A Point Mutation V419L in the Sodium Channel Gene from Natural Populations of Aedes aegypti Is Involved in Resistance to λ-Cyhalothrin in Colombia
Abstract
:1. Introduction
2. Materials and Methods
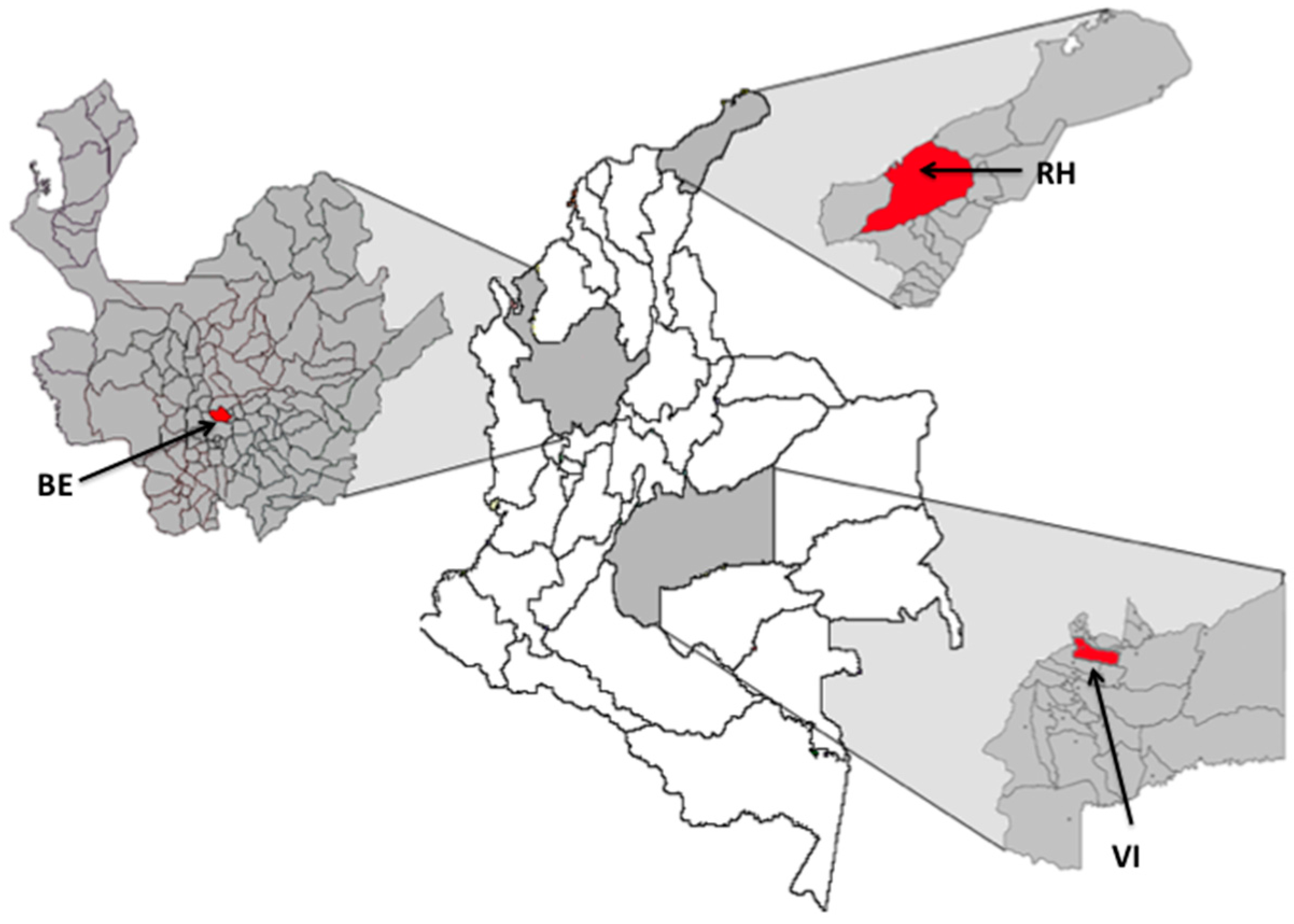
2.1. Mosquito Collection
2.2. Insecticide Bioassays
2.3. RNA Extraction and cDNA Synthesis
2.4. cDNA Sequencing
2.5. Allele-Specific PCR (AS-PCR) Genotyping
2.6. Correlation of Resistance with the Frequencies of Different Mutations
3. Results
3.1. λ-Cyhalothrin Bioassays and Adult Genotyping
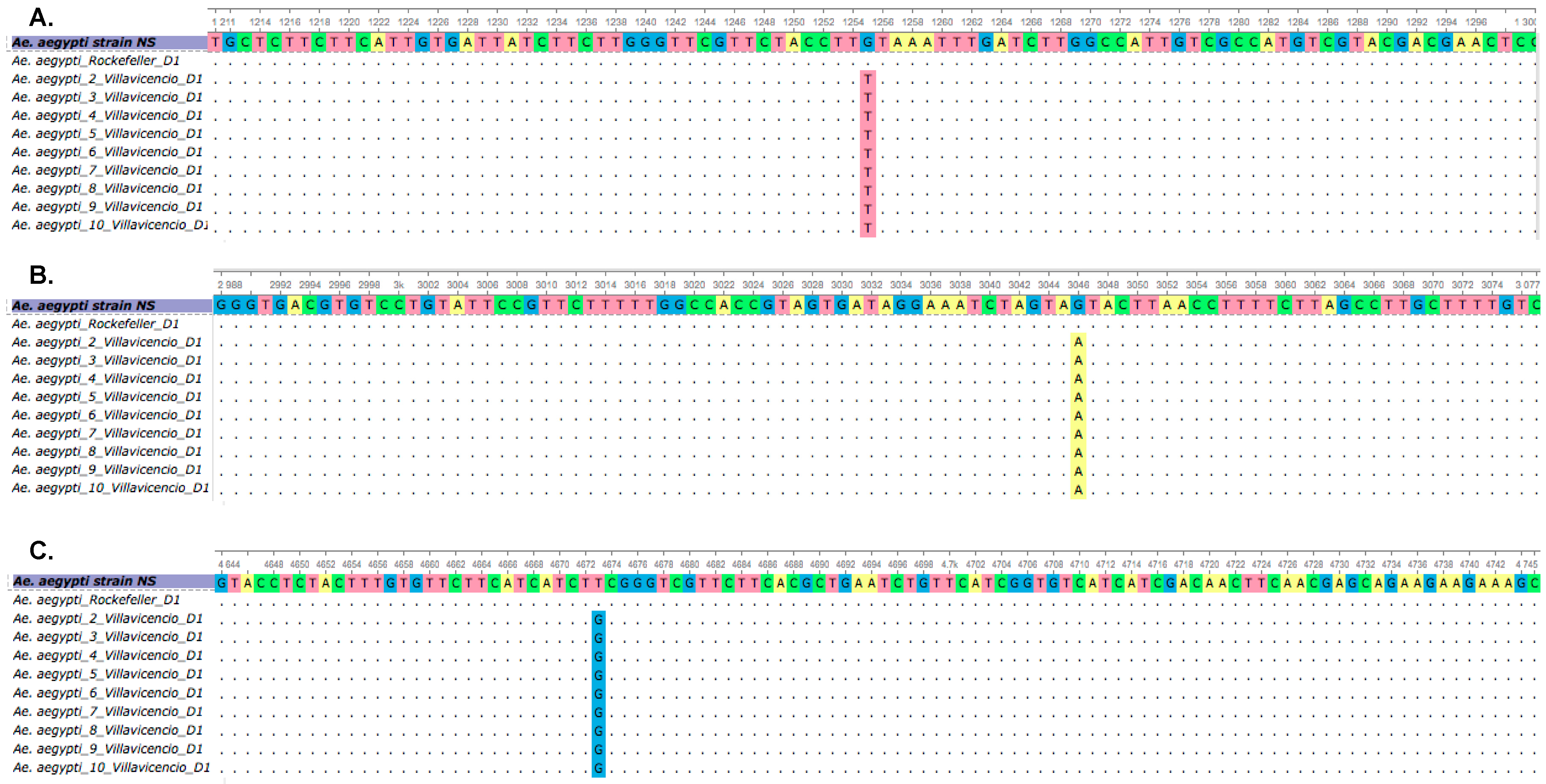
3.2. Comparison of cDNA Region from Susceptible and Resistant Mosquitoes and Identification of Sodium Channel Gene Mutations
3.3. AS-PCR for the V419L, V1016I and F1534C Mutations
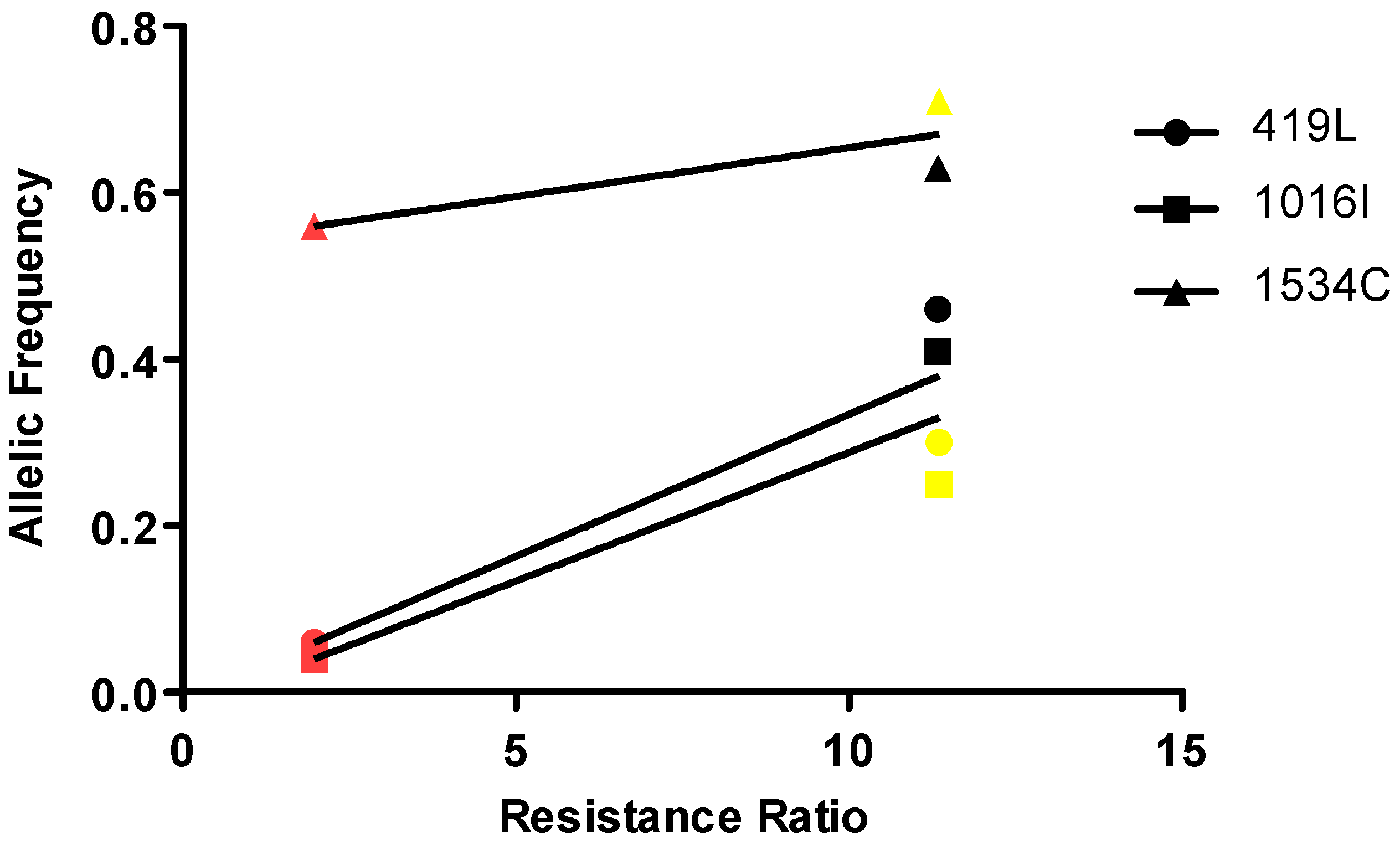
3.4. Correlation of Resistance with the Frequencies of the Different Mutations
3.5. Mutations in Combination
3.6. Allelic Frequencies in Absence of Insecticide
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Funding
References
- Akter, R.; Naish, S.; Hu, W.; Tong, S. Socio-demographic, ecological factors and dengue infection trends in Australia. PLoS ONE 2017, 12, e0185551. [Google Scholar] [CrossRef] [PubMed]
- Struchiner, C.J.; Rocklöv, J.; Wilder-Smith, A.; Massad, E. Increasing dengue incidence in Singapore over the Past 40 Years: Population growth, climate and mobility. PLoS ONE 2015, 10, e0136286. [Google Scholar] [CrossRef] [PubMed]
- Instituto Nacional de Salud. Boletín Epidemiológico Semanal. In Semana Epidemiológica Número 33 de 2016, 14—20 Agosto; Instituto Nacional de Salud: Bogota, Colombia, 2016; (In Spanish). Available online: http://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2016 Boletin epidemiologico semana 33.pdf (accessed on 9 October 2017).
