Comparative Flight Activities and Pathogen Load of Two Stocks of Honey Bees Reared in Gamma-Irradiated Combs
Abstract
:1. Introduction
2. Materials and Methods
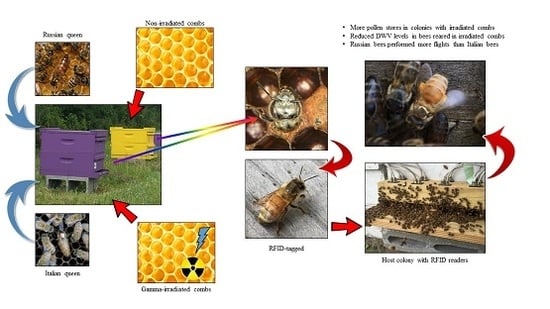
2.1. Establishing Test Colonies
2.2. Monitoring Flight Activities
2.3. RFID-Tagging and Paint-Marking of Bees
2.4. Host Colonies for RFID-Tagged and Paint-Marked Bees
2.5. Viral Load, Nosema Spore Count, and Amount of Pollen Stores
2.6. Data Analyses
3. Results
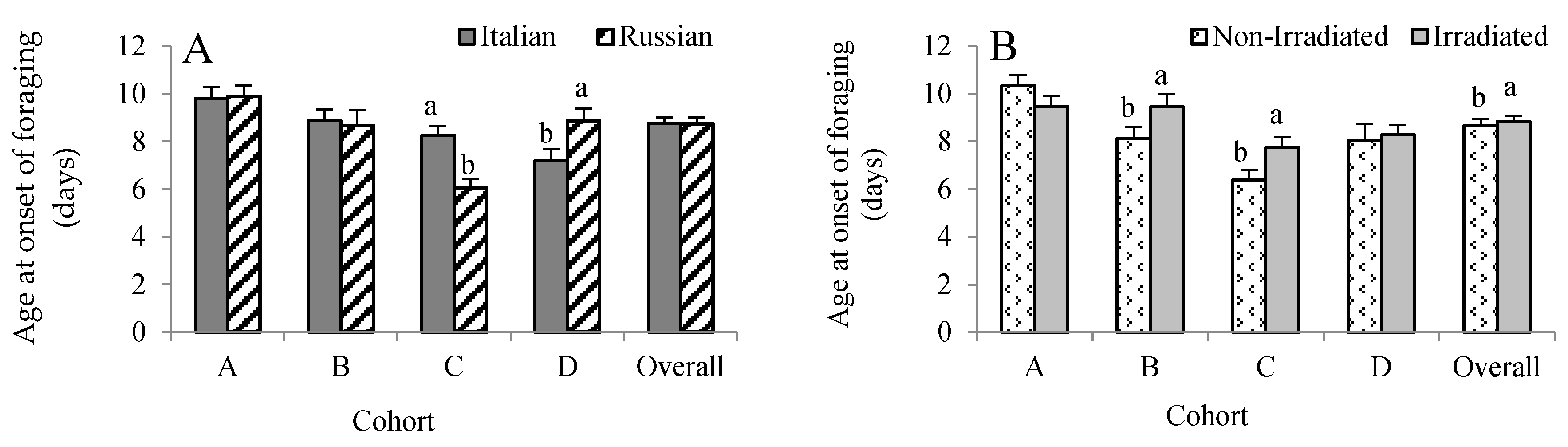
3.1. Effects of Bee Genotype and Comb Irradiation on Age at Onset of Foraging
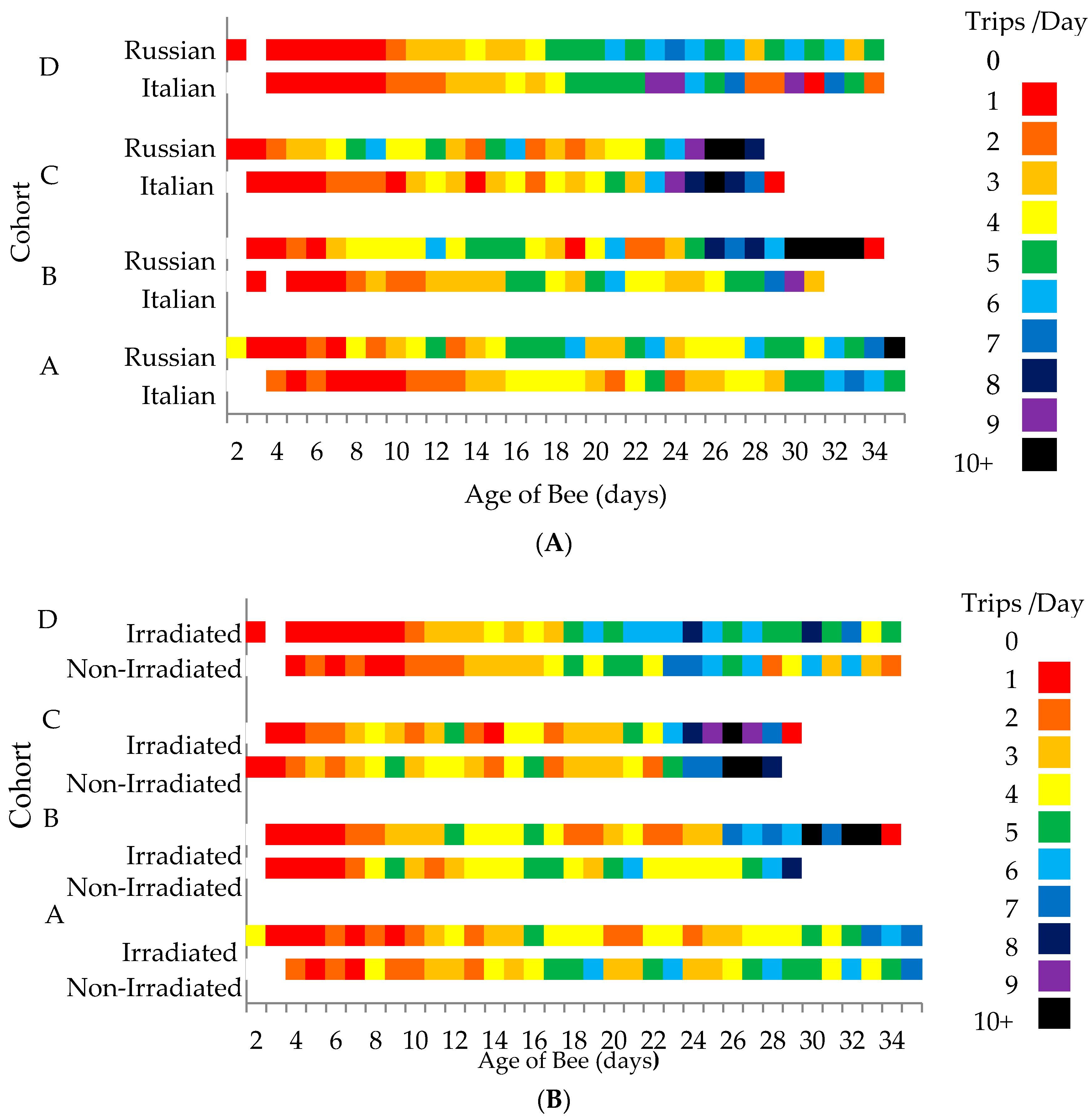
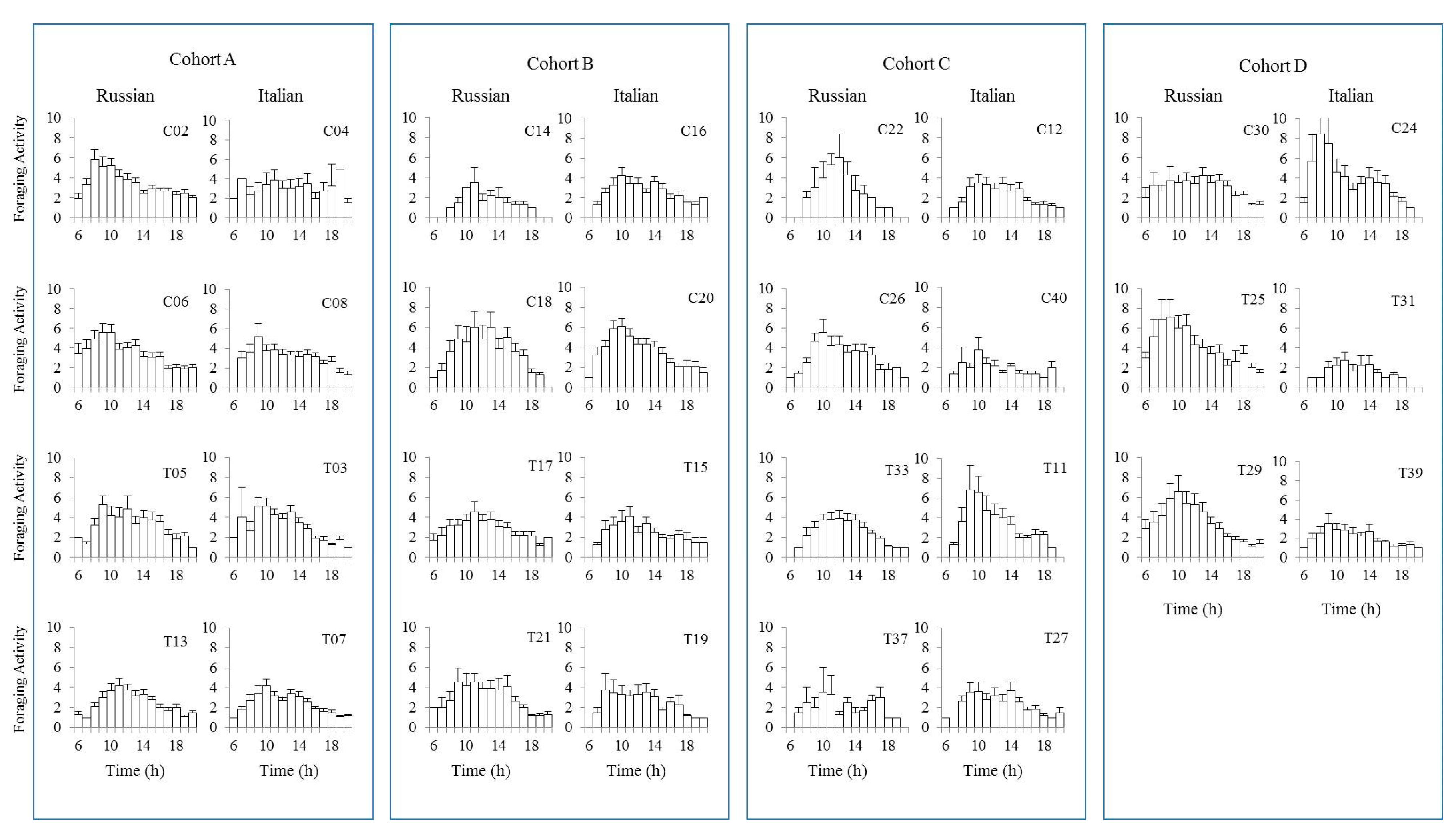
3.2. Effects of Bee Genotype and Comb Irradiation on Foraging Flights
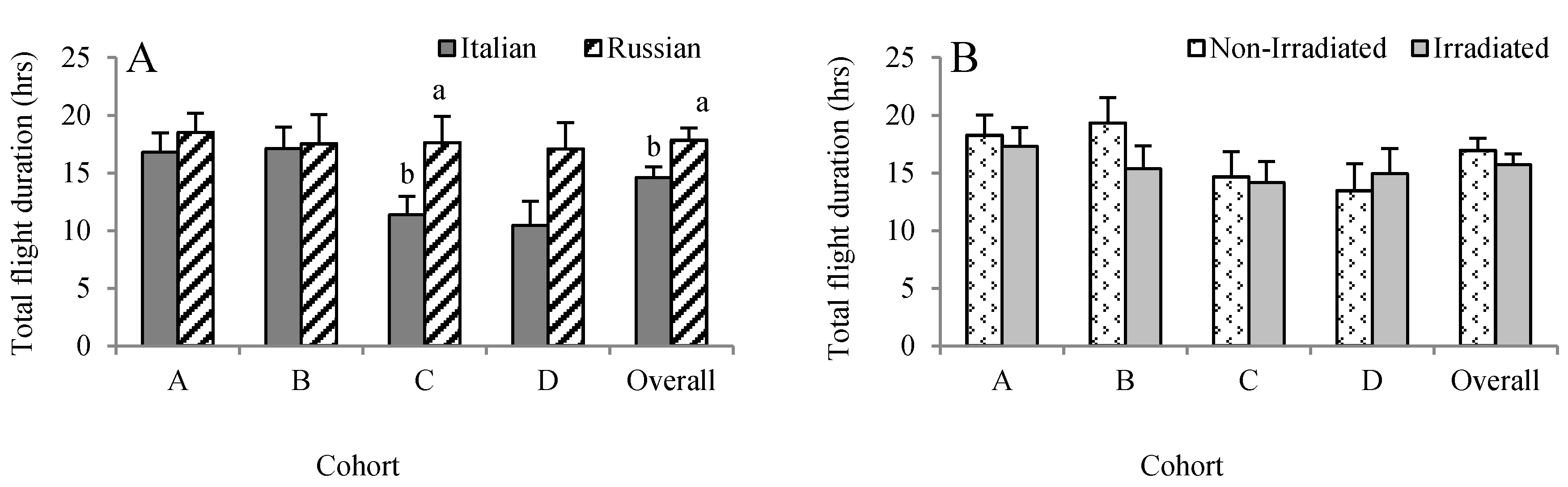
3.3. Effects of Bee Genotype and Comb Irradiation on Flight Duration
3.4. Effects of Bee Genotype and Comb Irradiation on Weight at Bee Emergence and Bee Survival
3.4.1. Weight at Bee Emergence
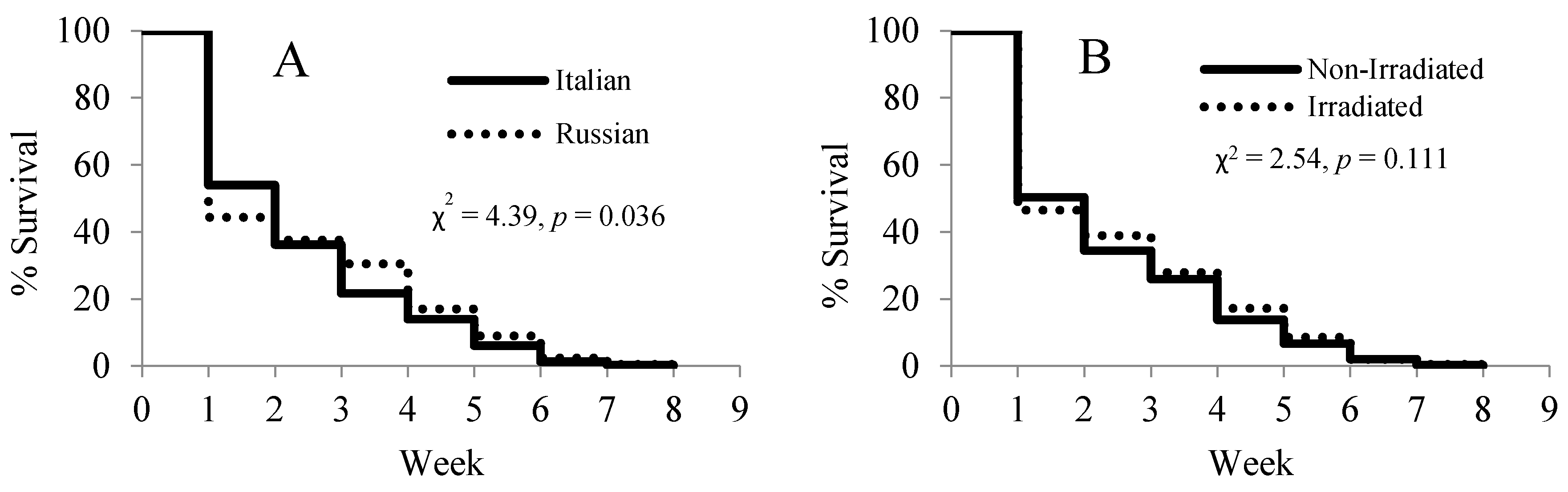
3.4.2. Survival of RFID-Tagged Bees
3.4.3. Survival of Paint-Marked Bees
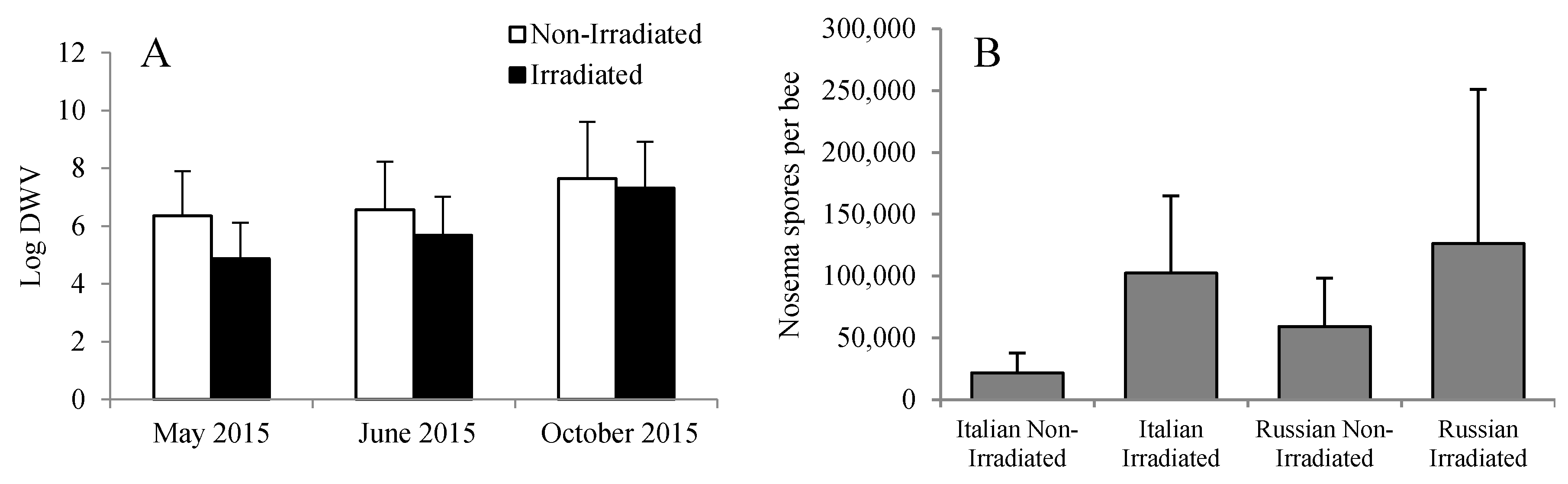
3.5. Effects of Bee Genotype and Comb Irradiation on Viral Load, Nosema Spore Count, and Stored Pollen
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- VanEngelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in europe and the united states and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef] [PubMed]
- Kralj, J.; Brockmann, A.; Fuchs, S.; Tautz, J. The parasitic mite varroa destructor affects non-associative learning in honey bee foragers, Apis mellifera L. J. Comp. Physiol. A 2007, 193, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Kralj, J.; Fuchs, S. Parasite-Host Interactions between Varroa Destructor Anderson and Trueman and Apis Mellifera L.: Influence of Parasitism on Flight Behaviour and on the Loss of Infested Foragers; Fachbereich Biologie und Informatik der Johann Wolfgang Goethe-Universität: Frankfurt, Germany, 2004. [Google Scholar]
