Investigation on the Mechanical Properties of Mg-Al Alloys (AZ41 and AZ51) and Its Composites
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microstructure
| Materials | Grain size (µm) | Microhardness (HV) |
|---|---|---|
| AZ41 | 3.8 ± 1.6 | 69 ± 2 |
| AZ51 | 2.0 ± 0.9 | 79 ± 1 |
| AZ41 + Y2O3 | 2.4 ± 1.1 | 82 ± 2 |
| AZ51 + Y2O3 | 3.6 ± 1.7 | 73 ± 1 |


2.2. XRD Analysis
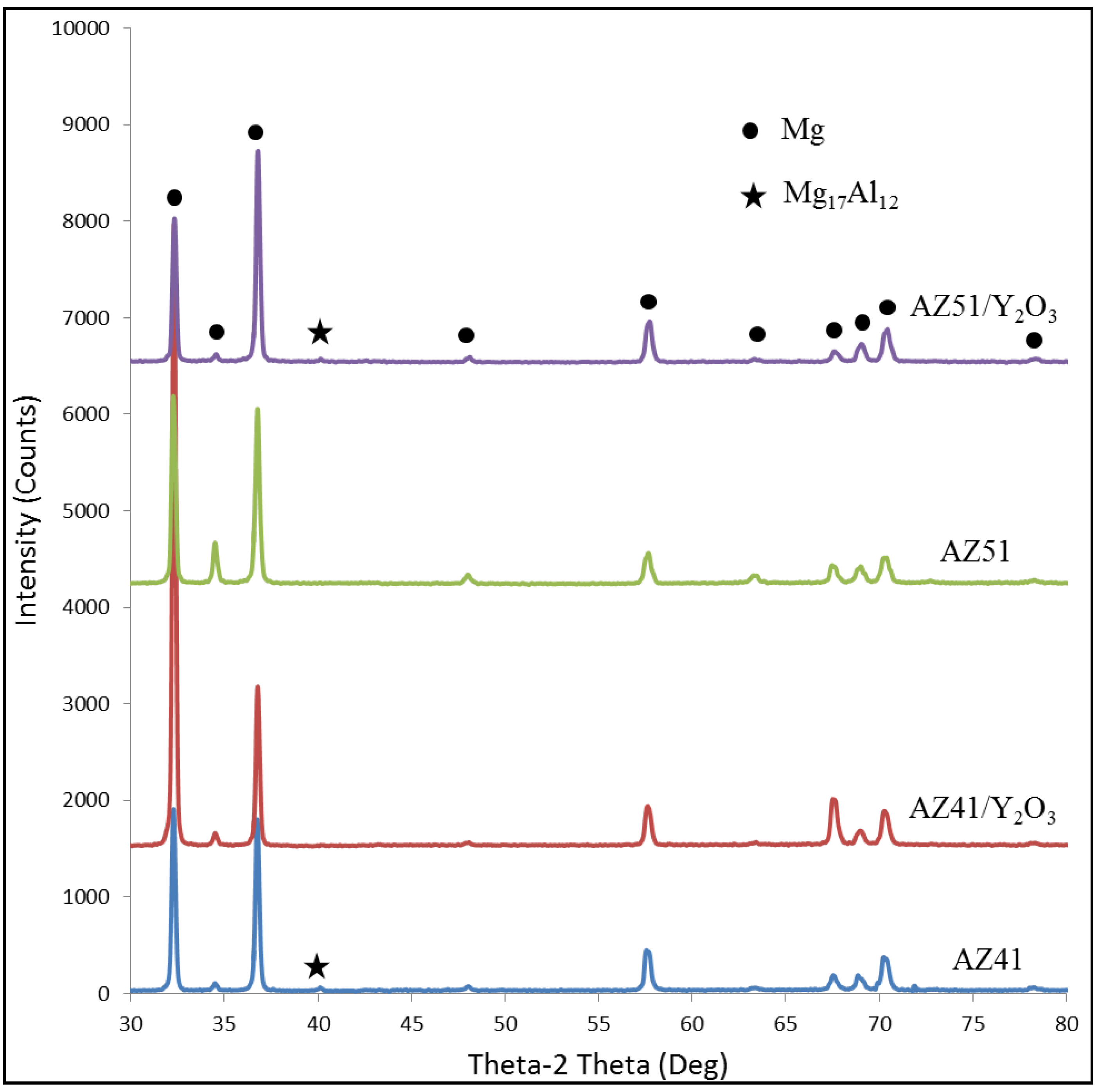
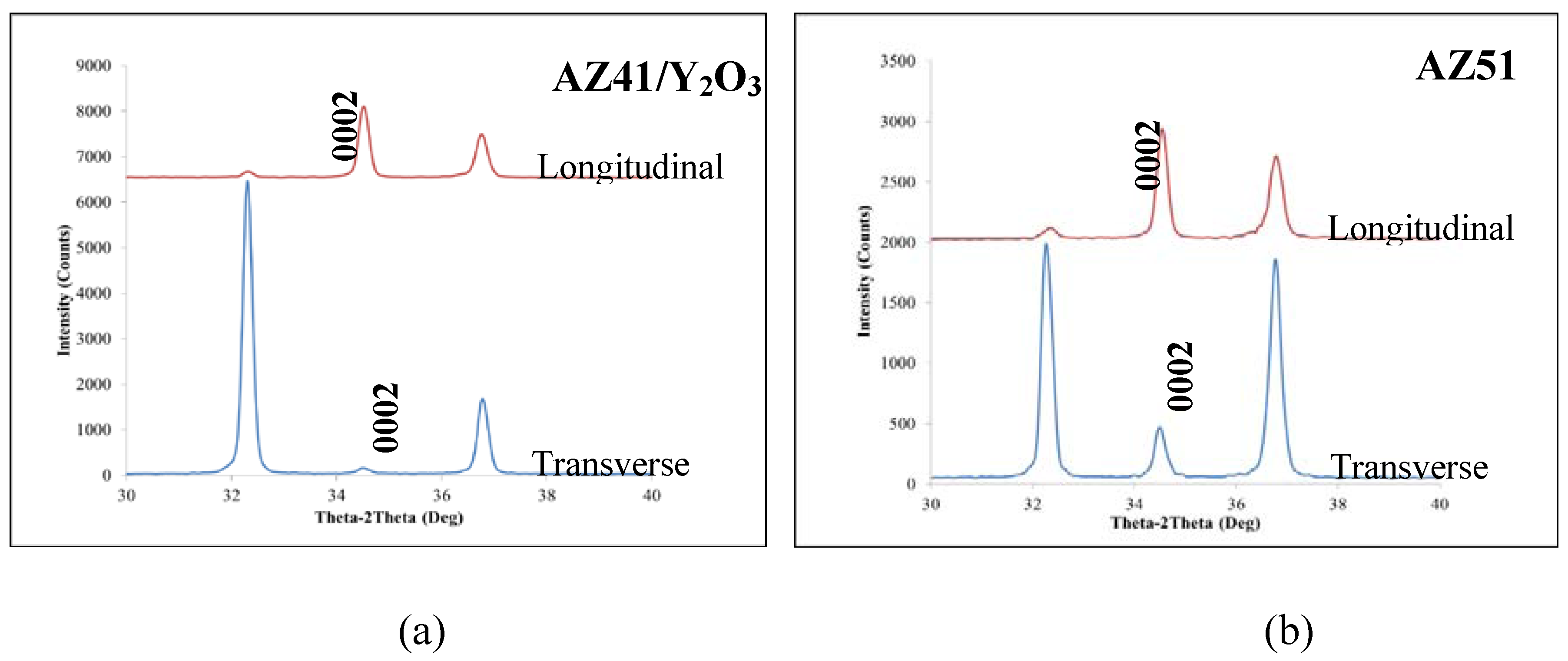
2.3. Microhardness
2.4. Tensile Properties
| Materials | 0.2%YS (MPa) | UTS (MPa) | Failure strain (%) |
|---|---|---|---|
| AZ41 | 184 ± 4 | 283 ± 4 | 13 ± 2 |
| AZ51 | 204 ± 4 | 300 ± 5 | 18 ± 2 |
| AZ41 + Y2O3 | 230 ± 2 | 311 ± 2 | 13 ± 2 |
| AZ51 + Y2O3 | 190 ± 4 | 295 ± 2 | 12 ± 1 |
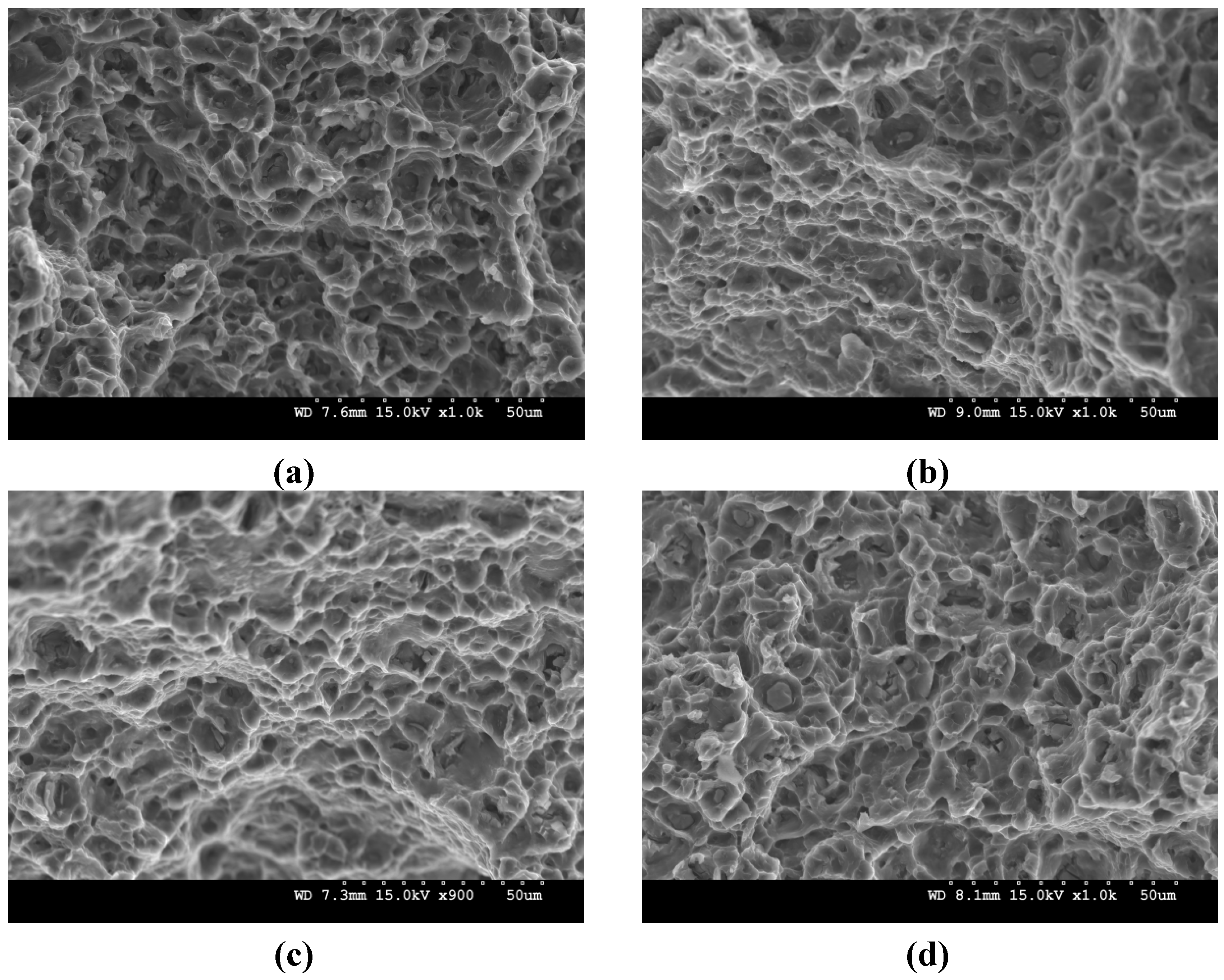
2.5. Compressive Properties
| Materials | 0.2%CYS (MPa) | UCS (MPa) | Failure Strain (%) |
|---|---|---|---|
| AZ41 | 165 ± 4 | 501 ± 8 | 17 ± 0.3 |
| AZ51 | 185 ± 11 | 508 ± 11 | 15 ± 1 |
| AZ41 + Y2O3 | 160 ± 13 | 451 ± 20 | 11 ± 1 |
| AZ51 + Y2O3 | 167 ± 6 | 564 ± 12 | 22 ± 1 |
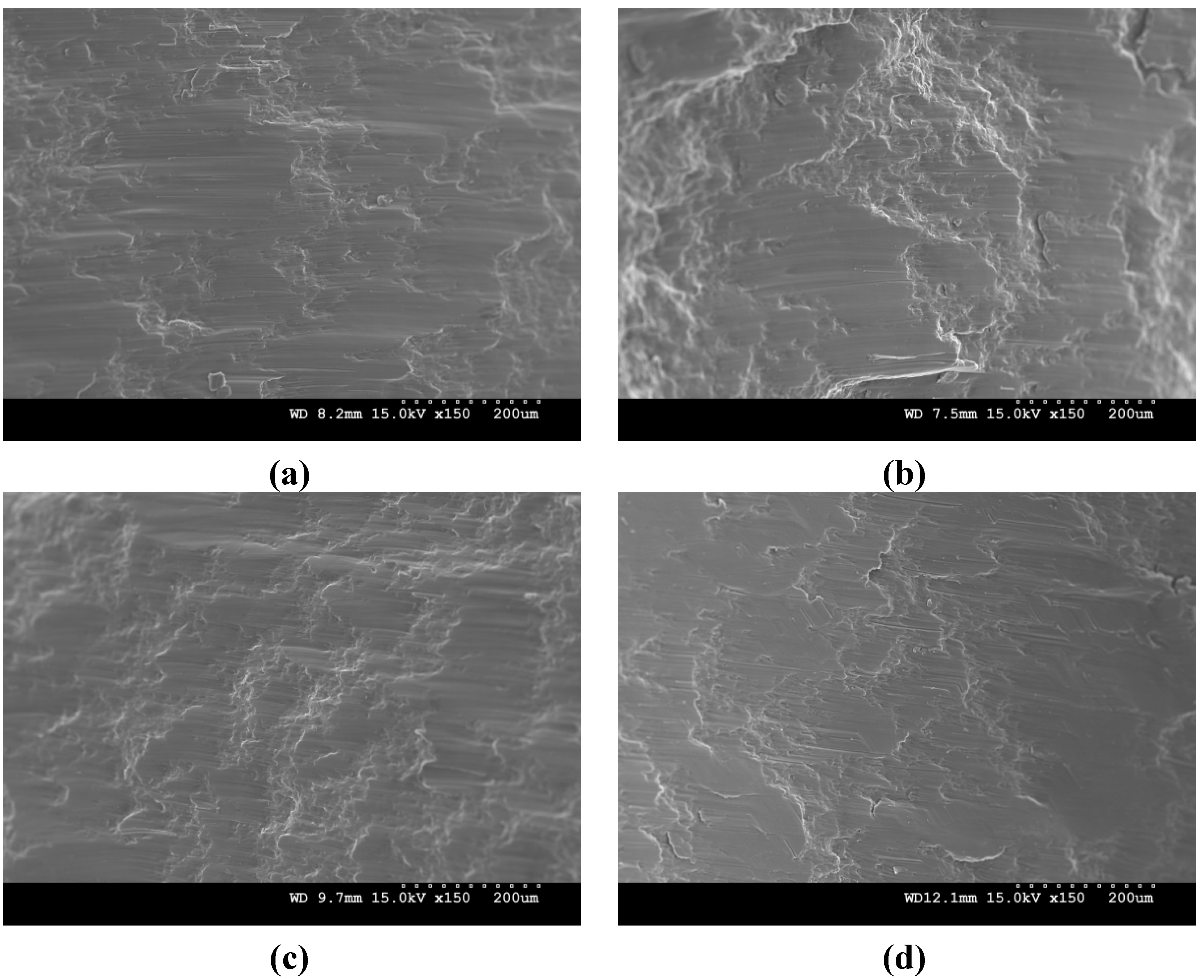
3. Materials and Experimental Procedures
4. Conclusions
- (1) Microstructure observation revealed the formation of discontinuous Mg17Al12 intermetallics in both AZ41 and AZ51 alloys. The intermetallics formed are of different morphologies: coarse, blocky intermetallics in AZ41 and combined form of coarse, blocky and fine, needle shaped intermetallics in AZ51. The coarse intermetallics in AZ41 