Potential of Extracted Locusta Migratoria Protein Fractions as Value-Added Ingredients
Abstract
:1. Introduction
2. Materials and Methods
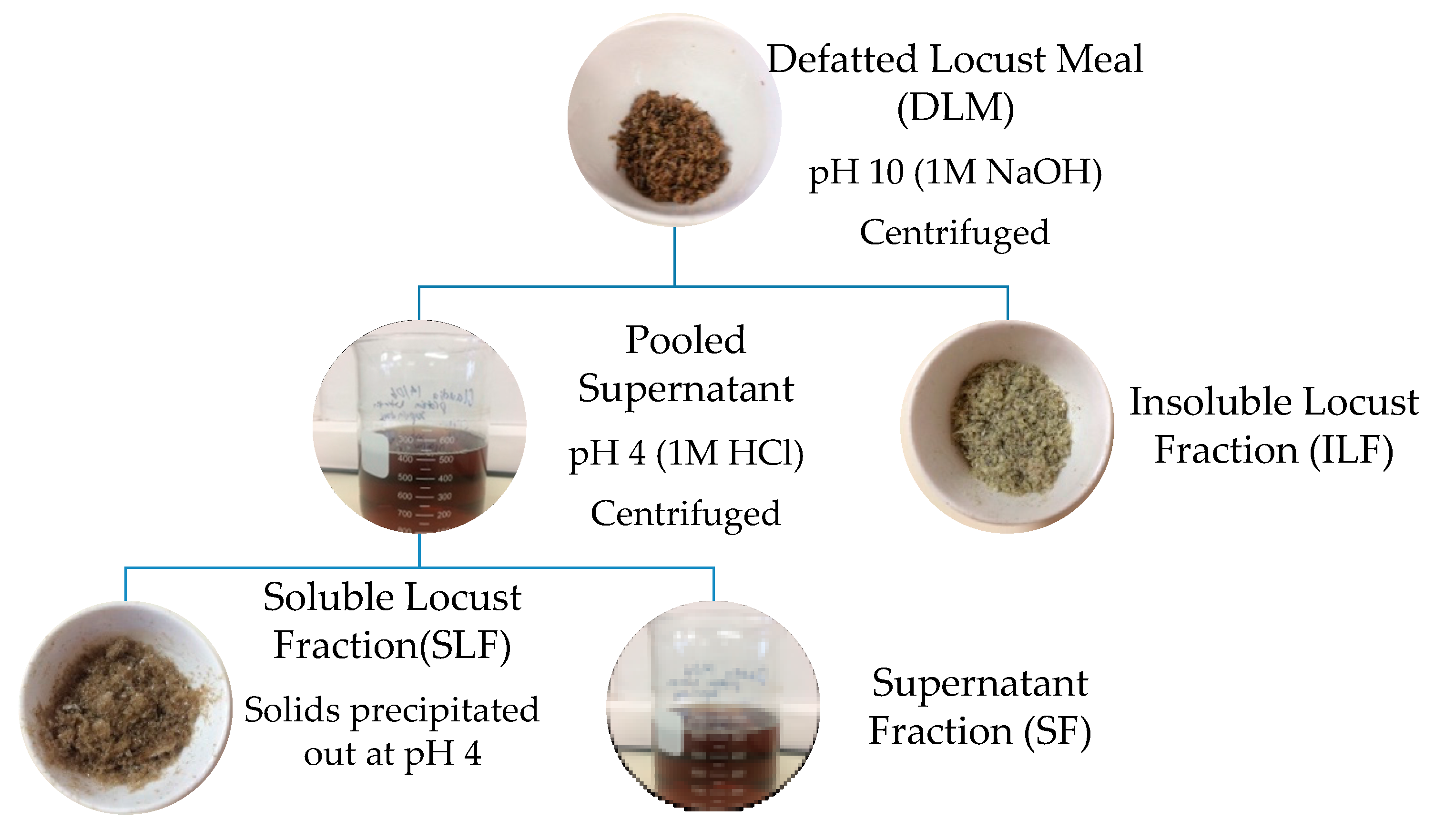
2.1. Preparation of Locust Meal
2.2. Proximate Analysis
2.3. Fatty Acid Composition
2.4. Protein Separation and Properties
2.4.1. Colour
2.4.2. Water and Oil Holding Capacity (WHC & OHC)
2.5. Data Analysis
3. Results
3.1. Proximate Composition
3.2. Fatty Acid Composition
3.3. Protein Properties
3.3.1. Protein Content and Yield of Extracted Fractions
3.3.2. Colour
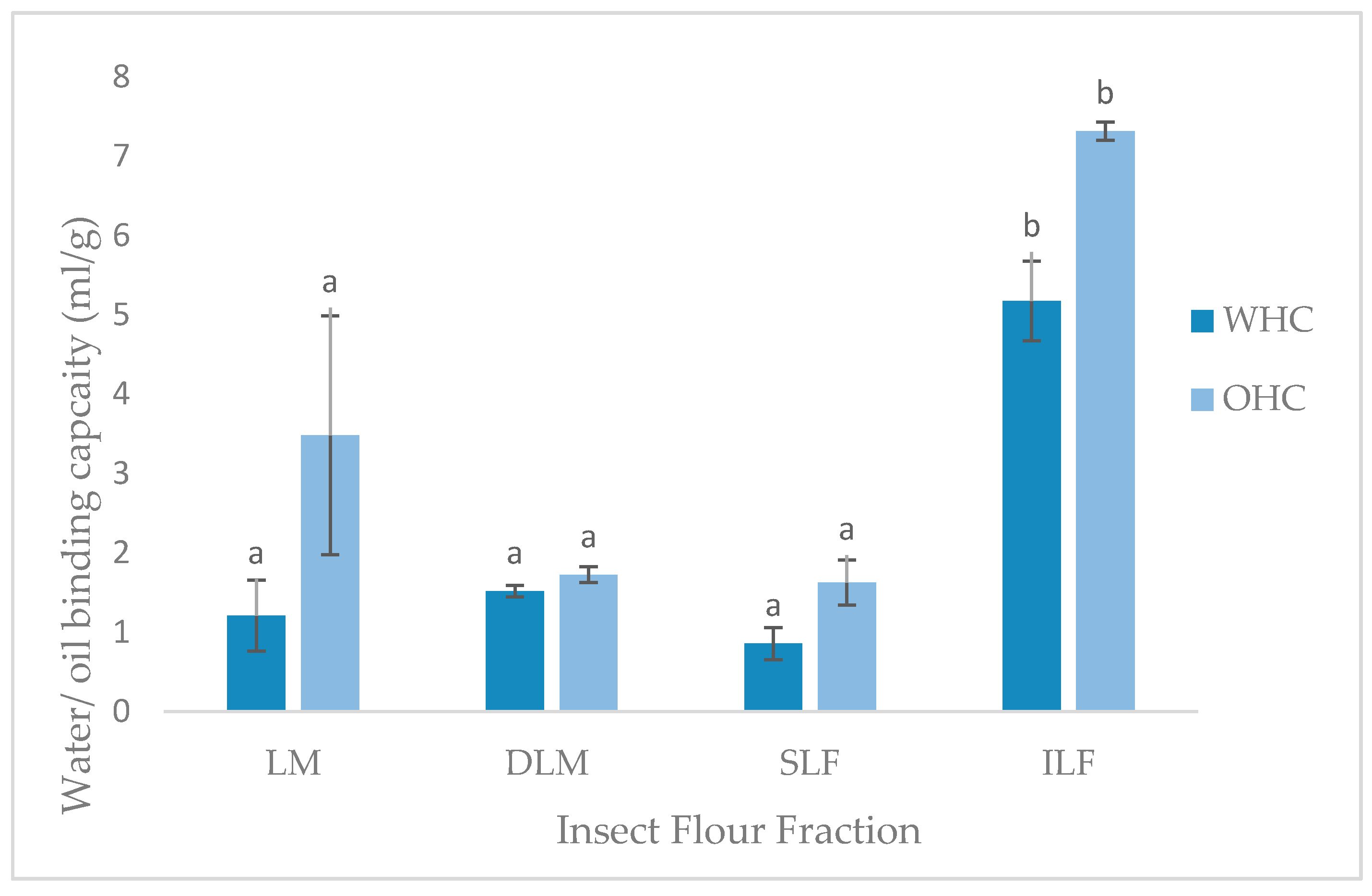
3.3.3. Water and Oil Holding Capacity
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mariod, A.A.; Saeed Mirghani, M.E.; Hussein, I. Chapter 44—Schistocerca gregaria (Desert Locust) and Locusta migratoria (Migratory Locust). In Unconventional Oilseeds and Oil Sources; Academic Press: Cambridge, MA, USA, 2017; pp. 293–297. ISBN 978-0-12-809435-8. [Google Scholar]
- Van Huis, A.; van Itterbeek, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, A.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; ISBN 978-92-5-107596-8. [Google Scholar]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An Exploration on Greenhouse Gas and Ammonia Production by Insect Species Suitable for Animal or Human Consumption. PLoS ONE 2011, 5, e14445. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G.; et al. Insect fatty acids: A comparison of lipids from three Orthopterans and Tenebrio molitor L. larvae. J. Asia-Pac. Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.H.A. Determination of Nutritive value of edible migratory locust Locusta migratoria, Linnaeus, 1758 (Orthoptera: Acrididae). Int. J. Adv. Pharm. Biol. Chem. 2015, 4, 144–148. [Google Scholar]
- Oonincx, D.G.A.B.; van der Poel, A.F.B. Effects of diet on the chemical composition of migratory locusts (Locusta migratoria). Zoo Biol. 2011, 30, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Garofalo, C.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Pasquini, M.; Mozzon, M.; Raffaelli, N.; Ruschioni, S.; et al. Insight into the proximate composition and microbial diversity of edible insects marketed in the European Union. Eur. Food Res. Technol. 2017, 243, 1157–1171. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food. Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Jonas-Levi, A.; Martinez, J.-J.I. The high level of protein content reported in insects for food and feed is overestimated. J. Food Compos. Anal. 2017, 62, 184–188. [Google Scholar] [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow Mealworm Protein for Food Purposes—Extraction and Functional Properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; van Boekel, M.A.J.S.; Boeren, S.; Lakemond, C.M.M. Protein identification and in vitro digestion of fractions from Tenebrio molitor. Eur. Food Res. Technol. 2016, 242, 1285–1297. [Google Scholar] [CrossRef]