- Kralj, J.; Fuchs, S. Parasitic varroa destructor mites influence flight duration and homing ability of infested apis mellifera foragers. Apidologie 2006, 37, 577–587. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, S48–S61. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, E.V.; Fannon, J.M.; Moore, J.D.; Wood, G.R.; Evans, D.J. The iflaviruses sacbrood virus and deformed wing virus evoke different transcriptional responses in the honeybee which may facilitate their horizontal or vertical transmission. PeerJ 2016, 4, e1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möckel, N.; Gisder, S.; Genersch, E. Horizontal transmission of deformed wing virus: Pathological consequences in adult bees (Apis mellifera) depend on the transmission route. J. Gen. Virol. 2011, 92, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Katznelson, H.; Jamieson, C.; Lawton, E.; Bellamy, W. Studies on the treatment of contaminated combs and honey with high velocity electrons. Can. J. Technol. 1952, 30, 95–103. [Google Scholar]
- Hansen, H.; Brødsgaard, C.J. American foulbrood: A review of its biology, diagnosis and control. Bee World 1999, 80, 5–23. [Google Scholar] [CrossRef]
- Gilliam, M.; Prest, D.B. Microbiology of feces of the larval honey bee, apis mellifera. J. Invertebr. Pathol. 1987, 49, 70–75. [Google Scholar] [CrossRef]
- Bailey, L. The transmission of nosema disease. Bee World 1953, 34, 171–172. [Google Scholar] [CrossRef]
- Ribière, M.; Lallemand, P.; Iscache, A.-L.; Schurr, F.; Celle, O.; Blanchard, P.; Olivier, V.; Faucon, J.-P. Spread of infectious chronic bee paralysis virus by honeybee (Apis mellifera L.) feces. Appl. Environ. Microbiol. 2007, 73, 7711–7716. [Google Scholar] [CrossRef] [PubMed]
- Donzé, G.; Herrmann, M.; Bachofen, B.; Guerin, P.R.M. Effect of mating frequency and brood cell infestation rate on the reproductive success of the honeybee parasite varroa jacobsoni. Ecol. Entomol. 1996, 21, 17–26. [Google Scholar] [CrossRef]
