Differential Regulation of Immune Signaling and Survival Response in Drosophila melanogaster Larvae upon Steinernema carpocapsae Nematode Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Strains
2.2. Nematodes
2.3. Gene Transcript Level Analysis
2.4. Survival Experiments
2.5. Statistical Analysis
3. Results
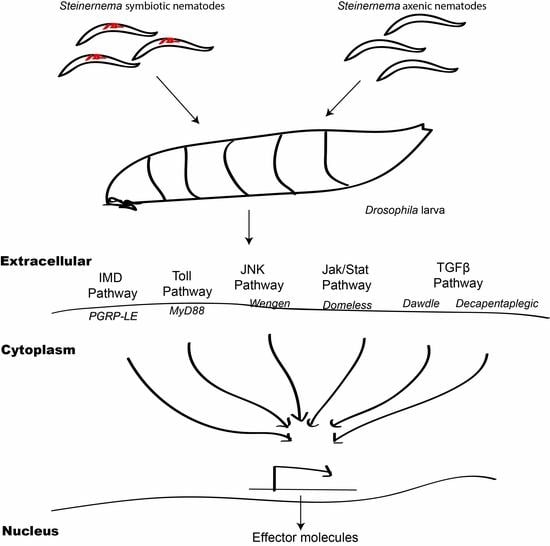
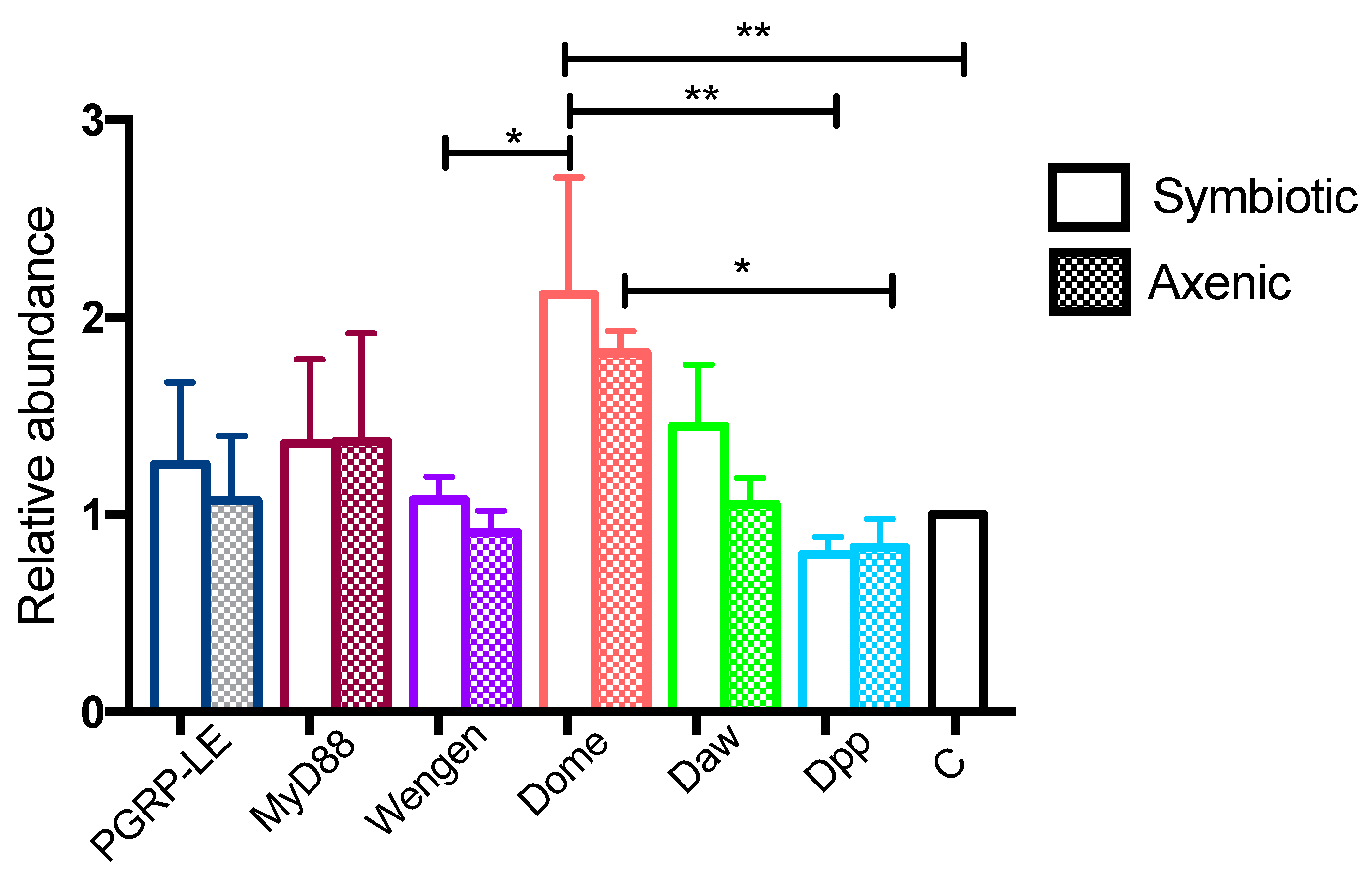
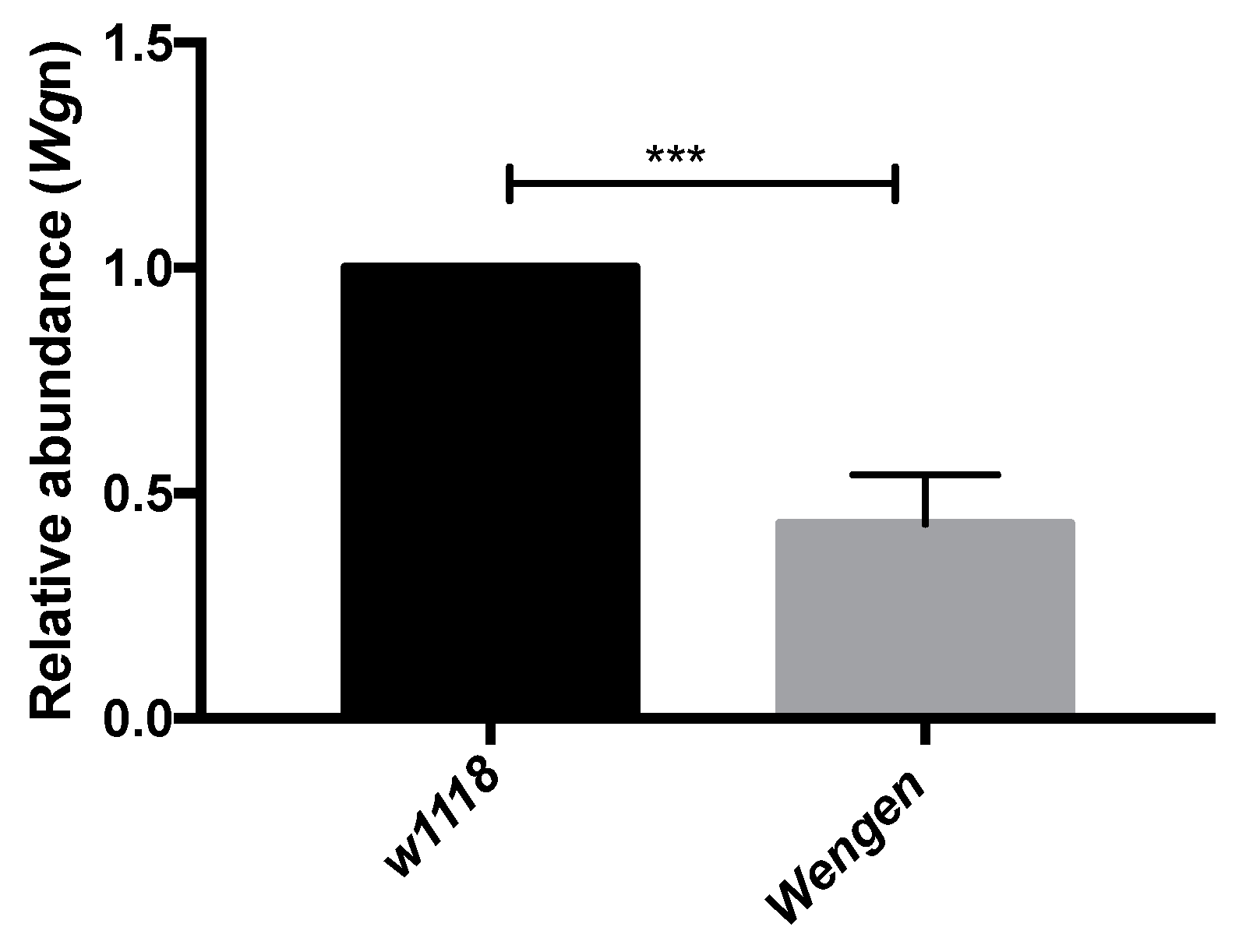
3.1. Infection with S. carpocapsae Axenic Nematodes Results in Elevated Transcript Levels of Immune-Related Signaling Pathway Genes in D. melanogaster Larvae
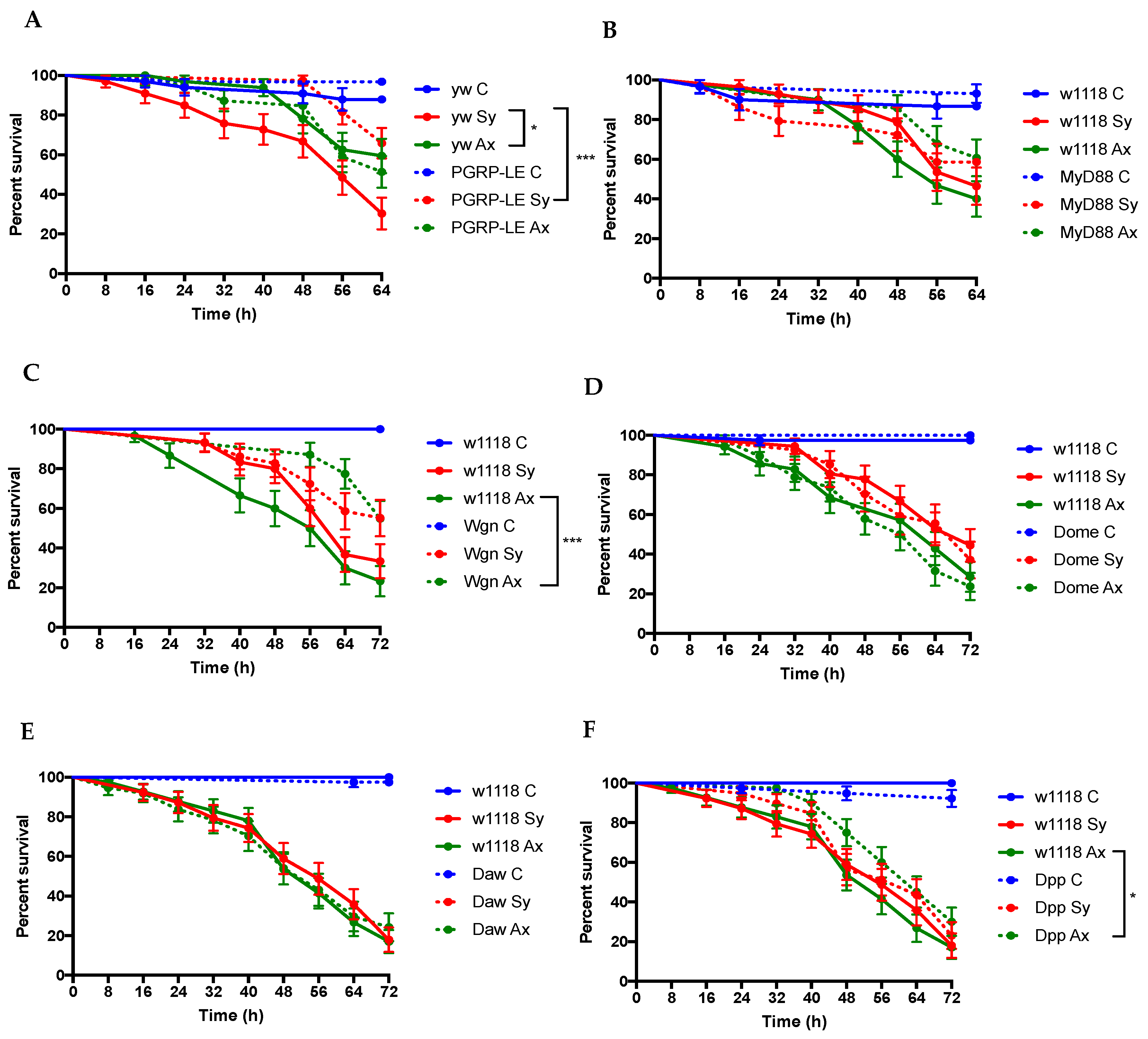
3.2. D. melanogaster Imd, Jnk, and TGFβ Pathway Mutants Exhibit Enhanced Survival to Infection by S. carpocapsae Nematodes
4. Discussion
5. Introduction
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix B
Validation of RNA-Seq Data Using qRT-PCR
| Gene | Mutant Genotype | Nature of Mutation | Background Strain |
|---|---|---|---|
| PGRP-LE | y1 w67c23 PGRP-LE112 | P-element activity | yw |
| MyD88 | NA | P-element activity | w1118 |
| Wengen | P{GD3427}v9152 | Transposable element insertion | w1118 |
| Domeless | NA | Homozygous viable | w1118 |
| Dawdle | w*; P{GawB}dawNP4661/CyO | P-element insertion | w1118 |
| Decapentaplegic | NA | Spontaneous | w1118 |
| Gene | Accession Number | Primer | Sequence | Tm (°C) |
|---|---|---|---|---|
| PGRP-LE | CG8995 | Forward Reverse | ATTGCAGAGTCCTCGGTTGTG TTCACTGGTATTTTGGTCGGC | 61 |
| MyD88 | CG2078 | Forward Reverse | ATCTGGAACACTTCCTGGGC CCACGAGAGCAGTCTGTCG | 61 |
| Wengen | CG6531 | Forward Reverse | ACCATCTGCGGTTCCATATACG CTGCTCATACTCGGAGGACTT | 61 |
| Domeless | CG14226 | Forward Reverse | GGCGGCGACTTTAATCTGAG GGTGTTGTTCAGGATTCGGAT | 61 |
| Dawdle | CG16987 | Forward Reverse | GGTGGATCAGCAGAAGGACT CCCACTGATCCAGTGTTTGA | 61 |
| Decapentaplegic | CG9885 | Forward Reverse | CCTTGGAGCCTCTGTCGAT TGCACTCTGATCTGGGATTTT | 61 |