- Tan, H.S.G.; Fischer, A.R.H.; van Trijp, H.C.M.; Stieger, M. Tasty but nasty? Exploring the role of sensory-liking and food appropriateness in the willingness to eat unusual novel foods like insects. Food Qual. Preference 2016, 48, 293–302. [Google Scholar] [CrossRef]
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and techno-functionality of flours and proteins from two edible insect species: Meal worm (Tenebrio molitor) and black soldier fly (Hermetia illucens) larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef] [PubMed]
- Ndiritu, A.K.; Kinyuru, J.N.; Kenji, G.M.; Gichuhi, P.N. Extraction technique influences the physico-chemical characteristics and functional properties of edible crickets (Acheta domesticus) protein concentrate. J. Food Meas. Charact. 2017, 11, 2013–2021. [Google Scholar] [CrossRef]
- Del Valle, F.R.; Mena, M.H.; Bourges, H. An investigation into insect protein. J. Food Process. Preserv. 1982, 6, 99–110. [Google Scholar] [CrossRef]
- Purschke, B.; Meinlschmidt, P.; Horn, C.; Rieder, O.; Jäger, H. Improvement of techno-functional properties of edible insect protein from migratory locust by enzymtaic hydrolysis. Eur. Food Res. Technol. 2017. [Google Scholar] [CrossRef]
- Purschke, B.; Tanzmeister, H.; Meinlschmidt, P.; Baumgartner, S.; Lauter, K.; Jäger, H. Recovery of soluble proteins from migratory locust (Locusta migratoria) and characterisation of their comopositional and techno-functional properties. Food Res. Int. 2018, 106, 271–279. [Google Scholar] [CrossRef]
- Poma, G.; Cuykx, M.; Amato, E.; Calaprice, C.; Focant, J.F.; Covaci, A. Evaluation of hazardous chemicals in edible insects and insect-based food intended for human consumption. Food Chem. Toxicol. 2017, 100, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, N.T.; Klein, G. Microbiology of processed edible insect products—Results of a preliminary survey. Int. J. Food. Microbiol. 2017, 243, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Caparros Megido, R.; Desmedt, S.; Blecker, C.; Béra, F.; Haubruge, É.; Alabi, T.; Francis, F. Microbiological Load of Edible Insects Found in Belgium. Insects 2017, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Vandeweyer, D.; Crauwels, S.; Lievens, B.; van Campenhout, L. Microbial counts of mealworm larvae (Tenebrio molitor) and crickets (Acheta domesticus and Gryllodes sigillatus) from different rearing companies and different production batches. Int. J. Food. Microbiol. 