- Kralj, J.; Fuchs, S. Nosema sp. Influences flight behavior of infected honey bee (Apis mellifera) foragers. Apidologie 2010, 41, 21–28. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic stress in the honeybee apis mellifera from nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Y.; Zhang, S.; Chen, S.; Li, W.; Yan, L.; Shi, L.; Wu, L.; Sohr, A.; Su, S. Viral infection affects sucrose responsiveness and homing ability of forager honey bees, Apis mellifea L. PLoS ONE 2013, 8, e77354. [Google Scholar]
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Beslay, D.; Costagliola, G.; Soubeyrand, S.; Kretzchmar, A.; Le Conte, Y. Flight behavior and pheromone changes associated to nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invertebr. Pathol. 2013, 113, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Benaets, K.; Van Geystelen, A.; Cardoen, D.; De Smet, L.; de Graaf, D.C.; Schoofs, L.; Larmuseau, M.H.D.; Brettell, L.E.; Martin, S.J.; Wenseleers, T. Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proc. R. Soc. B Biol. Sci. 2017, 284. [Google Scholar] [CrossRef] [PubMed]
- Dosselli, R.; Grassl, J.; Carson, A.; Simmons, L.W.; Baer, B. Flight behaviour of honey bee (Apis mellifera) workers is altered by initial infections of the fungal parasite nosema apis. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hallman, G.J. Control of stored product pests by ionizing radiation. J. Stored Prod. Res. 2013, 52, 36–41. [Google Scholar] [CrossRef]
- Kume, T.; Furuta, M.; Todoriki, S.; Uenoyama, N.; Kobayashi, Y. Status of food irradiation in the world. Radiat. Phys. Chem. 2009, 78, 222–226. [Google Scholar] [CrossRef]
- Hornitzky, M.A.Z. Commercial use of gamma radiation in the beekeeping industry. Bee World 1994, 75, 135–142. [Google Scholar] [CrossRef]
- Gochnauer, T.A.; Hamilton, H.A. Disinfection of honeybee combs by gamma irradiation I. American foul brood disease. J. Apic. Res. 1970, 9, 87–94. [Google Scholar] [CrossRef]
- Gosselin, P.; Charbonneau, R. Disinfection of the bee hive’s american foulbrood by gamma radiation from cobalt-60. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1990, 35, 292–295. [Google Scholar] [CrossRef]
- Baggio, A.; Gallina, A.; Dainese, N.; Manzinello, C.; Mutinelli, F.; Serra, G.; Colombo, R.; Carpana, E.; Sabatini, A.G.; Wallner, K.; et al. Gamma radiation: A sanitating treatment of afb-contaminated beekeeping eqiupment. Apiacta 2005, 40, 22–27. [Google Scholar]
