Black Border Increases Stomoxys calcitrans Catch on White Sticky Traps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trap Design
2.2. Experimental Design
2.3. Data Analyses
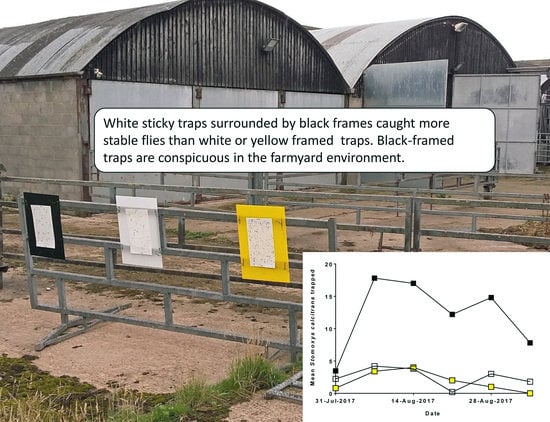
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taylor, D.B.; Moon, R.D.; Mark, D.R. Economic impact of stable flies (Diptera: Muscidae) on dairy and beef cattle production. J. Med. Entomol. 2012, 49, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Baldacchino, F.; Muenworn, V.; Desquesnes, M.; Desoli, F.; Charoenviriyaphap, T.; Duvallet, G. Transmission of pathogens by Stomoxys flies (Diptera, Muscidae): A review. Parasite 2013, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Broce, A.B. An improved alsynite trap for stable flies, Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 1988, 25, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. Sticky traps for sampling populations of Stomoxys calcitrans. J. Econ. Entomol. 1973, 66, 1279–1280. [Google Scholar] [CrossRef] [PubMed]
- Agee, H.R.; Patterson, R.S. Spectral sensitivity of stable, face, and horn flies and behavioral responses of stable flies to visual traps (Diptera: Muscidae). Environ. Entomol. 1983, 12, 1823–1828. [Google Scholar] [CrossRef]
- Beresford, D.V.; Sutcliffe, J.F. Studies on the effectiveness of Coroplast sticky traps for sampling stable flies (Diptera: Muscidae), including a comparison to alsynite. J. Econ. Entomol. 2006, 99, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Cilek, J.E. Attraction of colored plasticized corrugated boards to adult stable flies, Stomoxys calcitrans (Diptera: Muscidae). Fla. Entomol. 2003, 86, 420–423. [Google Scholar] [CrossRef]
- Zhu, J.J.; Zhang, Q.-h.; Taylor, D.B.; Friesen, K.A. Visual and olfactory enhancement of stable fly trapping. Pest Manag. Sci. 2016, 72, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Pospisil, J.; Zdarek, J. On the visual orientation of the stable fly (Stomoxys calcitrans L.) to colours. Acta Entomol. Bohemoslov 1965, 62, 85–91. [Google Scholar]
- Allan, S.A.; Day, J.F.; Edman, J.D. Visual ecology of biting flies. Ann. Rev. Entomol. 1987, 32, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Gatehouse, A.; Lewis, C. Host location behaviour of Stomoxys calcitrans. Entomol. Exp. Appl. 1973, 16, 275–290. [Google Scholar] [CrossRef]
- Cilek, J.E. Attractiveness of beach ball decoys to adult Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 2002, 39, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Geden, C.J. Visual targets for capture and management of house flies, Musca domestica L. J. Vector Ecol. 2006, 31, 152–157. [Google Scholar] [CrossRef]
- Schofield, S. Responses to electrified targets and daily activity of Stomoxys spp. (Diptera: Muscidae) in Zimbabwe. Bull. Entomol. Res. 1998, 88, 627–632. [Google Scholar] [CrossRef]
- Brady, J.; Shereni, W. Landing responses of the tsetse fly Glossina morsitans morsitans Westwood and the stable fly Stomoxys calcitrans (L.) (Diptera: Glossinidae & Muscidae) to black-and-white patterns: A laboratory study. Bull. Entomol. Res. 1988, 78, 301–311. [Google Scholar]
- Beresford, D.V.; Sutcliff, J.F. Stable fly (Stomoxys calcitrans: Diptera, Muscidae) trap response to changes in effective trap height caused by growing vegetation. J. Vector Ecol. 2008, 33, 40–45. [Google Scholar] [CrossRef]
- Solorzano, J.A.; Gilles, J.; Bravo, O.; Vargas, C.; Gomez-Bonilla, Y.; Bingham, G.V.; Taylor, D.B. Biology and trapping of stable flies (Diptera: Muscidae) developing in pineapple residues (Ananas comosus) in Costa Rica. J. Insect Sci. 2015, 15, 145. [Google Scholar] [CrossRef] [PubMed]
- Broce, A.B.; Hogsette, J.; Paisley, S. Winter feeding sites of hay in round bales as major developmental sites of Stomoxys calcitrans (Diptera: Muscidae) in pastures in spring and summer. J. Econ. Entomol. 2005, 98, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Foil, L.; Hogsette, J. Biology and control of tabanids, stable flies and horn flies. Rev. Sci. Tech. Off. Int. Epizoot. 1994, 13, 1125–1158. [Google Scholar] [CrossRef]
