Metal Foaming Investigated by X-ray Radioscopy
Abstract
:1. Introduction
2. X-ray Synchrotron Radioscopy in Material Science
2.1. Solidification of Metals
2.2. Diffusion in Metals
2.3. Flow of Melts
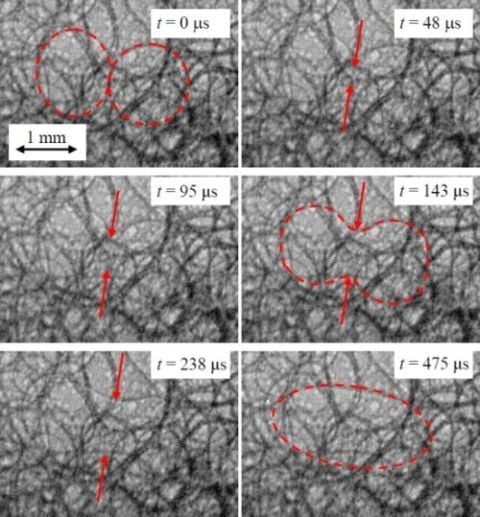
3. X-ray Synchrotron Radioscopy on Metal Foams

Fast Synchrotron X-ray Radioscopy

4. X-ray Laboratory Radioscopy—Selected Results
4.1. Process Control

4.2. New Effects

4.3. Cell Wall Rupture
4.4. Foaming Under Controlled Atmosphere

4.5. Foaming Under Microgravity


5. Summary
Acknowledgments
References
- Banhart, J. Manufacture, characterization and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Banhart, J.; Weaire, D. On the road again: Metal foams find favor. Phys. Today 2002, 55, 37–42. [Google Scholar] [CrossRef]
- Banhart, J.; Borbély, A.; Dzieciol, K.; García-Moreno, F.; Manke, I.; Kardjilov, N.; Kaysser-Pyzalla, A.R.; Strobl, M.; Treimer, W. X-ray and neutron imaging—Complementary techniques for materials science and engineering. Int. J. Mater. Res. 2010, 101, 1069–1079. [Google Scholar] [CrossRef]
- Mathiesen, R.H.; Arnberg, L.; Ramsøskar, K.; Weitkamp, T.; Raur, C.; Snigirev, A. Time-resolved X-ray imaging of Aluminum alloy solidification process. Metall. Mater. Trans. B 2002, 33, 613–623. [Google Scholar]
- Buffet, A.; Reinhardt, G.; Schenk, T.; Nguyen-Thi, H.; Gastaldi, J.; Mangelinck-Noel, N.; Bergeon, N.; Jung, H.; Hartwig, J.; Baruchel, J. Real-time and in situ solidification of Al-based alloys investigated by synchrotron radiation: a unique experimental set-up combining radiography and topography techniques. Phys. Status Solidi A 2007, 204, 2721–2727. [Google Scholar]
- Arnberg, L.; Mathiesen, R.H. The real time, high-resolution X-ray video microscopy of solidification in aluminum alloys. JOM J. Miner. Met. Mater. Soc. 2007, 59, 20–26. [Google Scholar]
- Nguyen-Thi, H.; Reinhardt, G.; Buffet, A.; Schenk, T.; Mangelinck-Noel, N.; Jung, H.; Bergeon, N.; Billia, B.; Hartwig, J.; Baruchel, J. In situ and real time analysis of TGZM phenomena by synchrotron X-ray radiography. J. Cryst. Growth 2008, 310, 2906–2914. [Google Scholar] [CrossRef]
- Yasuda, H.; Yamamoto, Y.; Nakatsuka, N.; Yoshiya, M.; Nagira, T.; Sugiyama, A.; Ohnaka, I.; Uesugi, K.; Umetani, K. In situ observation of solidification in A-Cu and Fe-Si-Al alloys. Int. J. Cast. Met. Res. 2009, 22, 15–21. [Google Scholar] [CrossRef]
- Griesche, A.; Garcia-Moreno, F.; Macht, M.-P.; Frohberg, G. Chemical diffusion experiments in AlNiCe-melts. Mater. Sci. Forum 2006, 508, 567–572. [Google Scholar] [CrossRef]
- Zabler, S.; Rueda, A.; Rack, A.; Riesemeier, H.; Zaslansky, P.; Manke, I.; Garcia-Moreno, F.; Banhart, J. Coarsening of grain refined semi-solid Al-Ge32 alloy: X-ray micotomography and in-situ radiography. Acta Mater. 2007, 55, 5045–5055. [Google Scholar]