- De Guzman, Z.M.; Cervancia, C.R.; Dimasuay, K.G.B.; Tolentino, M.M.; Abrera, G.B.; Cobar, M.L.C.; Fajardo, A.C., Jr.; Sabino, N.G.; Manila-Fajardo, A.C.; Feliciano, C.P. Radiation inactivation of Paenibacillus larvae and sterilization of american foul brood (afb) infected hives using co-60 gamma rays. Appl. Radiat. Isot. 2011, 69, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Hume, A.; Ames, J.; Rennick, L.; Duprex, W.; Marzi, A.; Tonkiss, J.; Mühlberger, E. Inactivation of rna viruses by gamma irradiation: A study on mitigating factors. Viruses 2016. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.C.; Davies, A.G.; Dulac, G.C.; Willis, N.G.; Papp-Vid, G.; Girard, A. Gamma ray inactivation of some animal viruses. Can. J. Comp. Med. 1981, 45, 397–399. [Google Scholar] [PubMed]
- Katznelson, H.; Robb, J. The use of gamma radiation from cobalt-60 in the control of diseases of the honeybee and the sterilization of honey. Can. J. Microbiol. 1962, 8, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Pankiw, P.; Bailey, L.; Gochnauer, T.A.; Hamilton, H.A. Disinfection of honeybee combs by gamma irradiation. II. European foul brood disease. J. Apic. Res. 1970, 9, 165–168. [Google Scholar] [CrossRef]
- Spivak, M.; Reuter, G.S. Resistance to american foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie 2001, 32, 555–565. [Google Scholar] [CrossRef]
- Rinderer, T.E.; Harris, J.W.; Hunt, G.; de Guzman, L.I. Breeding for resistance to Varroa destructor in north america. Apidologie 2010, 41, 409–424. [Google Scholar] [CrossRef]
- Büchler, R.; Berg, S.; Le Conte, Y. Breeding for resistance to Varroa destructor in europe. Apidologie 2010, 41, 393–408. [Google Scholar] [CrossRef]
- Invernizzi, C.; Rivas, F.; Bettucci, L. Resistance to chalkbrood disease in apis mellifera l. (hymenoptera: Apidae) colonies with different hygienic behaviour. Neotrop. Entomol. 2011, 40, 28–34. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Guzman-Novoa, E.; Gary, N.E. Genotypic variability of components of foraging behavior in honey bees (hymenoptera: Apidae). J. Econ. Entomol. 1993, 86, 715–721. [Google Scholar] [CrossRef]