| RpL32 | CG7939 | Forward Reverse | GATGACCATCCGCCCAGCA CGGACCGACAGCTGCTTGGC | 61 |
References
- Poinar, G.O., Jr. The Natural History of Nematodes; Prentice Hall: Englewood Cliffs, NJ, USA, 1983. [Google Scholar]
- Lacey, L.A.; Georgis, R. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J. Nematol. 2012, 44, 218–225. [Google Scholar] [PubMed]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Reynolds, S.E.; Eleftherianos, I. Insect immune responses to nematode parasites. Trends Parasitol. 2011, 27, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Eleftherianos, I. Parasitic nematode immunomodulatory strategies: Advances and perspectives. Pathogens 2016, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.M.; Carrillo, M.A.; Hallem, E.A. Variation in the susceptibility of Drosophila to different entomopathogenic nematodes. Infect. Immun. 2015, 8, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Daugherty, S.; Shetty, A.C.; Eleftherianos, I. RNAseq analysis of the Drosophila response to the entomopathogenic nematode Steinernema. G3 (Bethesda) 2017, 7, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Goodrich-Blair, H. The Steinernema carpocapsae intestinal vesicle contains a subcellular structure with which Xenorhabdus nematophila associates during colonization initiation. Cell Microbiol. 2005, 7, 1723–1735. [Google Scholar] [CrossRef] [PubMed]
- Herbert, E.E.; Goodrich-Blair, H. Friend and foe: The two faces of Xenorhabdus nematophila. Nat. Rev. Microbiol. 2007, 5, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Goodrich-Blair, H. They’ve got a ticket to ride: Xenorhabdus nematophila-Steinernema carpocapsae symbiosis. Curr. Opin. Microbiol. 2007, 10, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Montiel, R.; Abubucker, S.; Mitreva, M.; Simões, N. Transcripts analysis of the entomopathogenic nematode Steinernema carpocapsae induced in vitro with insect hemolymph. Mol. Biochem. Parasitol. 