- Maestre-Serrano, R.; Gomez-Camargo, D.; Ponce-Garcia, G.; Flores, A.E. Susceptibility to insecticides and resistance mechanisms in Aedes aegypti from the Colombian Caribbean Region. Pestic. Biochem. Physiol. 2014, 116, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Ngoagouni, C.; Kamgang, B.; Brengues, C.; Yahouedo, G.; Paupy, C.; Nakouné, E.; Kazanji, M.; Chandre, F. Susceptibility profile and metabolic mechanisms involved in Aedes aegypti and Aedes albopictus resistant to DDT and deltamethrin in the Central African Republic. Parasit. Vectors 2016, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Moyes, C.L.; Vontas, J.; Martins, A.J.; Ng, L.C.; Koou, S.Y.; Dusfour, I.; Raghavendra, K.; Pinto, J.; Corbel, V.; David, J.P.; et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Nomura, Y.; Zhorov, B.S.; Dong, K. Sodium Channel Mutations and Pyrethroid Resistance in Aedes aegypti. Insects 2016, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Brengues, C.; Hawkes, N.J.; Chandre, F.; McCarroll, L.; Duchon, S.; Guillet, P.; Manguin, S.; Morgan, J.C.; Hemingway, J. Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutations in the voltage-gated sodium channel gene. Med. Vet. Entomol. 2003, 17, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Saavedra-Rodriguez, K.; Urdaneta-Marquez, L.; Rajatileka, S.; Moulton, M.; Flores, A.E.; Fernandez-Salas, I.; Bisset, J.; Rodriguez, M.; Mccall, P.J.; Donnelly, M.J.; et al. A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol. Biol. 2007, 16, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Plernsub, S.; Saingamsook, J.; Yanola, J.; Lumjuan, N.; Tippawangkosol, P.; Walton, C.; Somboon, P. Temporal frequency of knockdown resistance mutations, F1534C and V1016G, in Aedes aegypti in Chiang Mai city, Thailand and the impact of the mutations on the efficiency of thermal fogging spray with pyrethroids. Acta Trop. 2016, 162, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Plernsub, S.; Saingamsook, J.; Yanola, J.; Lumjuan, N.; Tippawangkosol, P.; Sukontason, K.; Walton, C.; Somboon, P. Additive effect of knockdown resistance mutations, S989P, V1016G and F1534C, in a heterozygous genotype conferring pyrethroid resistance in Aedes aegypti in Thailand. Parasit. Vectors 2016, 9, 417. [Google Scholar] [CrossRef] [PubMed]