- Pankiw, T.; Tarpy, D.R.; Page, R.E. Genotype and rearing environment affect honeybee perception and foraging behaviour. Anim. Behav. 2002, 64, 663–672. [Google Scholar] [CrossRef]
- Scheiner, R.; Page, R.E.; Erber, J. Responsiveness to sucrose affects tactile and olfactory learning in preforaging honey bees of two genetic strains. Behav. Brain Res. 2001, 120, 67–73. [Google Scholar] [CrossRef]
- Lach, L.; Kratz, M.; Baer, B. Parasitized honey bees are less likely to forage and carry less pollen. J. Invertebr. Pathol. 2015, 130, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Rinderer, T.E.; de Guzman, L.I.; Delatte, G.T.; Stelzer, J.A.; Kuznetsov, V.N.; Beaman, L.D.; Watts, R.; Harris, J. Resistance to the parasitic mite Varroa destructor in honey bees from far-eastern russia. Apidologie 2001, 32, 381–394. [Google Scholar] [CrossRef]
- Rinderer, T.E.; de Guzman, L.I.; Danka, R. A new phase begins for the usda-ars russian honey bee breeding program. Am. Bee J. 2005, 145, 579–582. [Google Scholar]
- Stelzer, R.J.; Chittka, L. Bumblebee foraging rhythms under the midnight sun measured with radiofrequency identification. BMC Biol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.J.H.; Feinerman, O.; Franks, N.R. Flexible task allocation and the organization of work in ants. Proc. R. Soc. B Biol. Sci. 2009, 276, 4373–4380. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Devillers, J.; Aupinel, P.; Brun, F.; Bagnis, C.; Fourrier, J.; Gauthier, M. Honeybee tracking with microchips: A new methodology to measure the effects of pesticides. Ecotoxicology 2011, 20, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Tenczar, P.; Lutz, C.C.; Rao, V.D.; Goldenfeld, N.; Robinson, G.E. Automated monitoring reveals extreme interindividual variation and plasticity in honeybee foraging activity levels. Anim. Behav. 2014, 95, 41–48. [Google Scholar] [CrossRef]
