Red Spinach Extract Increases Ventilatory Threshold during Graded Exercise Testing
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Study Design
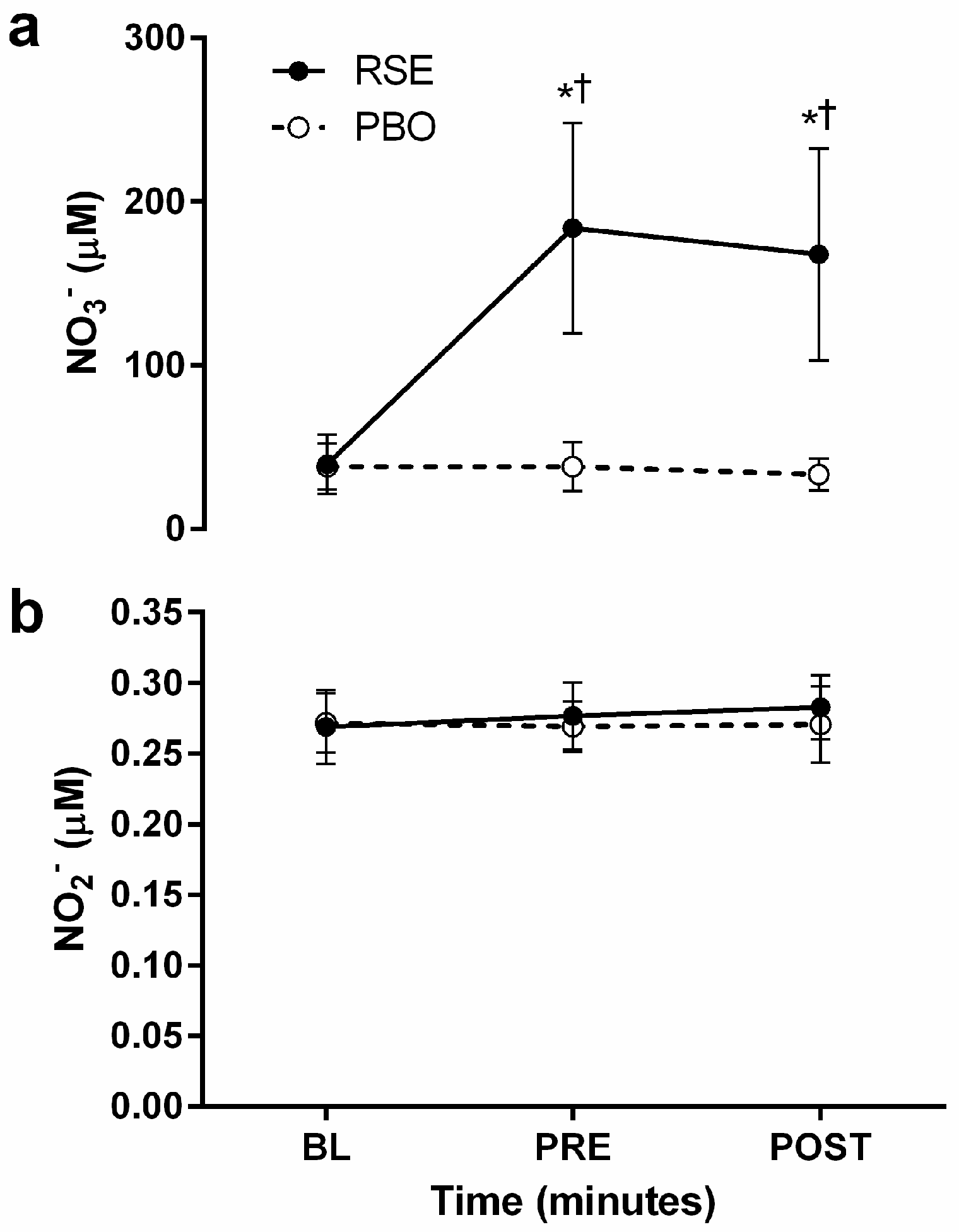
2.3. Blood Collection and Humoral Nitrate/Nitrite Concentrations
2.4. Supplementation: Red Spinach Extract and Placebo
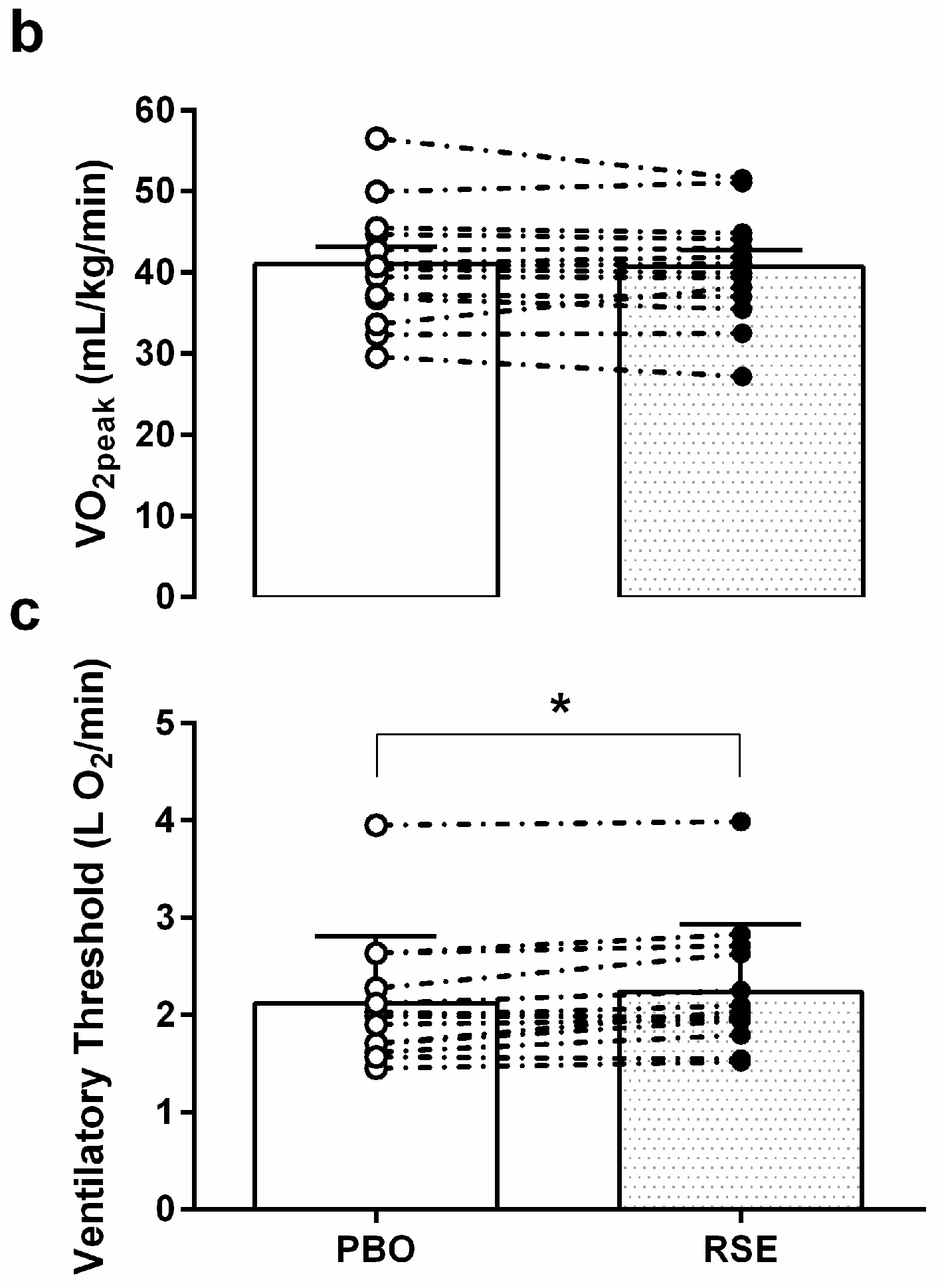
2.5. Graded Exercise Testing
2.6. Statistical Analysis
3. Results
3.1. Participants
3.2. Circulating Concentrations of Nitrate and Nitrite
3.3. Graded Exercise Testing Performance
4. Discussion
Practical Applications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Gibala, M.J.; Van Loon, L.J. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic. Biol. Med. 2010, 48, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Bescós, R.; Ferrer-Roca, V.; Galilea, P.A.; Roig, A.; Drobnic, F.; Sureda, A.; Martorell, M.; Cordova, A.; Tur, J.A.; Pons, A. Sodium nitrate supplementation does not enhance performance of endurance athletes. Med. Sci. Sports Exerc. 2012, 44, 2400–2409. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Res, P.; Stinkens, R.; Lundberg, J.O.; Gibala, M.J.; van Loon, L.J. No improvement in endurance performance after a single dose of beetroot juice. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Peacock, O.; Tjønna, A.E.; James, P.; Wisløff, U.; Welde, B.; Böhlke, N.; Smith, A.; Stokes, K.; Cook, C.; Sandbakk, Ø. Dietary nitrate does not enhance running performance in elite cross-country skiers. Med. Sci. Sports Exerc. 2012, 44, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; George, T.W.; Lovegrove, J.A. The effects of dietary nitrate on blood pressure and endothelial function: A review of human intervention studies. Nutr. Res. Rev. 2013, 26, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Wylie, L.J.; Kelly, J.; Bailey, S.J.; Blackwell, J.R.; Skiba, P.F.; Winyard, P.G.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013, 115, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, D.; Gupta, S. Pharmacokinetic study of amaranth extract in healthy human subjects: A randomized trial. Nutrition 2016, 32, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef] [PubMed]