- Yanola, J.; Somboon, P.; Walton, C.; Nachaiwieng, W.; Somwang, P.; Prapanthadara, L. High-throughput assays for detection of the F1534C mutation in the voltage-gated sodium channel gene in permethrin-resistant Aedes aegypti and the distribution of this mutation throughout Thailand. Trop. Med. Int. Health 2011, 16, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Obando, O.A.; Martins, A.J.; Navarro-Silva, M.A. First report of the Phe1534Cys kdr mutation in natural populations of Aedes albopictus from Brazil. Parasit. Vectors 2017, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, L.C.; Ponce, G.; Saavedra-Rodriguez, K.; Lopez, B.; Flores, A.E. Frequency of V1016I and F1534C mutations in the voltage-gated sodium channel gene in Aedes aegypti in Venezuela. Pest Manag. Sci. 2015, 71, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Atencia, M.C.; Pérez, M.D.J.; Jaramillo, M.C.; Caldera, S.M.; Cochero, S.; Bejarano, E.E. First report of the F1534C mutation associated with cross-resistance to DDT and pyrethroids in Aedes aegypti from Colombia. Biomedica 2016, 36, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Shen, W.-K.; Wang, T.-T.; Lin, Y.-H.; Hsu, E.-L.; Dai, S.-M. A novel amino acid substitution in a voltage-gated sodium channel is associated with knockdown resistance to permethrin in Aedes aegypti. Insect Biochem. Mol. Biol. 2009, 39, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Srisawat, R.; Komalamisra, N.; Apiwathnasorn, C.; Paeporn, P.; Roytrakul, S.; Rongsriyam, Y.; Eshita, Y. Field-collected permethrin-resistant Aedes aegypti from central Thailand contain point mutations in the domain IIS6 of the sodium channel gene (KDR). Southeast Asian J. Trop. Med. Public Health 2012, 43, 1380–1386. [Google Scholar] [PubMed]
- Pang, S.C.; Chiang, L.P.; Tan, C.H.; Vythilingam, I.; Lam-Phua, S.G.; Ng, L.C. Low efficacy of delthamethrin-treated net against Singapore Aedes aegypti is associated with kdr-type resistance. Trop. Biomed. 2015, 32, 140–150. [Google Scholar] [PubMed]