- Schneider, C.W.; Tautz, J.; Grünewald, B.; Fuchs, S. Rfid tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of apis mellifera. PLoS ONE 2012, 7, e30023. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.; Coulson, M.; Ruddle, N.; Wilkins, S.; Harkin, S. Thiamethoxam: Assessing flight activity of honeybees foraging on treated oilseed rape usinf radio frequency identification technology. Environ. Toxicol. Chem. 2016, 35, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Pankiw, T.; Page, R.E. Genotype and colony environment affect honeybee (Apis mellifera L.) development and foraging behavior. Behav. Ecol. Sociobiol. 2001, 51, 87–94. [Google Scholar]
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.D.; Pettis, J.; vanEngelsdorp, D. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012, 58, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, H.; Miranda, L.; Lin, S. Serious overestimation in quantitative pcr by circular (supercoiled) plasmid standard: Microalgal pcna as the model gene. PLoS ONE 2010, 5, e9545. [Google Scholar] [CrossRef] [PubMed]
- Cavigli, I.; Daughenbaugh, K.F.; Martin, M.; Lerch, M.; Banner, K.; Garcia, E.; Brutscher, L.M.; Flenniken, M.L. Pathogen prevalence and abundance in honey bee colonies involved in almond pollination. Apidologie 2016, 47, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Fries, I.; Chauzat, M.-P.; Chen, Y.-P.; Doublet, V.; Genersch, E.; Gisder, S.; Higes, M.; McMahon, D.P.; Martín-Hernández, R.; Natsopoulou, M.; et al. Standard methods for nosema research. J. Apic. Res. 2013, 52, 1–28. [Google Scholar] [CrossRef]
- Rogers, L.E.; Gilbert, R.O.; Burgett, M. Sampling honeybee colonies for brood production: A double sampling technique. J. Apic. Res. 1983, 22, 232–241. [Google Scholar] [CrossRef]
- SAS Institute. Sas Technical Report P-229, Sas/Stat Software: Changes and Enhancements, Release 6.07; SAS Institute: Cary, NC, USA, 1992; p. 620. [Google Scholar]
- Beling, I. Über das zeitgedächtnis der bienen. Z. Vgl. Physiol. 1929, 9, 259–338. [Google Scholar] [CrossRef]
