and insect density of Dicyphus tamaninii Wagner (Heteroptera: Miridae) on progeny. J. Pest Sci. 2009, 82, 241–246. [Google Scholar] [CrossRef]
- Franco, K.; Jauset, A.; Castañé, C. Monogamy and polygamy in two species of mirid bugs: A functional-based approach. J. Insect Physiol. 2011, 57, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Ukai, Y. SHAPE: A computer program package for quantitative evaluation of biological shapes based on elliptic Fourier descriptors. J. Hered. 2002, 93, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Freeman, H. Computer processing of line drawing images. Comp. Survs. 1974, 6, 57–97. [Google Scholar] [CrossRef]
- Crampton, J. Elliptic Fourier shape analysis of fossil bivalves: some practical considerations. Lethaia 1995, 28, 179–186. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.L.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.J.; Berlocher, S.H. Endless Forms: Species and Speciation; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Paterson, H. The recognition of cryptic species among economically important insects. In Heliothis: Research Methods and Prospects; Myron, Z., Ed.; Springer: New York, NY, USA, 1991; pp. 1–10. [Google Scholar]
- Winker, K. Sibling species were first recognized by William Derham (1718). Auk 2005, 122, 706–707. [Google Scholar] [CrossRef]
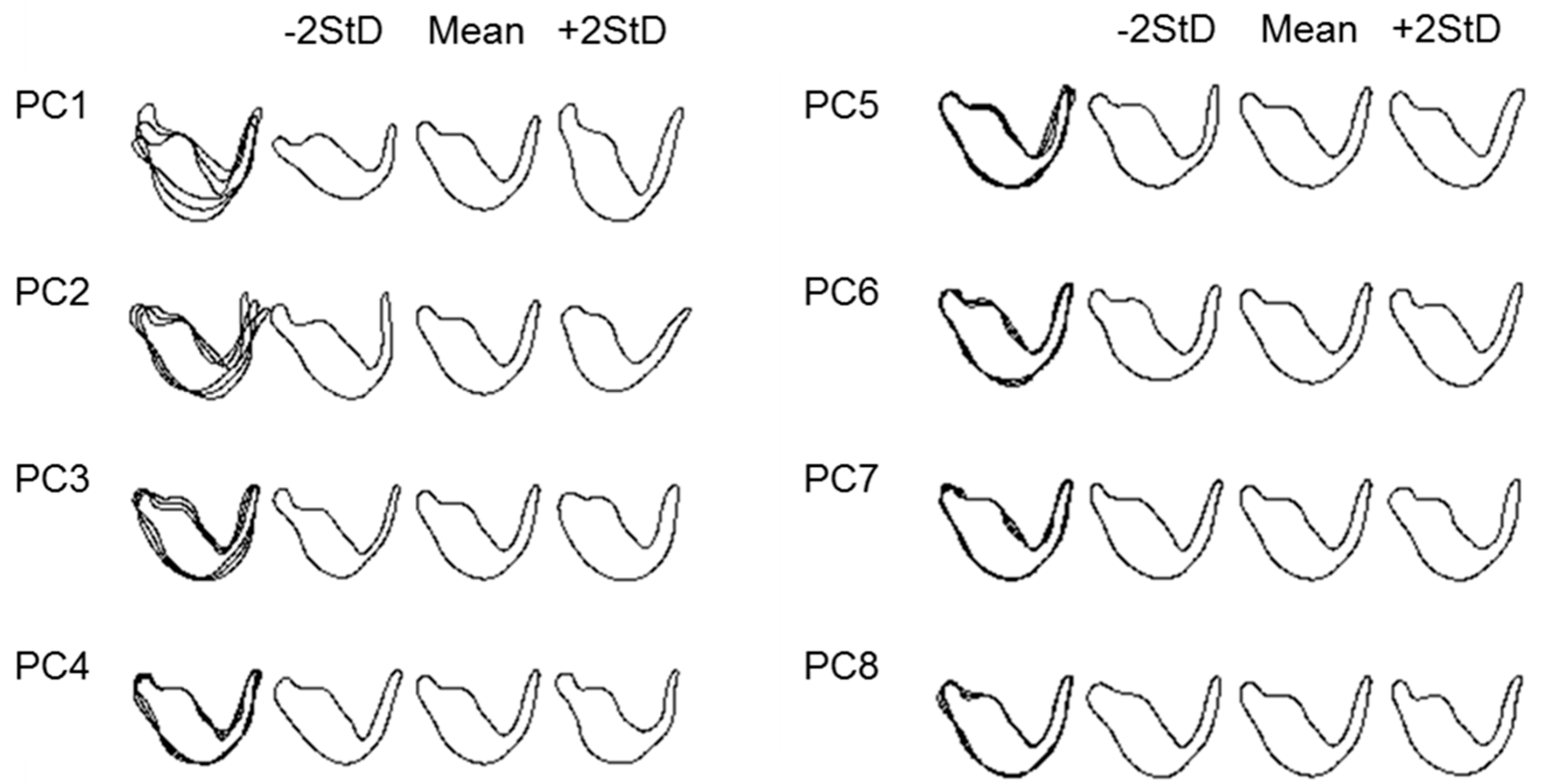
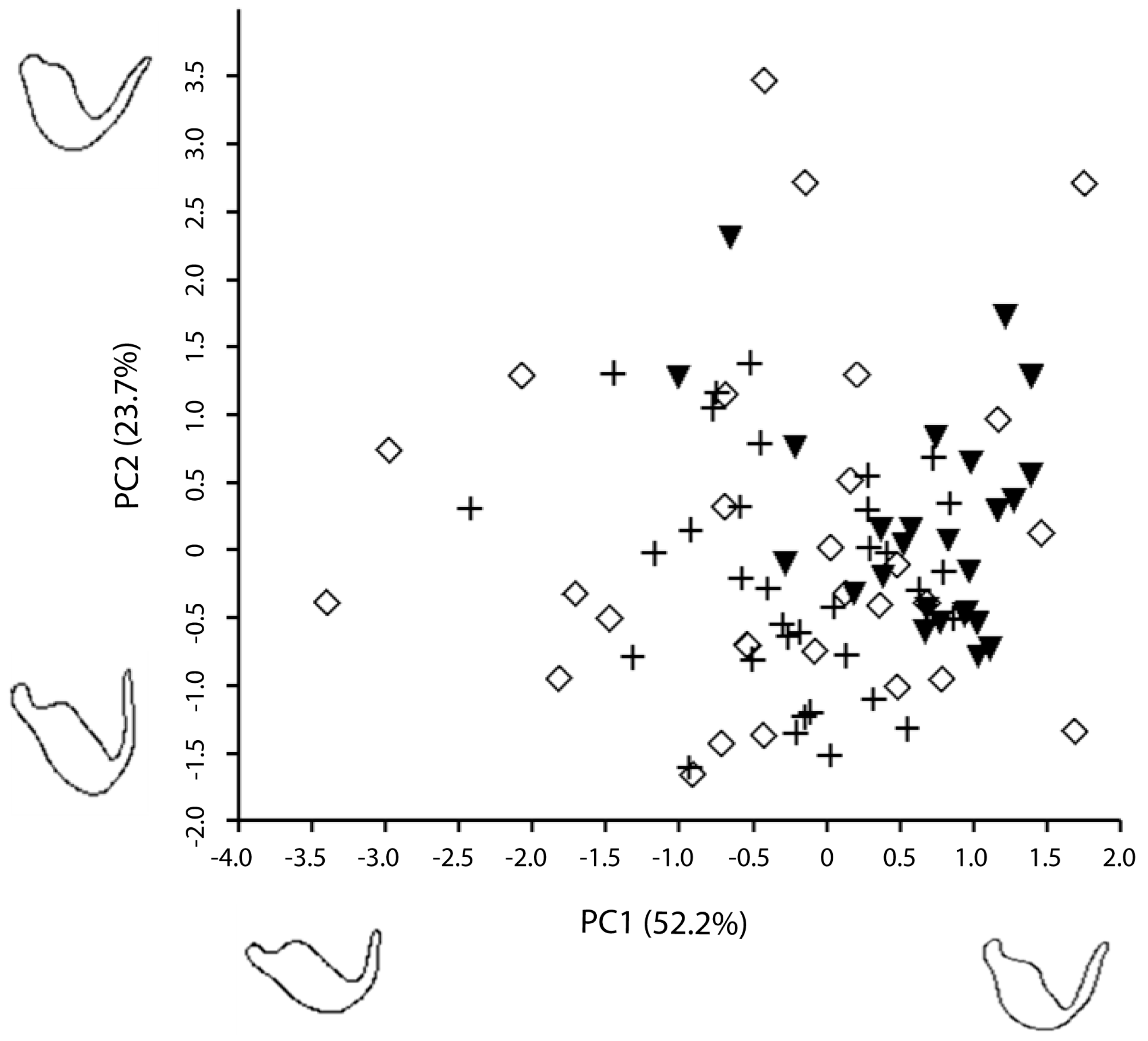
| PC | Eigenvalue | % Variance |
|---|---|---|
| 1 | 1.14269e12 | 52.1680 |
| 2 | 5.18737e11 | 23.6820 |
| 3 | 1.54099e11 | 7.0352 |
| 4 | 1.01518e11 | 4.6347 |
| 5 | 7.52726e10 | 3.4365 |
| 6 | 3.77691e10 | 1.7243 |
| 7 | 3.15860e10 | 1.4420 |
| 8 | 2.49245e10 | 1.1379 |
| Cumulative variance (%) | 95.24 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jauset, A.M.; Edo-Tena, E.; Parés-Casanova, P.M.; Castañé, C.; Agustí, N.; Alomar, O. Elliptic Fourier Analysis in the Study of the Male Genitalia to Discriminate Three Macrolophus Species (Hemiptera: Miridae). Insects 2017, 8, 120. https://doi.org/10.3390/insects8040120
Jauset AM, Edo-Tena E, Parés-Casanova PM, Castañé C, Agustí N, Alomar O. Elliptic Fourier Analysis in the Study of the Male Genitalia to Discriminate Three Macrolophus Species (Hemiptera: Miridae). Insects. 2017; 8(4):120. https://doi.org/10.3390/insects8040120
Chicago/Turabian StyleJauset, A. M., E. Edo-Tena, P. M. Parés-Casanova, C. Castañé, N. Agustí, and O. Alomar. 2017. "Elliptic Fourier Analysis in the Study of the Male Genitalia to Discriminate Three Macrolophus Species (Hemiptera: Miridae)" Insects 8, no. 4: 120. https://doi.org/10.3390/insects8040120