were partially broken down into fine intermetallics due to the presence of Y2O3 in AZ41/Y2O3 composite. However, the formation of coarse intermetallics and absence of needle shaped intermetallics were observed in AZ51/Y2O3 composite regardless of having added the same amount of Y2O3 into AZ51.
- (2) The microhardness was increased in AZ41/Y2O3 composite, whereas a decrease in microhardness was observed in AZ51/Y2O3 composites when compared to that of AZ41 and AZ51. The highest microhardness was observed in AZ41 + Y2O3 composite among all synthesized materials.
- (3) An improvement in both 0.2% yield strength and ultimate tensile strength was observed in AZ51, AZ41/Y2O3 and AZ51/Y2O3 composites through the addition of Al and/or Y2O3 into AZ41. The failure strain was increased in AZ51 which is correlated to the formation of needle shaped intermetallics and texture changes. Being the same basal texture, the failure strain remained at a similar level in the rest of the synthesized materials but it was lower than that of AZ51.
- (4) Similar compressive yield strength was observed in the synthesized materials except AZ51. The improvement in yield strength particularly in AZ51 was associated with the observed random texture. The compressive failure strain in AZ41 and AZ51/Y2O3 composite was found to be higher than that of AZ51 and AZ41/Y2O3 composite.Variation in compressive failure strain was affected by the difference in grain sizes.
Acknowledgments
Conflict of Interest
References
