Mechanical Properties and Deformation Behavior of Bulk Metallic Glasses
Abstract
:1. Introduction
2. Mechanical Properties and Deformation of Bulk Metallic Glasses and Composites at Room Temperature
| Element | Content, at % | σy | σf | E | HV | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |||||
| Al | La | Cu | 35 | 55 | 1 | 880 | 43 | 256 | [10] | |
| Al | La | Cu | 30 | 50 | 20 | 750 | 32 | 240 | [10] | |
| Al | La | Cu | 25 | 55 | 20 | 600 | 32 | 213 | [10] | |
| Al | La | Cu | 25 | 50 | 25 | 535 | 29 | 208 | [10] | |
| Cu | Hf | Al | 50 | 42.5 | 7.5 | 2370 | 128 | 673 | [11] | |
| Cu | Hf | Al | 52.5 | 40 | 7.5 | 2345 | 125 | 661 | [11] | |
| Cu | Hf | Al | 52.5 | 40 | 7.5 | 2344 | 125 | 661 | [12] | |
| Cu | Hf | Al | 50 | 45 | 5 | 2262 | 121 | 627 | [11] | |
| Cu | Hf | Al | 50 | 45 | 5 | 2260 | 121 | 627 | [12] | |
| Cu | Hf | Ti | 60 | 25 | 15 | 2010 | 2160 | 124 | [13] | |
| Cu | Hf | Ti | 60 | 25 | 15 | 2024 | 2088 | 124 | [12] | |
| Cu | Hf | Ti | 60 | 25 | 15 | 1920 | 2130 | 120 | [14] | |
| Cu | Zr | Ag | 50 | 45 | 5 | 1940 | 112 | 599 | [15] | |
| Cu | Zr | Ag | 50 | 45 | 5 | 1940 | 112 | 599 | [15] | |
| Cu | Zr | Ag | 45 | 47.5 | 7.5 | 1820 | 108 | 556 | [15] | |
| Cu | Zr | Ag | 45 | 45 | 10 | 1810 | 108 | 542 | [15] | |
| Cu | Zr | Ag | 42.5 | 47.5 | 10 | 1780 | 106 | 534 | [15] | |
| Cu | Zr | Ag | 45 | 50 | 5 | 1885 | 111 | 585 | [15] | |
| Cu | Zr | Al | 47.5 | 47.5 | 5 | 1547 | 2265 | 87 | [16] | |
| Cu | Zr | Al | 55 | 40 | 5 | 2210 | 115 | 581 | [17] | |
| Cu | Zr | Al | 52.5 | 42.5 | 5 | 2115 | 111 | 573 | [17] | |
| Cu | Zr | Al | 50 | 45 | 5 | 1885 | 102 | 546 | [17] | |
| Cu | Zr | Al | 46 | 46 | 8 | 1894 | 2250 | 580 | [18] | |
| Cu | Zr | Al | 55 | 40 | 5 | 2210 | 115 | 581 | [19,20] | |
| Cu | Zr | Al | 52.5 | 42.5 | 5 | 2115 | 111 | 573 | [19,20] | |
| Cu | Zr | Al | 50 | 45 | 5 | 1885 | 102 | 546 | [19,20] | |
| Cu | Zr | Al | 48 | 48 | 4 | 1199 | 1882 | 103 | [21] | |
| Cu | Zr | Al | 47 | 47 | 6 | 1733 | 2250 | 580 | [22] | |
| Cu | Zr | Ga | 52.5 | 42.5 | 5 | 1940 | 105 | 552 | [23] | |
| Cu | Zr | Ga | 55 | 40 | 5 | 2025 | 109 | 565 | [23] | |
| Cu | Zr | Ga | 52.5 | 40 | 7.5 | 2130 | 111 | 581 | [23] | |
| Cu | Zr | Ga | 57.5 | 40 | 2.5 | 1910 | 105 | 547 | [23] | |
| Cu | Zr | Ti | 60 | 30 | 10 | 1785 | 2150 | 114 | [24] | |
| Gd | Al | Ni | 60 | 30 | 10 | 1330 | 67 | [25] | ||
| Gd | Al | Ni | 55 | 25 | 20 | 1300 | 65 | [26] | ||
| Gd | Al | Ni | 65 | 25 | 10 | 1300 | 63 | [26] | ||
| Gd | Co | Al | 60 | 30 | 10 | 1186 | 60 | [25] | ||
| Gd | Co | Al | 60 | 25 | 15 | 1250 | 63 | [25] | ||
| Gd | Co | Al | 60 | 20 | 20 | 1240 | 63 | [25] | ||
| Gd | Ni | Al | 60 | 25 | 15 | 1280 | 64 | [25] | ||
| Gd | Ni | Al | 60 | 20 | 20 | 1240 | 63 | [25] | ||
| Gd | Ni | Al | 50 | 25 | 25 | 1320 | 66 | [26] | ||
| La | Al | Ni | 45 | 45 | 10 | 1080 | 795 | 52 | 330 | [27] |
| La | Al | Ni | 45 | 35 | 20 | 995 | 720 | 46 | 305 | [27] |
| La | Al | Ni | 50 | 35 | 15 | 950 | 715 | 41 | 290 | [27] |
| La | Al | Ni | 50 | 30 | 20 | 930 | 715 | 41 | 285 | [27] |
| La | Al | NI | 55 | 25 | 20 | 735 | 515 | 34 | 225 | [22] |