2010, 169, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.R.; Goodrich-Blair, H. Master of conquest and pillage: Xenorhabdus nematophila global regulators control transitions from virulence to nutrient acquisition. Cell Microbiol. 2009, 11, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- McMullen, J.G.; Stock, S.P. In vivo and in vitro rearing of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae). J. Vis. Exp. 2014, 22, 52096. [Google Scholar]
- Yadav, S.; Shokal, U.; Forst, S.; Eleftherianos, I. An improved method for generating axenic entomopathogenic nematodes. BMC Res. Notes 2015, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Kounatidis, I.; Ligoxygakis, P. Drosophila as a model system to unravel the layers of innate immunity to infection. Open Biol. 2012, 2, 120075. [Google Scholar] [CrossRef] [PubMed]
- Arefin, B.; Kucerova, L.; Dobes, P.; Markus, R.; Strnad, H.; Wang, Z.; Hyrsl, P.; Zurovec, M.; Theopold, U. Genome-wide transcriptional analysis of Drosophila larvae infected by entomopathogenic nematodes shows involvement of complement, recognition and extracellular matrix proteins. J. Innate Immun. 2014, 6, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Creasy, T.; Kumari, P.; Shetty, A.; Shokal, U.; Tallon, L.J.; Eleftherianos, I. Drosophila anti-nematode and antibacterial immune regulators revealed by RNA-seq. BMC Genom. 2015, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Binda-Rossetti, S.; Mastore, M.; Protasoni, M.; Brivio, M.F. Effects of an entomopathogen nematode on the immune response of the insect pest red palm weevil: Focus on the antimicrobial response. J. Invertebr. Pathol. 2016, 133, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Tauszig-Delmasure, S.; Bilak, H.; Capovilla, M.; Hoffmann, J.A.; Imler, J.L. Drosophila MyD88 is required for the response to fungal and Gram-positive bacterial infections. Nat. Immunol. 