- Mordike, B.L.; Kainer, K.U. Magnesium Alloys and Their Applications; Werkstoff-Insformations gesellschaft: Frankfu, Germany, 1998. [Google Scholar]
- Kainer, K.U. Magnesium Alloys and Technology; WILEY-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Polmear, I.J. Magnesium alloys and applications. Mater. Sci. Technol. 1994, 10, 1–16. [Google Scholar] [CrossRef]
- Friendich, H.; Schumanm, S. Research for a new age of magnesium in the automotive industry. J. Mater. Process. Technol. 2001, 117, 276–281. [Google Scholar] [CrossRef]
- Mordike, B.L.; Ebert, T. Magnesum: Properties, applications, potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.P.; Zhang, J.X.; Lorimer, G.W.; Robson, J. Review on research and development of magnesium alloys. Acta Metall. Sin. 2008, 21, 313–328. [Google Scholar]
- Agnew, S.R.; Tome, C.N.; Brown, D.W.; Holden, T.M.; Vogel, S.C. Study of slip mechanisms in a magnesium alloy by neutron diffraction and modelling. Scripta Mater. 2003, 48, 1003–1008. [Google Scholar] [CrossRef]
- Barnett, M.R.; Keshavarz, Z.; Beer, A.G.; Atwell, D. Influence of grain size on the compressive deformation of wrought Mg-3Al-1Zn. Acta Mater. 2004, 52, 5093–5103. [Google Scholar] [CrossRef]
- Mukai, T.; Yamanoi, M.; Watanabe, H.; Higashi, K. Ductility enhancement in AZ31 magnesium alloy by controlling its grain structure. Scripta Mater. 2001, 45, 89–94. [Google Scholar]
- Chino, Y.; Kimura, K.; Hakamada, M.; Mabuchi, M. Mechanical anisotropy due to twinning in an extruded AZ31 Mg alloy. Mater. Sci. Eng. A 2008, 485, 311–317. [Google Scholar] [CrossRef]