- Bellinato, D.F.; Viana-Medeiros, P.F.; Araújo, S.C.; Martins, A.J.; Lima, J.B.P.; Valle, D. Resistance Status to the Insecticides Temephos, Deltamethrin, and Diflubenzuron in Brazilian Aedes aegypti Populations. BioMed Res. Int. 2016, 2016, 8603263. [Google Scholar] [CrossRef] [PubMed]
- Honório, N.A.; Loureno̧-de-Oliveiria, R. Frequency of Aedes aegypti and Aedes albopictus larvae and pupae in traps, Brazil. Rev. Saude Publica 2001, 35, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Collet, M.L.; Frizzo, C.; Orlandin, E.; Rona, L.D.P.; Nascimento, J.C.; Montano, M.A.E.; Müller, G.A.; Wagner, G. Frequency of the Val1016Ile mutation on the kdr gene in Aedes aegypti (Diptera: Culicidae) in south Brazil. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef] [PubMed]
- Vera-Maloof, F.Z.; Saavedra-Rodriguez, K.; Elizondo-Quiroga, A.E.; Lozano-Fuentes, S.; Black IV, W.C. Coevolution of the Ile1,016 and Cys1,534 Mutations in the Voltage Gated Sodium Channel Gene of Aedes aegypti in Mexico. PLoS Negl. Trop. Dis. 2015, 9, e0004263. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-González, I.; Quiñones, M.L.; Lenhart, A.; Brogdon, W.G. Insecticide resistance status of Aedes aegypti (L.) from Colombia. Pest Manag. Sci. 2011, 67, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, C.B.; Salazar-Terreros, M.J.; Mina, N.J.; McAllister, J.; Brogdon, W. Insecticide resistance status of Aedes aegypti in 10 localities in Colombia. Acta Trop. 2011, 118, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Santacoloma Varon, L.; Chaves Cordoba, B.; Brochero, H. Susceptibility of Aedes aegypti to DDT, deltamethrin, and lambdacyhalothrin in Colombia. Rev. Panam. Salud Publica 2010, 27, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Maestre, S.R.; Rey, V.G.; De Las Salas, A.J.; Vergara, S.C.; Santacoloma, V.L.; Goenaga, O.S.; Carrasquilla, F.M.C. Susceptibility status of Aedes aegypti to insecticides in Atlántico (Colombia). Rev. Colomb. Entomol. 2010, 36, 242–248. [Google Scholar]