- Rinkevich, F.D.; Margotta, J.W.; Pittman, J.M.; Danka, R.G.; Tarver, M.R.; Ottea, J.A.; Healy, K.B. Genetics, synergists, and age affect insecticide sensitivity of the honey bee, apis mellifera. PLoS ONE 2015, 10, e0139841. [Google Scholar] [CrossRef] [PubMed]
- Waddington, K.D.; Herbert, T.J.; Visscher, P.K.; Richter, M.R. Comparisons of forager distributions from matched honey bee colonies in suburban environments. Behav. Ecol. Sociobiol. 1994, 35, 423–429. [Google Scholar] [CrossRef]
- Tubbs, H.; Harper, C.; Bigalk, M.; Bernard, S.J.; Delatte, G.T.; Sylvester, H.A.; Rinderer, T.E. Commercial management of ars russian honey bees. Am. Bee J. 2003, 143, 819–820. [Google Scholar]
- Rinderer, T.E.; de Guzman, L.I.; Harper, C. The effects of co-mingled russian and italian honey bee stocks and sunny or shaded apiaries on varroa mite infestation level, worker bee population and honey production. Am. Bee J. 2004, 144, 481–485. [Google Scholar]
- Rinderer, T.E.; de Guzman, L.I.; Delatte, G.T.; Stelzer, J.A.; Lancaster, V.A.; Williams, J.L.; Beaman, L.D.; Kuznetsov, V.; Bigalk, M.; Bernard, S.J.; et al. Multi-state field trials of ars russian honey bees 2. Honey production 1999, 2000. Am. Bee J. 2001, 141, 726–729. [Google Scholar]
- Visscher, P.K.; Dukas, R. Survivorship of foraging honey bees. Insectes Sociaux 1997, 44, 1–5. [Google Scholar] [CrossRef]
- Janmaat, A.F.; Winston, M.L. The influence of pollen storage area and varroa jacobsoni oudemans parasitism on temporal caste structure in honey bees (Apis mellifera L.). Insectes Sociaux 2000, 47, 177–182. [Google Scholar] [CrossRef]
- Colwell, M.J.; Currie, R.W.; Pernal, S.F. Viruses in unexpected places: New transmission routes of european honey bee (Apis mellifera) viruses. In Proceedings of the American Bee Research Conference, Galveston, TX, USA, 12–13 January 2017; Simone-Finstrom, M., Ed.; Bee World: Galveston, TX, USA, 2017. [Google Scholar]
- Goblirsch, M.; Huang, Z.Y.; Spivak, M. Physiological and behavioral changes in honey bees (Apis mellifera) induced by nosema ceranae infection. PLoS ONE 2013, 8, e58165. [Google Scholar] [CrossRef] [PubMed]