- Zabler, S.; Rack, A.; Rueda, A.; Helfen, L.; Garcia-Moreno, F.; Banhart, J. Direct observation of flow in semi-solid alloys by synchrotron-based X-ray micro-radioscopy. Phys. Status Solidi A 2010, 207, 718–723. [Google Scholar]
- Wang, Y.; Liu, X.; Im, K.S.; Lee, W.K.; Wang, J.; Fezzaa, K.; Hung, D.L.S.; Winkelman, J.R. Ultrafast X-ray study of dense-liquid-jet flow dynamics using structure-tracking velocimetry. Nat. Phys. 2008, 4, 305–309. [Google Scholar] [CrossRef]
- 13. Rack, A.; Garcia-Moreno, F.; Schmitt, C.; Betz, O.; Cecilia, A.; Ershov, A.; Rack, T.; Banhart, J.; Zabler, S. On the possibilities of hard X-ray imaging with high spatio-temporal resolution using polychromatic synchrotron radiation. J. X-ray Sci. Technol. 2010, 18, 429–444. [Google Scholar]
- Garcia-Moreno, F.; Rack, A.; Helfen, L.; Baumbach, T.; Zabler, S.; Babcsán, N.; Banhart, J.; Martin, T.; Ponchut, C.; di Michiel, M. Fast processes in liquid metal foams investigated by high-speed synchrotron X-ray micro-radioscopy. Appl. Phys. Lett. 2008, 92, 134104–134106. [Google Scholar]
- Rack, A.; Garcia-Moreno, F.; Baumbach, T.; Banhart, J. Synchrotron-based radioscopy employing spatio-temporal micro-resolution for studying fast phenomena in liquid metal foams. J. Synchrotron Radiat. 2009, 16, 432–434. [Google Scholar] [CrossRef]
- Stanzick, H.; Banhart, J.; Helfen, L.; Baumbach, T. In-situ monitoring of metal foam evolution and decay. In Foams and Emulsions; Zitha, P., Banhart, J., Verbist, G., Eds.; MIT-Verlag: Bremen, Germany, 2000; pp. 290–296. [Google Scholar]
- Banhart, J.; Stanzick, H.; Helfen, L.; Baumbach, T.; Nijhof, K. Real-time X-ray investigation of Aluminium Foam Sandwich production. Adv. Eng. Mater. 2001, 3, 407–411. [Google Scholar]
- Stanzick, H.; Wichmann, M.; Weise, J.; Helfen, L.; Baumbach, T.; Banhart, J. Process control in aluminium foam production using real-time X-ray radioscopy. Adv. Eng. Mater. 2002, 4, 814–823. [Google Scholar] [CrossRef]
- Gergely, V.; Clyne, T.W. Drainage in standing liquid metal foams: Modelling and experimental observations. Acta Mater. 2004, 52, 3047–3058. [Google Scholar] [CrossRef]
- Garcia Moreno, F.; Fromme, M.; Banhart, J. Real-time X-ray radioscopy on metallic foams using a compact micro-focus source. Adv. Eng. Mater. 2004, 6, 416–420. [Google Scholar] [CrossRef]
- Garcia-Moreno, F.; Solórzano, E.; Banhart, J. Kinetics of coalescence in liquid aluminium foams. Soft Matter 2011, 7, 9216–9223. [Google Scholar]
- Brunke, O.; Odenbach, S. In situ observation and numerical calculations of the evolution of metallic foams. J. Phys. Condens. Matter 2006, 18, 6493–6506. [Google Scholar] [CrossRef]