- McMahon, N.F.; Leveritt, M.D.; Pavey, T.G. The effect of dietary nitrate supplementation on endurance exercise performance in healthy adults: A systematic review and meta-analysis. Sports Med. 2017, 47, 735–756. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Blood, and Lung Institute. Low Nitrate Diet. Available online: https:// web.archive.org/web/20061003110844/http://www.nhlbi.nih.gov/labs/7east/lowNdiet.htm (accessed on 6 October 2017).
- Petersson, J.; Carlström, M.; Schreiber, O.; Phillipson, M.; Christoffersson, G.; Jägare, A.; Roos, S.; Jansson, E.A.; Persson, A.E.; Lundberg, J.O.; et al. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic. Biol. Med. 2009, 46, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Haun, C.T.; Kephart, W.C.; Holland, A.M.; Mobley, C.B.; McCloskey, A.E.; Shake, J.J.; Pascoe, D.D.; Roberts, M.D.; Martin, J.S. Differential vascular reactivity responses acutely following ingestion of a nitrate rich red spinach extract. Eur. J. Appl. Physiol. 2016, 116, 2267–2279. [Google Scholar] [CrossRef] [PubMed]
- Dunham, A.J.; Barkley, R.M.; Sievers, R.E. Aqueous nitrite ion determination by selective reduction and gas phase nitric oxide chemiluminescence. Anal. Chem. 1995, 67, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Pinder, A.G.; Rogers, S.C.; Khalatbari, A.; Ingram, T.E.; James, P.E. The measurement of nitric oxide and its metabolites in biological samples by ozone-based chemiluminescence. Redox Med. Signal Transduct. Methods Protoc. 2009, 10–27. [Google Scholar]