- Aguirre-Obando, O.A.; Dalla B., A.C.; Duque L., J.E.; Navarro-Silva, M.A. Insecticide resistance and genetic variability in natural populations of Aedes (Stegomyia) aegypti (Diptera: Culicidae) from Colombia. Zoologia 2015, 32, 14–22. [Google Scholar] [CrossRef]
- Jaimes-Dueñez, J.; Arboleda, S.; Triana-Chávez, O.; Gómez-Palacio, A. Spatio-Temporal Distribution of Aedes aegypti (Diptera: Culicidae) Mitochondrial Lineages in Cities with Distinct Dengue Incidence Rates Suggests Complex Population Dynamics of the Dengue Vector in Colombia. PLoS Negl. Trop. Dis. 2015. [Google Scholar] [CrossRef] [PubMed]
- Coto, M.M.; Lazcano, J.A.; de Fernández, D.M.; Soca, A. Malathion resistance in Aedes aegypti and Culex quinquefasciatus after its use in Aedes aegypti control programs. J. Am. Mosq. Control Assoc. 2000, 16, 324–330. [Google Scholar] [PubMed]
- Kuno, G. Early History of Laboratory Breeding of Aedes aegypti (Diptera: Culicidae) Focusing on the Origins and Use of Selected Strains. J. Med. Entomol. 2010, 47, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.H.; Mendez, M.A.; Rasmussen, M.O.; Mehaffey, P.C.; Besansky, N.J.; Finnerty, V. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 1987, 37, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-X.; Kaufman, P.E.; Xue, R.-D.; Zhao, M.-H.; Wang, G.; Yan, T.; Guo, X.-X.; Zhang, Y.-M.; Dong, Y.-D.; Xing, D.; et al. Relationship between insecticide resistance and kdr mutations in the dengue vector Aedes aegypti in Southern China. Parasit. Vectors 2015, 8, 325. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.; Rousset, F. GENEPOP version 1.2: Population genetics software for exact tests and ecumenicism. J. Hered. 1995, 82, 248–249. [Google Scholar] [CrossRef]