2002, 3, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kronhamn, J.; Ekström, J.O.; Korkut, G.G.; Hultmark, D. JAK/STAT signaling in Drosophila muscles controls the cellular immune response against parasitoid infection. EMBO Rep. 2015, 16, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Gramates, S.L.; Marygold, S.J.; dos Santos, G.; Urbano, J.; Antonazzo, G.; Matthews, B.B.; Rey, A.J.; Tabone, C.J.; Crosby, M.A.; Ennert, D.B.; et al. FlyBase at 25: Looking into the future. Nucleic Acid Res. 2017, 45, D663–D671. [Google Scholar] [CrossRef] [PubMed]
- Ekengren, S.; Tryselius, Y.; Dushay, M.S.; Liu, G.; Steiner, H.; Hultmark, D. A humoral stress response in Drosoph. Curr. Biol. 2001, 11, 714–718. [Google Scholar] [CrossRef]
- White, G.F. A method for obtaining infective nematode larvae from cultures. Science 1927, 66, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.R. ‘Validation’ in genome-scale research. J. Biol. 2009, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Shokal, U.; Eleftherianos, I. Immune gene transcription in Drosophila adult flies infected by entomopathogenic nematodes and their mutualistic bacteria. J. Insect Physiol. 2013, 59, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Park, Y.; Kim, Y.; Hwang, J.; Lee, D. An entomopathogenic bacterium, Xenorhabdus nematophila, suppresses expression of antimicrobial peptides controlled by Toll and Imd pathways by blocking eicosanoid biosynthesis. Arch. Insect Biochem. Physiol. 2013, 83, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Hussa, E.A.; Casanova-Torres, A.M.; Goodrich-Blair, H. The global transcription factor Lrp controls virulence modulation in Xenorhabdus nematophila. J. Bacteriol. 2015, 197, 3015–3025. [Google Scholar] [CrossRef] [PubMed]