- Gilles, J.; David, J.F.; Duvallet, G.; De La Rocque, S.; Tillard, E. Efficiency of traps for Stomoxys calcitrans and Stomoxys niger niger on Reunion Island. Med. Vet. Entomol. 2007, 21, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Ose, G.A.; Hogsette, J.A. Spatial distribution, seasonality and trap preference of stable fly, Stomoxys calcitrans L. (Diptera: Muscidae), adults on a 12-hectare zoological park. Zoo Biol. 2014, 33, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Beresford, D.V.; Sutcliffe, J.F. Evidence for sticky-trap avoidance by stable fly, Stomoxys calcitrans (Diptera: Muscidae), in response to trapped flies. J. Am. Mosq. Control Assoc. 2017, 33, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.J.; Wall, R. Effects of contrast on attraction of the housefly, Musca domestica, to visual targets. Med. Vet. Entomol. 1998, 12, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Buschman, L.L.; Patterson, R.S. Assembly, mating, and thermoregulating behavior of stable flies under field conditions. Environ. Entomol. 1981, 10, 16–21. [Google Scholar] [CrossRef]
- Schofield, S.; Torr, S.J. Behavioural modalities of ‘non-vector’ biting Diptera: From olfaction to feeding. In Olfaction in Vector-Host Interactions. Ecology and Control of Vector-Borne Diseases; Takken, W., Knols, B.G.J., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2010; Volume 2, pp. 291–308. [Google Scholar]
- Parr, H. Studies on Stomoxys calcitrans (L.) in Uganda, East Africa. II.—Notes on life-history and behaviour. Bull. Entomol. Res. 1962, 53, 437–443. [Google Scholar] [CrossRef]
- Waage, J.K. How the zebra got its stripes-biting flies as selective agents in the evolution of zebra coloration. J. Entomol. Soc. South. Afr. 1981, 44, 351–358. [Google Scholar]
- Gibson, G. Do tsetse flies ‘see’ zebras? A field study of the visual response of tsetse to striped targets. Physiol. Entomol. 1992, 17, 141–147. [Google Scholar] [CrossRef]
- Caro, T.; Izzo, A.; Reiner, R.C., Jr.; Walker, H.; Stankowich, T. The function of zebra stripes. Nat. Commun. 2014, 5, 3535. [Google Scholar] [CrossRef] [PubMed]
- Aak, A.; Birkemoe, T.; Mehl, R. Blowfly (Diptera, Calliphoridae) damage on stockfish in northern Norway: Pest species, damage assessment and the potential of mass trapping. J. Pest Sci. 2010, 83, 329–337. [Google Scholar] [CrossRef]
- Goulson, D.; Hughes, W.O.H.; Chapman, J.W. Fly populations associated with landfill and composting sites used for household refuse disposal. Bull. Entomol. Res. 1999, 89, 493–498. [Google Scholar] [CrossRef]
- Bilaniuk, V.; Beresford, D.V. Sampling adult blow flies (Diptera: Calliphoridae) at pig carcasses with sticky traps: Effects of trap colour, height, and inclination. Can. Soc. Forensic Sci. J. 2010, 43, 181–190. [Google Scholar] [CrossRef]
- Lunau, K. Visual ecology of flies with particular reference to colour vision and colour preferences. J. Comp. Physiol. A 2014, 200, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, M.; Lunau, K.; Dorin, A.; Schulze, B.; Bischoff, M.; Burd, M.; Dyer, A.G. Floral colours in a world without birds and bees: The plants of Macquarie Island. Plant. Biol. 2016, 18, 842–850. [Google Scholar] [CrossRef] [PubMed]

| Species | Site Habitat | Frame Colour | Mean No. of Flies per Week | Confidence Intervals |
|---|---|---|---|---|
| Stomoxys calcitrans | Farmyard | Black | 20.29 | (9.98–41.25) |
| White | 4.13 | (1.97–8.67) | ||
| Yellow | 3.12 | (1.47–6.61) | ||
| Pasture | Black | 0.83 | (0.29–2.38) | |
| White | 0.17 | (0.03–0.85) | ||
| Yellow | 0.17 | (0.03–0.85) | ||
| Pollenia sp. | Farmyard | Black | 0.17 | (0.03–0.87) |
| White | 0.06 | (0.01–0.60) | ||
| Yellow | 0.63 | (0.17–2.29) | ||
| Pasture | Black | 2.17 | (0.53–8.92) | |
| White | 0.42 | (0.08–2.10) | ||
| Yellow | 6.58 | (1.66–26.17) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murchie, A.K.; Hall, C.E.; Gordon, A.W.; Clawson, S. Black Border Increases Stomoxys calcitrans Catch on White Sticky Traps. Insects 2018, 9, 13. https://doi.org/10.3390/insects9010013
Murchie AK, Hall CE, Gordon AW, Clawson S. Black Border Increases Stomoxys calcitrans Catch on White Sticky Traps. Insects. 2018; 9(1):13. https://doi.org/10.3390/insects9010013
Chicago/Turabian StyleMurchie, Archie K., Carol E. Hall, Alan W. Gordon, and Sam Clawson. 2018. "Black Border Increases Stomoxys calcitrans Catch on White Sticky Traps" Insects 9, no. 1: 13. https://doi.org/10.3390/insects9010013