- Gaskill, S.E.; Ruby, B.C.; Walker, A.J.; Sanchez, O.A.; Serfass, R.C.; Leon, A.S. Validity and reliability of combining three methods to determine ventilatory threshold. Med. Sci. Sports Exerc. 2001, 33, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Frank, M.H.; Whipp, B.J.; Wasserman, K. Anaerobic threshold alterations caused by endurance training in middle-aged men. J. Appl. Physiol. 1979, 46, 1039–1046. [Google Scholar] [PubMed]
- Beaver, W.L.; Wasserman, K.; Whipp, B.J. A new method for detecting anaerobic threshold by gas exchange. J. Appl. Physiol. 1986, 60, 2020–2027. [Google Scholar] [PubMed]
- Ekkekakis, P.; Lind, E.; Hall, E.E.; Petruzzello, S.J. Do regression-based computer algorithms for determining the ventilatory threshold agree? J. Sports Sci. 2008, 26, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.H.; Molitoris, B.A. A statistical method for determining the breakpoint of two lines. Anal. Biochem. 1984, 141, 287–290. [Google Scholar] [CrossRef]
- Rosenthal, R.; Cooper, H.; Hedges, L. Parametric measures of effect size. In The Handbook of Research Synthesis; Russel Sage Foundation: New York, NY, USA, 1994; pp. 231–244. [Google Scholar]
- Hunter, R.A.; Storm, W.L.; Coneski, P.N.; Schoenfisch, M.H. Inaccuracies of nitric oxide measurement methods in biological media. Anal. Chem. 2013, 85, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.W.; Johnson, N.A.; Chapman, P.G.; Burke, L.M. The effect of nitrate supplementation on exercise performance in healthy individuals: A systematic review and meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.L.; Brooks, E.; Powers, S.K.; Tulley, R. The effects of caffeine on graded exercise performance in caffeine naive versus habituated subjects. Eur. J. Appl. Physiol. Occup. Physiol. 1991, 62, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Kenjale, A.A.; Ham, K.L.; Stabler, T.; Robbins, J.L.; Johnson, J.L.; VanBruggen, M.; Privette, G.; Yim, E.; Kraus, W.E.; Allen, J.D. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. J. Appl. Physiol. 2011, 110, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Govoni, M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 2004, 37, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D. Role of potassium in the regulation of systemic physiological function during exercise. Acta Physiol. 1996, 156, 287–294. [Google Scholar] [CrossRef] [PubMed]


| Stage | Time (min) | Speed (km/h) | Grade (%) |
|---|---|---|---|
| 0 | −3–0 | 2.7 | 0 |
| 1 | 0–3 | 2.7 | 10 |
| 2 | 3–6 | 4.0 | 12 |
| 3 | 6–9 | 5.5 | 14 |
| 4 | 9–12 | 6.8 | 16 |
| 5 | 12–15 | 8.0 | 18 |
| 6 | 15–18 | 8.9 | 20 |
| 7 | 18–21 | 9.7 | 22 |
| Characteristics | Overall (n = 14) | Males (n = 7) | Females (n = 7) |
|---|---|---|---|
| Age, yrs | 23.7 ± 3.2 | 23.5 ± 2.3 | 23.9 ± 4.1 |
| Height, m | 1.72 ± 0.09 | 1.77 ± 0.08 | 1.68 ± 0.09 |
| Weight, kg | 81.7 ± 17.3 | 88.1 ± 13.3 | 75.3 ± 19.4 |
| BMI, kg/m2 | 27.3 ± 3.8 | 28.2 ± 2.9 | 26.4 ± 4.6 |
| VO2 peak, mL/kg/min | 40.9 ± 7.2 | 44.7 ± 7.3 | 37.2 ± 4.9 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, A.N.; Haun, C.T.; Kephart, W.C.; Holland, A.M.; Mobley, C.B.; Pascoe, D.D.; Roberts, M.D.; Martin, J.S. Red Spinach Extract Increases Ventilatory Threshold during Graded Exercise Testing. Sports 2017, 5, 80. https://doi.org/10.3390/sports5040080
Moore AN, Haun CT, Kephart WC, Holland AM, Mobley CB, Pascoe DD, Roberts MD, Martin JS. Red Spinach Extract Increases Ventilatory Threshold during Graded Exercise Testing. Sports. 2017; 5(4):80. https://doi.org/10.3390/sports5040080
Chicago/Turabian StyleMoore, Angelique N., Cody T. Haun, Wesley C. Kephart, Angelia M. Holland, Christopher B. Mobley, David D. Pascoe, Michael D. Roberts, and Jeffrey S. Martin. 2017. "Red Spinach Extract Increases Ventilatory Threshold during Graded Exercise Testing" Sports 5, no. 4: 80. https://doi.org/10.3390/sports5040080