- Aymeric, J.L.; Givaudan, A.; Duvic, B. Imd pathway is involved in the interaction of Drosophila melanogaster with the entomopathogenic bacteria, Xenorhabdus nematophila and Photorhabdus luminescens. Mol. Immunol. 2012, 47, 2342–2348. [Google Scholar] [CrossRef] [PubMed]
- Hallem, E.A.; Rengarajan, M.; Ciche, T.A.; Sternberg, P.W. Nematodes, bacteria, and flies: A tripartite model for nematode parasitism. Curr. Biol. 2007, 17, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Castillo, J.C.; Patrnogic, J. TGF-β signaling regulates resistance to parasitic nematode infection in Drosophila melanogaster. Immunobiology 2016, 221, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Noselli, S.; Angès, F. Roles of the JNK signaling pathway in Drosophila morhogenesis. Curr. Opin. Genet. Dev. 1999, 9, 466–472. [Google Scholar] [CrossRef]
- Stronach, B.E.; Perrimon, N. Stress signaling in Drosophila. Oncogene 1999, 18, 6172–6182. [Google Scholar] [CrossRef] [PubMed]
- Rämet, M.; Lanot, R.; Zachary, D.; Manfruelli, P. JNK signaling pathway is required for efficient wound healing in Drosophila. Dev. Biol. 2002, 241, 145–156. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, S.; Gupta, S.; Eleftherianos, I. Differential Regulation of Immune Signaling and Survival Response in Drosophila melanogaster Larvae upon Steinernema carpocapsae Nematode Infection. Insects 2018, 9, 17. https://doi.org/10.3390/insects9010017
Yadav S, Gupta S, Eleftherianos I. Differential Regulation of Immune Signaling and Survival Response in Drosophila melanogaster Larvae upon Steinernema carpocapsae Nematode Infection. Insects. 2018; 9(1):17. https://doi.org/10.3390/insects9010017
Chicago/Turabian StyleYadav, Shruti, Sonali Gupta, and Ioannis Eleftherianos. 2018. "Differential Regulation of Immune Signaling and Survival Response in Drosophila melanogaster Larvae upon Steinernema carpocapsae Nematode Infection" Insects 9, no. 1: 17. https://doi.org/10.3390/insects9010017