Honeybees Tolerate Cyanogenic Glucosides from Clover Nectar and Flowers
Abstract
:1. Introduction
2. Materials and Methods
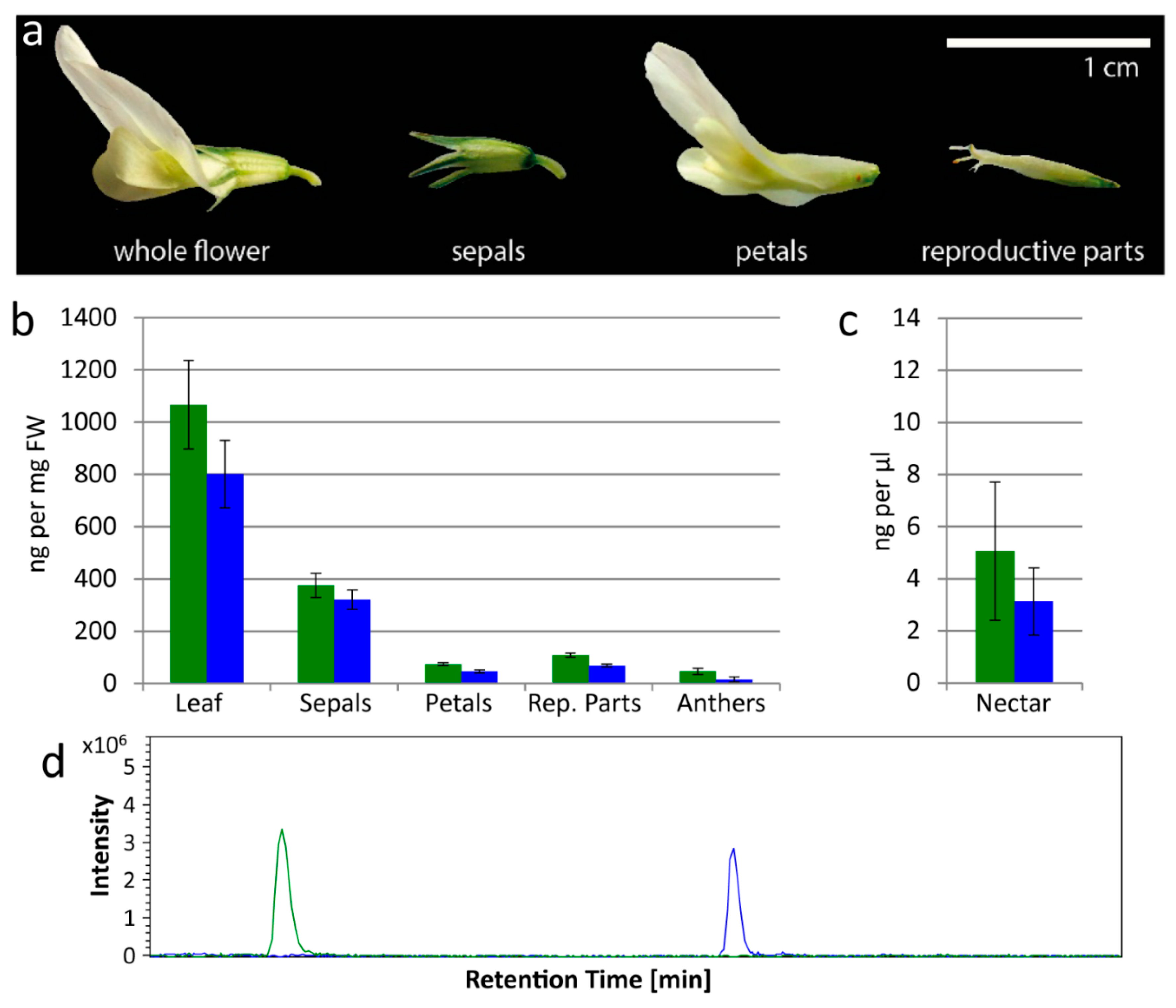
2.1. Plant Material
2.2. Nectar Sampling
2.3. Bee Material
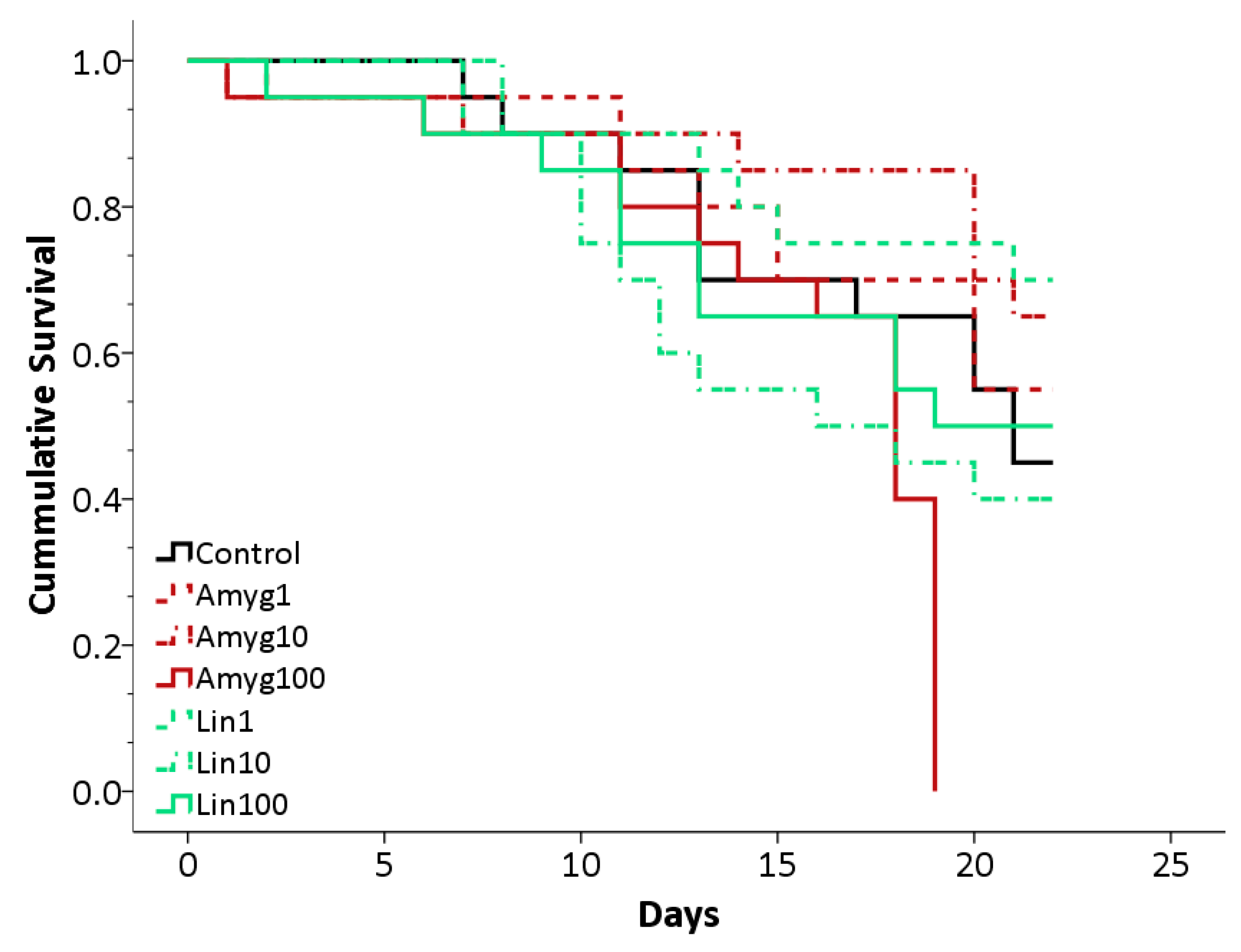
2.4. Chronic Feeding of Cyanogenic Glucosides
2.5. LC-MS Analysis
2.6. β-Glucosidase Activity Assay
2.7. Genes Involved in Degradation and Detoxification of Cyanogenic Glucosides in the Honeybee Genome
2.8. Statistical Analysis
3. Results
3.1. Cyanogenic Glucosides in White Clover
3.2. Honeybee Survival and Consumption
3.3. Degradation and Detoxification of Cyanogenic Glucosides by Bees
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Brodschneider, R.; Crailsheim, K. Nutrition and health in honey bees. Apidologie 2010, 41, 278–294. [Google Scholar] [CrossRef]
- Baker, H.G. Non-sugar chemical constituents of nectar. Apidologie 1977, 8, 349–356. [Google Scholar] [CrossRef]
- Pentzold, S.; Zagrobelny, M.; Bjarnholt, N.; Kroymann, J.; Vogel, H.; Olsen, C.E.; Møller, B.L.; Bak, S. Metabolism, excretion and avoidance of cyanogenic glucosides in insects with different feeding specialisations. Insect Biochem. Mol. Biol. 2015, 66, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Pentzold, S.; Zagrobelny, M.; Roelsgaard, P.S.; Møller, B.L.; Bak, S. The multiple strategies of an insect herbivore to overcome plant cyanogenic glucoside defence. PLoS ONE 2014, 9, e91337. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.S. The ecological significance of toxic nectar. Oikos 2000, 91, 409–420. [Google Scholar] [CrossRef]
- Hawkins, J.; De Vere, N.; Griffith, A.; Ford, C.R.; Allainguillaume, J.; Hegarty, M.J.; Baillie, L.; Adams-Groom, B. Using DNA metabarcoding to identify the floral composition of honey: A new tool for investigating honey bee foraging preferences. PLoS ONE 2015, 10, e0134735. [Google Scholar] [CrossRef] [PubMed]