- Garcia-Moreno, F.; Holm, P.; Banhart, J. Metallic foam experiments under microgravity. ESA Spec. Publ. 2007, 647, 389–392. [Google Scholar]
- Garcia-Moreno, F.; Jiménez, C.; Mukherjee, M.; Holm, P.; Weise, J.; Banhart, J. Experiments on metallic foams under gravity and microgravity. Colloids Surf. A 2009, 344, 101–106. [Google Scholar]
- Garcia-Moreno, F.; Raffaele, N.; Banhart, J. Optimisation of mould filling during PM metal foaming. In Proceedings of the CellMet08, Dresden, Germany, 8–10 October 2008; Stephani, G., Ed.; pp. 33–138.
- Mukherjee, M.; Garcia-Moreno, F.; Banhart, J. Solidification of metal foams. Acta Mater. 2010, 58, 6358–6370. [Google Scholar]
- Mukherjee, M.; Garcia-Moreno, F.; Banhart, J. Collapse of aluminium foam in two different atmospheres. Metall. Mater. Trans. B 2010, 41, 500–504. [Google Scholar] [CrossRef]
- Körner, C.; Berger, F.; Arnold, M.; Stadelmann, C.; Singer, R.F. Influence of processing conditions on morphology of metal foams produced from metal powder. Mater. Sci. Technol. 2000, 16, 781–784. [Google Scholar]
- Garcia-Moreno, F.; Babscán, N.; Banhart, J. X-ray radioscopy of liquid metal foams: Influence of heating profile, atmosphere and pressure. Colloids Surf. A 2005, 263, 290–294. [Google Scholar]
- Garcia-Moreno, F.; Babcsán, N.; Banhart, J. The role of the gas pressure on the foaming of metals following the PM-route. In Porous Metals and Metal Foaming Technology; Nakajima, H., Kanetake, N., Eds.; The Japan Institute of Metals: Sendai, Japan, 2006; pp. 129–132. [Google Scholar]
- Garcia-Moreno, F.; Banhart, J. Foaming of blowing agent-free aluminium powder compacts. Colloids Surf. A 2007, 309, 264–269. [Google Scholar] [CrossRef]
- Neu, T. Schäumen von Magnesium und Magnesiumlegierungen. Bachelor Thesis, Technical University Berlin, Berlin, Germany, 2011. [Google Scholar]
- Garcia-Moreno, F.; Mukherjee, M.; Jiménez, C.; Banhart, J. X-ray radioscopy of liquid metal foams under microgravity. Trans. Indian Inst. Met. 2009, 62, 451–454. [Google Scholar] [CrossRef]
- Mukherjee, M.; Garcia-Moreno, F.; Jiménez, C.; Banhart, J. Al and Zn foams blown by an intrinsic gas source. Adv. Eng. Mater. 2010, 12, 472–477. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Garcia-Moreno, F.; Mukherjee, M.; Jiménez, C.; Rack, A.; Banhart, J. Metal Foaming Investigated by X-ray Radioscopy. Metals 2012, 2, 10-21. https://doi.org/10.3390/met2010010
Garcia-Moreno F, Mukherjee M, Jiménez C, Rack A, Banhart J. Metal Foaming Investigated by X-ray Radioscopy. Metals. 2012; 2(1):10-21. https://doi.org/10.3390/met2010010
Chicago/Turabian StyleGarcia-Moreno, Francisco, Manas Mukherjee, Catalina Jiménez, Alexander Rack, and John Banhart. 2012. "Metal Foaming Investigated by X-ray Radioscopy" Metals 2, no. 1: 10-21. https://doi.org/10.3390/met2010010