- Yin, S.M.; Wang, C.H.; Diao, Y.D.; Wu, S.D.; Li, S.X. Influence of grain size and texture on the yield asymmetry of Mg-3Al-1Zn alloy. J. Mater. Sci. Technol. 2011, 27, 29–34. [Google Scholar]
- Laser, T.; Hartig, C.; Nurnberg, M.R.; Letzig, D.; Bormann, R. The influence of calcium and cerium mischmetal on the microstructural evolution of Mg-3Al-1Zn during extrusion and resulting mechanical properties. Acta Mater. 2008, 56, 2791–2798. [Google Scholar] [CrossRef]
- Morisada, Y.; Fujii, H.; Nagaoka, T.; Fukusumi, M. MWCNTs/AZ31 surface composites fabricated by friction stir processing. Mater. Sci. Eng. A 2006, 419, 344–348. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Gupta, M. Increasing significantly the failure strain and work of fracture of solidification processed AZ31B using nano-Al2O3 particulates. J. Alloy. Compd. 2008, 459, 244–250. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Gupta, M. Enhancing compressive response of AZ31B magnesium alloy using alumina nanoparticulates. Comp. Sci. Technol. 2008, 68, 2185–2192. [Google Scholar] [CrossRef]
- Nguyen, Q.B.; Tun, K.S.; Chan, J.; Kwok, R.; Kumar, J.V.M.; Phung, H.T.; Gupta, M. Simultaneous effect of nano-Al2O3 and micrometre Cu particulates on microstructure and mechanical properties of magnesium alloy AZ31. Mater. Sci. Technol. 2012, 28, 227–233. [Google Scholar]