- Bruni, I.; Galimberti, A.; Caridi, L.; Scaccabarozzi, D.; De Mattia, F.; Casiraghi, M.; Labra, M. A DNA barcoding approach to identify plant species in multiflower honey. Food Chem. 2015, 170, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.D.; Draguleasa, M.A.; Tan, G.M. Flowers with caffeinated nectar receive more pollination. Arthropod-Plant Interact. 2015, 9, 1–7. [Google Scholar] [CrossRef]
- Cutler, G.C.; Rix, R.R. Can poisons stimulate bees? Appreciating the potential of hormesis in bee-pesticide research. Pest Manag. Sci. 2015, 71, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Ayestaran, A.; Giurfa, M.; de Brito Sanchez, M.G. Toxic but drank: Gustatory aversive compounds induce post-ingestional malaise in harnessed honeybees. PLoS ONE 2010, 5, e15000. [Google Scholar] [CrossRef] [PubMed]
- Hurst, V.; Stevenson, P.C.; Wright, G.A. Toxins induce ‘malaise’ behaviour in the honeybee (Apis mellifera). J. Comp. Physiol. A 2014, 200, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Singaravelan, N.; Nee’man, G.; Inbar, M.; Iszhaki, I. Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectars. J. Chem. Ecol. 2005, 31, 2791–2804. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.A.; Mustard, J.A.; Simcock, N.K.; Ross-Taylor, A.A.R.; McNicholas, L.D.; Popescu, A.; Marion-Poll, F. Parallel reinforcement pathways for conditioned food aversions in the honeybee. Curr. Biol. 2010, 20, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.A.; Dews, L.; Brugato, A.; Dey, K.; Wright, G.A. Consumption of an acute dose of caffeine reduces acquisition but not memory in the honey bee. Behav. Brain Res. 2012, 232, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Pontoh, J.; Low, N.H. Purification and characterization of β-glucosidase from honey bees (Apis mellifera). Insect Biochem. Mol. 2002, 32, 679–690. [Google Scholar] [CrossRef]
- Zagrobelny, M.; Bak, S.; Møller, B.L. Cyanogenesis in plants and arthropods. Phytochemistry 2008, 69, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Kjærgaard, T. A plant that changed the world: The rise and fall of clover 1000–2000. Landsc. Res. 2003, 28, 41–49. [Google Scholar] [CrossRef]