- Fuentes-Vallejo, M. Space and space-time distributions of dengue in a hyper-endemic urban space: The case of Girardot, Colombia. BMC Infect. Dis. 2017, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Nomura, Y.; Satar, G.; Hu, Z.; Nauen, R.; He, S.Y.; Zhorov, B.S.; Dong, K. Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc. Natl. Acad. Sci. USA 2013, 110, 11785–11790. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.F.; Rajatileka, S.; Ranson, H. Pyrethroid resistance in Aedes aegypti from Grand Cayman. Am. J. Trop. Med. Hyg. 2010, 83, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kushwah, R.B.S.; Dykes, C.L.; Kapoor, N.; Adak, T.; Singh, O.P. Pyrethroid-Resistance and Presence of Two Knockdown Resistance (kdr) Mutations, F1534C and a Novel Mutation T1520I, in Indian Aedes aegypti. PLoS Negl. Trop. Dis. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Haddi, K.; Tomé, H.V.V.; Du, Y.; Valbon, W.R.; Nomura, Y.; Martins, G.F.; Dong, K.; Oliveira, E.E. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: A potential challenge for mosquito control. Sci. Rep. 2017, 7, 46549. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Nomura, Y.; Zhorov, B.S.; Dong, K. Evidence for Dual Binding Sites for 1,1,1-Trichloro-2,2-bis(p-chlorophenyl)ethane (DDT) in Insect Sodium Channels. J. Biol. Chem. 2016, 291, 4638–4648. [Google Scholar] [CrossRef] [PubMed]
- Ishak, I.H.; Jaal, Z.; Ranson, H.; Wondji, C.S. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit. Vectors 2015, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, Y. Genetic Structure Analysis of Colombian Aedes aegypti (Diptera: Culicidae) Populations Unveils Inbreeding of African Ancient Mitochondrial Lineages; Universidad de Antioquia: Antioquia, Colombia, 2016. [Google Scholar]
- Ponce-García, G.; Del Río-Galvan, S.; Barrera, R.; Saavedra-Rodriguez, K.; Villanueva-Segura, K.; Felix, G.; Amador, M.; Flores, A.E. Knockdown Resistance Mutations in Aedes aegypti (Diptera: Culicidae) from Puerto Rico. J. Med. Entomol. 2016, 53, 1410–1414. [Google Scholar] [CrossRef] [PubMed]