- Hassan, S.F.; Gupta, M. Development and characterization of ductile Mg/Y2O3 nanocomposites. J. Eng. Mat. Technol. 2007, 129, 462–467. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Properties and deformation behaviour of Mg-Y2O3 nanocomposites. Acta Mater. 2007, 55, 5115–5121. [Google Scholar] [CrossRef]
- Tun, K.S.; Gupta, M. Improving mechanical properties of magnesium using nano-Yttria reinforcement and microwave assisted powder metallurgy method. Comp. Sci. Technol. 2007, 67, 2657–2664. [Google Scholar] [CrossRef]
- Avedesian, M.M.; Baker, H. ASM Specialty Handbook: Magnesium and Magnesium Alloys; ASM International: Materials Park, OH, USA, 1999. [Google Scholar]
- StJohn, D.H.; Qian, M.; Easton, M.A.; Cao, P.; Hildebrand, Z. Grain refinement in Mg alloys. Metall. Mater. Trans. A 2005, 36, 1669–1679. [Google Scholar] [CrossRef]
- Lee, Y.C.; Dahle, A.K.; StJohn, D.H. The role of solute in grain refinement of magnesium. Metall. Mater. Trans. A 2000, 31, 2895–2906. [Google Scholar] [CrossRef]
- Song, C.J.; Han, Q.Y.; Zhai, Q.J. Review of grain refinement methods for as-cast microstructure of magnesium alloy. China Foundary 2009, 6, 93–103. [Google Scholar]
- Kang, Y.C.; Chan, S.L. Tensile properties of nanometric Al2O3 particulate-reinforced aluminum matrix composites. Mater. Chem. Phys. 2004, 85, 438–443. [Google Scholar] [CrossRef]
- Choi, H.; Sun, Y.; Slater, B.P.; Konishi, H.; Li, H. AZ91D/TiB2 nanocomposites fabricated by solidification nanoprocessing. Adv. Eng. Mater. 2012, 14, 291–295. [Google Scholar] [CrossRef]