- Atwood, S.S. Genetics of cross-incompatibility among self-incompatible plants of Trifolium repens. J. Am. Soc. Agron. 1940, 32, 955–968. [Google Scholar] [CrossRef]
- Goodman, R.D.; Williams, A.E. Honeybee pollination of white clover (Trifolium repens L.) cv. Haifa. Aust. J. Exp. Agric. 1994, 34, 1121–1123. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Legumes of World Economic Importance; Plenum Press: New York, NY, USA; London, UK, 1981. [Google Scholar]
- Collinge, D.B.; Hughes, M.A. Developmental and physiological studies on the cyanogenic glucosides of white clover, Trifolium repens L. J. Exp. Bot. 1982, 33, 154–161. [Google Scholar] [CrossRef]
- Muzashvili, T.; Moniuszko-Szajwaj, B.; Pecio, L.; Oleszek, W.; Stochmal, A. Ultraperformance liquid chromatography tandem mass spectroscopy determination of cyanogenic glucosides in Trifolium species. J. Agric. Food Chem. 2014, 62, 1777–1782. [Google Scholar] [CrossRef] [PubMed]
- London-Shafir, I.; Shafir, S.; Eisikowitch, D. Amygdalin in almond nectar and pollen—Facts and possible roles. Plant Syst. Evol. 2003, 238, 87–95. [Google Scholar] [CrossRef]
- Lai, D.; Picmanova, M.; Abou, H.M.; Motawia, M.S.; Olsen, C.E.; Møller, B.L.; Rook, F.; Takos, A.M. Lotus japonicus flowers are defended by a cyanogenic beta-glucosidase with highly restricted expression to essential reproductive organs. Plant Mol. Biol. 2015, 89, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stromgren, E.J.; Sullivan, T.P. Influence of pitfall versus Longworth livetraps, bait addition, and drift fences on trap success and mortality of shrews. Acta Theriol. 2014, 59, 203–210. [Google Scholar] [CrossRef]
- Møller, B.L.; Olsen, C.E.; Motawia, M.S. General and stereocontrolled approach to the chemical synthesis of naturally occurring cyanogenic glucosides. J. Nat. Prod. 2016, 79, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Zagrobelny, M.; (University of Copenhagen, Copenhagen, Denmark). Personnal Communication, 2017.