- Eiri, D.M.; Suwannapong, G.; Endler, M.; Nieh, J.C. Nosema ceranae can infect honey bee larvae and reduces subsequent adult longevity. PLoS ONE 2015, 10, e0126330. [Google Scholar] [CrossRef] [PubMed]
- Ptaszyńska, A.A.; Borsuk, G.; Anusiewicz, M.; Mułenko, W. Location of Nosema spp. Spores within the body of the honey bee. Med. Weter. 2012, 68, 618–621. [Google Scholar]
- Kurze, C.; Mayack, C.; Hirche, F.; Stangl, G.I.; Le Conte, Y.; Kryger, P.; Moritz, R.F.A. Nosema spp. Infections cause no energetic stress in tolerant honeybees. Parasitol. Res. 2016, 115, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, A.L.; Rinderer, T.E.; Sylvester, H.A.; Holloway, B.; Oldroyd, B.P. Patterns of Apis mellifera infestation by nosema ceranae support the parasite hypothesis for the evolution of extreme polyandry in eusocial insects. Apidologie 2012, 43, 539–548. [Google Scholar] [CrossRef]

| Honey Bee Stock | Comb Treatment | RFID-Tagged Bees | # Paint-Marked Bees | ||||
|---|---|---|---|---|---|---|---|
| Cohort A | Cohort B | Cohort C | Cohort D | Total | |||
| Italian | Non-irradiated | 38 (2) | 55 (2) | 30 (2) | 30 (1) | 153 (7) | 487 |
| Irradiated | 78 (2) | 33 (2) | 43 (2) | 42 (2) | 196 (8) | 646 | |
| Russian | Non-irradiated | 76 (2) | 16 (2) | 30 (2) | 29 (1) | 151 (7) | 584 |
| Irradiated | 52 (2) | 41 (2) | 38 (2) | 58 (2) | 189 (8) | 686 | |
| TOTAL | 244 (8) | 145 (8) | 141 (8) | 159 (6) | 689 (30) | 2403 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Guzman, L.I.; Frake, A.M.; Simone-Finstrom, M. Comparative Flight Activities and Pathogen Load of Two Stocks of Honey Bees Reared in Gamma-Irradiated Combs. Insects 2017, 8, 127. https://doi.org/10.3390/insects8040127
De Guzman LI, Frake AM, Simone-Finstrom M. Comparative Flight Activities and Pathogen Load of Two Stocks of Honey Bees Reared in Gamma-Irradiated Combs. Insects. 2017; 8(4):127. https://doi.org/10.3390/insects8040127
Chicago/Turabian StyleDe Guzman, Lilia I., Amanda M. Frake, and Michael Simone-Finstrom. 2017. "Comparative Flight Activities and Pathogen Load of Two Stocks of Honey Bees Reared in Gamma-Irradiated Combs" Insects 8, no. 4: 127. https://doi.org/10.3390/insects8040127