- Shanthi, M.; Tun, K.S.; Pandey, R.S.; Gupta, M. Enhancing overall tensile behavior or ductility of AZ91D using nano-Al2O3 and heat treatment. Met. Mater. 2011, 49, 197–205. [Google Scholar]
- Asla, K.M.; Tari, A.; Khomamizadeh, F. The effect of different content of Al, RE and Si element on the microstructure, mechanical and creep properties of Mg-Al alloys. Mater. Sci. Eng. A 2009, 523, 1–6. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–25. [Google Scholar] [CrossRef]
- Cullity, B.D. Element of X-Ray Diffraction, 2nd ed; Addison-Wesley: Reading, MA, USA, 1978; p. 414. [Google Scholar]
- McDanels, D.C. Analysis of stress-strain, fracture and ductility behavior of aluminum matrix composites containing discontinuous silicon carbide reinforcement. Metall. Trans. A 1985, 16, 1105–1115. [Google Scholar] [CrossRef]
- Goh, C.S.; Wei, J.; Lee, L.C.; Gupta, M. Ductility improvement and fatigue studies in Mg-CNT nanocomposites. Comp. Sci. Technol. 2008, 68, 1432–1439. [Google Scholar] [CrossRef]
- Fleck, N.A.; Muller, G.M.; Ashby, M.F.; Hutchinson, J.W. Strain gradient plasticity: Theory and experiment. Acta Metall. Mater. 1994, 42, 475–487. [Google Scholar] [CrossRef]
- Arsenault, R.J.; Shi, N. Dislocation generation due to differences between the coefficients of thermal expansion. Mater. Sci. Eng. 1986, 81, 175–187. [Google Scholar] [CrossRef]
- Somekawa, H.; Mukai, T. Effect of texture on fracture toughness in extruded AZ31 magnesium alloy. Scripta Mater. 2005, 53, 541–545. [Google Scholar] [CrossRef]