- Lambert, J.L.; Ramasamy, J.; Paukstelis, J.V. Stable reagents for the colorimetric determination of cyanide by modified könig reactions. Anal. Chem. 1975, 47, 916–918. [Google Scholar] [CrossRef]
- Zagrobelny, M.; Scheibye-Alsing, K.; Bjerg Jensen, N.; Møller, B.L.; Gorodkin, J.; Bak, S. 454 pyrosequencing based transcriptome analysis of Zygaena filipendulae with focus on genes involved in biosynthesis of cyanogenic glucosides. BMC Genom. 2009, 10, 574. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Jørgensen, K.; Vogel, H.; Møller, B.L.; Bak, S. Chemical defense balanced by sequestration and de novo biosynthesis in a lepidopteran specialist. PLoS ONE 2014, 9, e108745. [Google Scholar] [CrossRef] [PubMed]
- Cecen, S.; Gosterit, A.; Gurel, F. Pollination effects of the bumble bee and honey bee on white clover (Trifolium repens L.) seed production. J. Apic. Res. 2007, 46, 69–72. [Google Scholar] [CrossRef]
- Anderson, K.E.; Sheehan, T.; Mott, B.M.; Maes, P.; Snyder, L.; Schwan, M.; Walton, A.; Jones, B.; Corby-Harris, V. Microbial ecology of the hive and pollination landscape: Bacterial associates from floral nectar, the alimentary tract and stored food of honey bees (Apis mellifera). PLoS ONE 2013, 8, e83125. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.J.; Rusch, D.B.; Stewart, F.J.; Mattila, H.R.; Newton, I.L. Saccharide breakdown and fermentation by the honey bee gut microbiome. Environ. Microbiol. 2015, 17, 796–815. [Google Scholar] [CrossRef] [PubMed]
- Møller, B.L. Functional diversifications of cyanogenic glucosides. Curr. Opin. Plant Biol. 2010, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.S.; Otterstatter, M.C.; Thomson, J.D. Consumption of a nectar alkaloid reduces pathogen load in bumble bees. Oecologia 2010, 162, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.L.; Adler, L.S.; Leonard, A.S.; Andicoechea, J.; Regan, K.H.; Anthony, W.E.; Manson, J.S.; Irwin, R.E. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. B 2015, 282, 20142471. [Google Scholar] [CrossRef] [PubMed]
- Palmer-Young, E.C.; Tozkar, C.Ö.; Schwarz, R.S.; Chen, Y.; Irwin, R.E.; Adler, L.S.; Evans, J.D. Nectar and pollen phytochemicals stimulate honey bee (Hymenoptera: Apidae) immunity to viral infection. J. Econ. Entomol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Erler, S.; Denner, A.; Bobiş, O.; Forsgren, E.; Moritz, R.F.A. Diversity of honey stores and their impact on pathogenic bacteria of the honeybee, Apis mellifera. Ecol. Evol. 2014, 4, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
- Gherman, B.I.; Denner, A.; Bobiş, O.; Dezmirean, D.S.; Marghitas, L.A.; Schluns, H.; Moritz, R.F.A.; Erler, S. Pathogen-associated self-medication behaviour in the honeybee Apis mellifera. Behav. Ecol. Sociobiol. 2014, 68, 1777–1784. [Google Scholar] [CrossRef]
| HCN Emission | β-Glucosidase Activity in Whole Bees | β-Glucosidase Activity in Bee Gut | ||||
|---|---|---|---|---|---|---|
| Linamarin | Amygdalin | Control | Linamarin | Amygdalin | Control | |
| HCN emission (nmol ± s.d.) | 2.9 (±1.6) a | 7.1 (±3.0) a | 0.5 (±0.2) a | 6.6 (±1.9) a | 5.3 (±1.2) a | 0.3 (±0.0) a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lecocq, A.; Green, A.A.; Pinheiro De Castro, É.C.; Olsen, C.E.; Jensen, A.B.; Zagrobelny, M. Honeybees Tolerate Cyanogenic Glucosides from Clover Nectar and Flowers. Insects 2018, 9, 31. https://doi.org/10.3390/insects9010031
Lecocq A, Green AA, Pinheiro De Castro ÉC, Olsen CE, Jensen AB, Zagrobelny M. Honeybees Tolerate Cyanogenic Glucosides from Clover Nectar and Flowers. Insects. 2018; 9(1):31. https://doi.org/10.3390/insects9010031
Chicago/Turabian StyleLecocq, Antoine, Amelia A. Green, Érika Cristina Pinheiro De Castro, Carl Erik Olsen, Annette B. Jensen, and Mika Zagrobelny. 2018. "Honeybees Tolerate Cyanogenic Glucosides from Clover Nectar and Flowers" Insects 9, no. 1: 31. https://doi.org/10.3390/insects9010031