2017, 242, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Yen, A.L. Edible insects: Traditional knowledge or western phobia? Entomol. Res. 2009, 39, 289–298. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International; Association of Analytical Communities: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Van Wijngaarden, D. Modified rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem. 1967, 39, 848–849. [Google Scholar] [CrossRef]
- Amza, T.; Amadou, I.; Zhu, K.; Zhou, H. Effect of extraction and isolation on physicochemical and functional properties of an underutilized seed protein: Gingerbread plum (Neocarya macrophylla). Food. Res. Int. 2011, 44, 2843–2850. [Google Scholar] [CrossRef]
- Tirgar, M.; Silcock, P.; Carne, A.; Birch, E.J. Effect of extraction method on functional properties of flaxseed protein concentrates. Food Chem. 2017, 215, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Paoletti, M.G.; Norberto, L.; Cozzarini, E.; Musumeci, S. Binomium Chitin-Chitinase: Recent Issues. In Role of Chitinases in Human Stomach for Chitin Digestion: AMCase in the Gastric Digestion of Chitin and Chit in Gastric Pathologies; Nova Science Publishers, Inc.: New York, NY, USA, 2009; pp. 339–358. ISBN 978-1-60692-339-9. [Google Scholar]
- Mohamed, E.H.A. Fatty acids contents of the edible migratory locust Locusta migratoria, Linnaeus, 1758 (Orthoptera: Acrididae). Int. J. Adv. Pharm. Biol. Chem. 2015, 4, 746–750. [Google Scholar]
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Sánchez-Muros-Lozano, M.J.; García-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- Raksakantong, P.; Meeso, N.; Kubola, J.; Siriamornpun, S. Fatty acids and proximate composition of eight Thai edible terricolous insects. Food Res. Int. 2010, 43, 350–355. [Google Scholar] [CrossRef]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Shukla, V. Is our balanced food really balanced? Correcting ω6/ ω3 Ratio through Combating Oxidation is the Key to the Success for Better Health. J. Food Nutr. Popul. Health. 2017, 1, 18. [Google Scholar]
- Purschke, B.; Brüggen, H.; Scheibelberger, R.; Jäger, H. Effect of pre-treatment and drying method on physico-chemical properties and dry fractionation behaviour of mealworm larvae (Tenebrio molitor L.). Eur. Food Res. Technol. 2017. [Google Scholar] [CrossRef]