- Goindin, D.; Delannay, C.; Gelasse, A.; Ramdini, C.; Gaude, T.; Faucon, F.; David, J.-P.; Gustave, J.; Vega-Rua, A.; Fouque, F. Levels of insecticide resistance to deltamethrin, malathion, and temephos, and associated mechanisms in Aedes aegypti mosquitoes from the Guadeloupe and Saint Martin islands (French West Indies). Infect. Dis. Poverty 2017, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Ishak, I.H.; Kamgang, B.; Ibrahim, S.S.; Riveron, J.M.; Irving, H.; Wondji, C.S. Pyrethroid Resistance in Malaysian Populations of Dengue Vector Aedes aegypti is Mediated by CYP9 Family of Cytochrome P450 Genes. PLoS Negl. Trop. Dis. 2017, 11, e0005302. [Google Scholar] [CrossRef] [PubMed]

| Mutation | Primers | Sequence | Reference |
|---|---|---|---|
| V419L | PM1_Ext_419F | GATTCCTCCAGAACTCCACC | This paper |
| PM1_Ext_419R | TCAATGGATTTGGGTGACAA | ||
| PM1_F_419Wt | CTTGGGTTCGTTCTACCTTG | ||
| PM1_F_419Mut | CTTGGGTTCGTTCTACCTTT | ||
| V1016I | PM2_Ext_1016F | GCCACCGTAGTGATAGGAAATC | Li et al., 2015 [32] |
| PM2_Ext_1016R | CGGGTTAAGTTTCGTTTAGTAGC | ||
| PM2_F_1016Wt | GTTTCCCACTCGCACAGGT | ||
| PM2_F_1016Mut | GTTTCCCACTCGCACAGA | This paper | |
| F1534C | PM3_Ext_1534F | GGAGAACTACACGTGGGAGAAC | Li et al., 2015 [32] |
| PM3_Ext_1534R | CGCCACTGAAATTGAGAATAGC | ||
| PM3_R_1534WT | GCGTGAAGAACGACCCGA | ||
| PM3_R_1534Mut | GCGTGAAGAACGACCCGC |
| Mosquito Population | n | LC50 | Confidence Limit | RR50 | Phenotype |
|---|---|---|---|---|---|
| Rockefeller | 360 | 0.01614 | 0.009–0.02323 | - | - |
| Bello | 300 | 0.03182 | 0.0282–0.0363 | 1.97 | Susceptible |
| Villavicencio | 300 | 0.1831 | 0.1320–0.2764 | 11.34 | Resistant |
| Riohacha | 300 | 0.1768 | 0.1220–0.2926 | 10.96 | Resistant |
| Genotype (1016) | Hardy-Weinberg Parameters | |||||||
| Location | V/V | V/I | I/I | n | Ho | He | X2 | p |
| Villavicencio | 24 | 57 | 8 | 89 | 0.64 | 0.48 | 0.10 | 0.75 |
| Riohacha | 47 | 43 | 1 | 91 | 0.47 | 0.37 | 0.07 | 0.79 |
| Bello | 93 | 7 | 1 | 101 | 0.07 | 0.09 | 0.03 | 0.86 |
| Genotype (1534) | Hardy-Weinberg Parameters | |||||||