- Jiang, J.; Godfrey, A.; Liu, W.; Liu, Q. Microtexture evolution via deformation twinning and slip during compression of magnesium alloy AZ31. Mater. Sci. Eng. A 2008, 483–484, 576–579. [Google Scholar]
- Klimanek, P.; Potzsch, A. Microstructure evolution under compressive plastic deformation of magnesium at different temperatures and strain rates. Mater. Sci. Eng. A 2002, 324, 145–150. [Google Scholar] [CrossRef]
- Barnett, M.R. Influence of deformation conditions and texture on the high temperature flow stress of magnesium AZ31. J. Light Met. 2001, 1, 167–177. [Google Scholar] [CrossRef]
- Garces, G.; Rodriguez, M.; Perez, P.; Adeva, P. Effect of volume fraction and particle size on the microstructure and plastic deformation of Mg-Y2O3 composites. Mater. Sci. Eng. A 2006, 419, 357–364. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tun, K.S.; Minh, N.J.; Nguyen, Q.B.; Hamouda, A.M.; Gupta, M. Investigation on the Mechanical Properties of Mg-Al Alloys (AZ41 and AZ51) and Its Composites. Metals 2012, 2, 313-328. https://doi.org/10.3390/met2030313
Tun KS, Minh NJ, Nguyen QB, Hamouda AM, Gupta M. Investigation on the Mechanical Properties of Mg-Al Alloys (AZ41 and AZ51) and Its Composites. Metals. 2012; 2(3):313-328. https://doi.org/10.3390/met2030313
Chicago/Turabian StyleTun, Khin S., Ng J. Minh, Quy B. Nguyen, Abdel Magid Hamouda, and Manoj Gupta. 2012. "Investigation on the Mechanical Properties of Mg-Al Alloys (AZ41 and AZ51) and Its Composites" Metals 2, no. 3: 313-328. https://doi.org/10.3390/met2030313