- Kim, H.-W.; Setyabrata, D.; Lee, Y.J.; Jones, O.G.; Kim, Y.H.B. Pre-treated mealworm larvae and silkworm pupae as a novel protein ingredient in emulsion sausages. Innov.Food. Sci. Emerg. Technol. 2016, 38, 116–123. [Google Scholar] [CrossRef]
- Goodwin, T.W. The biochemistry of locust pigmentation. Biol. Rev. 1952, 27, 439–460. [Google Scholar] [CrossRef]
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and physicochemical characterization of Tenebrio molitor proteins. Food. Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef] [PubMed]
| Component | Mean (%) ± SD |
|---|---|
| Crude Protein | 50.79 ± 0.69 |
| Crude Fat | 34.93 ± 3.37 |
| Carbohydrate | 13.46 ± 4.53 |
| Ash | 2.42 ± 0.11 |
| Fatty Acid | Mean ± SD (%) |
|---|---|
| Myristic (C14:0) | 2.69 ± 0.90 |
| Palmitic (C16:0) | 27.30 ± 1.53 |
| Palmitoleic (C16:1) | 1.17 ± 0.14 |
| Stearic (C18:0) | 7.23 ± 0.99 |
| Oleic (C18:1n9c) | 37.02 ± 0.67 |
| Linoleic (C18:2n6c) | 8.94 ± 0.59 |
| α-Linolenic (C18:3n3c) | 15.64 ± 2.68 |
| Total SFA | 37.22 |
| Total MUFA | 38.49 ± 1.09 |
| Total PUFA | 24.57 ± 3.27 |
| SFA/UFA | 0.58 |
| N−6/n−3 | 0.55 |
| Fraction | Protein Content ± SD (%) | Yield (%) | Protein Recovery (% of Total Protein) |
|---|---|---|---|
| Locust meal | 50.79 ± 0.69 a | ||
| Insoluble locust fraction | 81.29 ± 4.43 b | 37.76 | 39.85 |
| Soluble locust fraction | 73.64 ± 10.64 b | 9.83 | 9.64 |
| Supernatant fraction | 69.13 ± 2.89 b | 56.60 | 52.17 |
| Component | ∆E | L* | a* | b* |
|---|---|---|---|---|
| Locust meal | - | 28.14 ± 2.62 a | 4.89 ± 0.64 b | 10.06 ± 1.46 a |
| Insoluble locust fraction | 9.21 ± 3.37 a | 35.48 ± 0.96 c | 5.76 ± 0.13 c | 15.56 ± 0.29 b |
| Soluble locust fraction | 6.94 ± 4.53 b | 30.80 ± 3.08 b | 8.87 ± 1.51 d | 15.09 ± 2.50 b |
| Supernatant fraction | 23.44 ± 0.11 c | 50.88 ± 1.26 d | 1.67 ± 0.07 a | 14.80 ± 0.42 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarkson, C.; Mirosa, M.; Birch, J. Potential of Extracted Locusta Migratoria Protein Fractions as Value-Added Ingredients. Insects 2018, 9, 20. https://doi.org/10.3390/insects9010020
Clarkson C, Mirosa M, Birch J. Potential of Extracted Locusta Migratoria Protein Fractions as Value-Added Ingredients. Insects. 2018; 9(1):20. https://doi.org/10.3390/insects9010020
Chicago/Turabian StyleClarkson, Claudia, Miranda Mirosa, and John Birch. 2018. "Potential of Extracted Locusta Migratoria Protein Fractions as Value-Added Ingredients" Insects 9, no. 1: 20. https://doi.org/10.3390/insects9010020