| Location | F/F | F/C | C/C | n | Ho | He | X2 | p |
| Villavicencio | 9 | 32 | 16 | 57 | 0.48 | 0.47 | 0.06 | 0.81 |
| Riohacha | 7 | 22 | 34 | 63 | 0.35 | 0.41 | 0.02 | 0.89 |
| Bello | 9 | 32 | 16 | 57 | 0.56 | 0.49 | 0.02 | 0.89 |
| Genotype (419) | Hardy-Weinberg Parameters | |||||||
| Location | V/V | V/L | L/L | n | Ho | He | X2 | p |
| Villavicencio | 26 | 33 | 20 | 79 | 0.42 | 0.50 | 0.03 | 0.86 |
| Riohacha | 32 | 24 | 7 | 63 | 0.38 | 0.42 | 0.01 | 0.92 |
| Bello | 52 | 5 | 1 | 58 | 0.09 | 0.11 | 0.06 | 0.81 |
| Haplotypes | Villavicencio | Riohacha | Bello |
|---|---|---|---|
| V419/V1016/F1534 | 0 | 4 | 7 |
| V419/V1016/1534C | 8 | 9 | 11 |
| V419/V1016/F1534C | 7 | 10 | 27 |
| 419L/V1016/F1534 | 0 | 1 | 0 |
| V419L/V1016/F1534 | 0 | 1 | 0 |
| V419L/V1016/1534C | 2 | 0 | 0 |
| V419L/V1016/F1534C | 0 | 2 | 0 |
| 419L/1016I/1534C | 6 | 1 | 1 |
| V419/V1016I/1534C | 4 | 4 | 0 |
| V419/V1016I/F1534C | 1 | 0 | 0 |
| 419L/V1016I/F1534 | 0 | 1 | 0 |
| 419L/V1016I/1534C | 9 | 2 | 0 |
| V419L/V1016I/1534C | 20 | 12 | 2 |
| V419L/V1016I/F1534C | 3 | 6 | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granada, Y.; Mejía-Jaramillo, A.M.; Strode, C.; Triana-Chavez, O. A Point Mutation V419L in the Sodium Channel Gene from Natural Populations of Aedes aegypti Is Involved in Resistance to λ-Cyhalothrin in Colombia. Insects 2018, 9, 23. https://doi.org/10.3390/insects9010023
Granada Y, Mejía-Jaramillo AM, Strode C, Triana-Chavez O. A Point Mutation V419L in the Sodium Channel Gene from Natural Populations of Aedes aegypti Is Involved in Resistance to λ-Cyhalothrin in Colombia. Insects. 2018; 9(1):23. https://doi.org/10.3390/insects9010023
Chicago/Turabian StyleGranada, Yurany, Ana María Mejía-Jaramillo, Clare Strode, and Omar Triana-Chavez. 2018. "A Point Mutation V419L in the Sodium Channel Gene from Natural Populations of Aedes aegypti Is Involved in Resistance to λ-Cyhalothrin in Colombia" Insects 9, no. 1: 23. https://doi.org/10.3390/insects9010023