| Mg | Cu | Y | 80 | 10 | 10 | 630 | 820 | 220 | [28] | |
| Mg | Cu | Y | 75 | 15 | 10 | 743 | 50 | [28] | ||
| Mg | Ni | Gd | 75 | 15 | 10 | 929 | [29] | |||
| Mg | Ni | Gd | 70 | 20 | 10 | 880 | [29] | |||
| Mg | Ni | Gd | 70 | 15 | 15 | 965 | [29] | |||
| Mg | Ni | Gd | 65 | 25 | 10 | 884 | [29] | |||
| Mg | Ni | Gd | 65 | 20 | 15 | 909 | [29] | |||
| Mg | Ni | Gd | 60 | 25 | 15 | 869 | [29] | |||
| Mg | Ni | Y | 82.5 | 12.5 | 5 | 610 | 44 | 212 | [30] | |
| Mg | Ni | Y | 80 | 15 | 5 | 830 | 46 | 224 | [30] | |
| Mg | Ni | Y | 85 | 10 | 5 | 640 | 40 | 193 | [30] | |
| Zr | Al | Ni | 70 | 10 | 20 | 1411 | 1335 | 61 | 432 | [31] |
| Zr | Al | Ni | 65 | 10 | 25 | 1581 | 1520 | 64.5 | 484 | [31] |
| Zr | Al | Ni | 65 | 15 | 20 | 1614 | 1640 | 70.5 | 494 | [31] |
| Zr | Al | Ni | 60 | 15 | 25 | 1640 | 1715 | 72.6 | 502 | [31] |
| Zr | Al | Ni | 60 | 20 | 20 | 1795 | 1720 | 78.2 | 549 | [31] |
| Zr | Co | Al | 55 | 30 | 15 | 1790 | 98 | 543 | [32] | |
| Zr | Co | Al | 55 | 25 | 20 | 1750 | 96 | 530 | [32] | |
| Zr | Co | Al | 55 | 25 | 20 | 1900 | 114 | [33] | ||
| Zr | Cu | Al | 50 | 40 | 10 | 1821 | 89 | [34] | ||
| Zr | Cu | Al | 50 | 40 | 10 | 1860 | 88 | 496 | [35] | |
| Zr | Cu | Al | 52.5 | 37.5 | 10 | 1840 | 86 | 485 | [32,36] | |
| Zr | Cu | Al | 50 | 37.5 | 12.5 | 1960 | 93 | 511 | [32,36] | |
| Zr | Cu | Al | 50 | 42.5 | 7.5 | 1820 | 86 | 475 | [32,36] | |
| Zr | Cu | Al | 55 | 35 | 10 | 1810 | 83 | 470 | [36] | |
| Zr | Cu | Al | 60 | 30 | 10 | 1720 | 80 | 446 | [36] | |
| Zr | Cu | Al | 47.5 | 42.5 | 10 | 1920 | 90 | 508 | [36] | |
| Zr | Ni | Al | 60 | 25 | 15 | 1760 | 88 | 495 | [31] | |
| Zr | Ni | Al | 55 | 25 | 20 | 1780 | 89 | 502 | [31,37] | |
| Zr | Ni | Al | 55 | 30 | 15 | 1820 | 99 | 514 | [31,37] | |
| Zr | Ni | Al | 60 | 20 | 20 | 1793 | 1720 | 78.2 | 549 | [38] |
| Zr | Ni | Al | 70 | 20 | 10 | 1411 | 1335 | 61 | 432 | [31,39] |
| Zr | Ni | Al | 65 | 25 | 10 | 1520 | 1581 | 64.5 | 484 | [31,39] |
| Zr | Ni | Al | 65 | 20 | 15 | 1614 | 1640 | 494 | [31,39] | |
| Zr | Ni | Al | 60 | 25 | 15 | 1640 | 1715 | 502 | [31,39] | |
| Zr | Ni | Ti | 40 | 37 | 23 | 1630 | 524 | [40] | ||
| Element | Content, at % | σy | σf | E | HV | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||||
| Ce | Al | Cu | Co | 68 | 10 | 20 | 2 | 1180 | 31.34 | [41] | ||
| Ce | Al | Cu | Fe | 68 | 10 | 20 | 2 | 1232 | 32.7 | [41] | ||
| Ce | Al | Cu | Nb | 68 | 10 | 20 | 2 | 1165 | 30.95 | [41] | ||
| Ce | Al | Cu | Ni | 68 | 10 | 20 | 2 | 1198 | 31.93 | [41] | ||
| Co | Fe | Ta | B | 38 | 25 | 5.5 | 31.5 | 5185 | 268 | [42] | ||
| Cu | Hf | Ti | Ta | 56.4 | 23.5 | 14.1 | 6 | 2125 | 2100 | 104 | [43] | |
| Cu | Zr | Ag | Al | 45 | 45 | 7 | 3 | 1836 | 110 | 540 | [44] | |
| Cu | Zr | Ag | Al | 45 | 45 | 5 | 5 | 1890 | 112 | 556 | [44] | |
| Cu | Zr | Ag | Al | 45 | 45 | 3 | 7 | 1912 | 112 | 561 | [44] | |
| Cu | Zr | Hf | Ag | 45 | 25 | 20 | 10 | 2000 | 122 | 579 | [41] | |
| Cu | Zr | Ti | Be | 55.5 | 27.75 | 9.25 | 7.5 | 2450 | 146 | 710 | [45] | |
| Cu | Zr | Ti | Y | 58.8 | 29.4 | 9.8 | 2 | 1780 | 2050 | 115 | [46] | |
| Fe | Si | B | Nb | 72 | 9.6 | 14.4 | 4 | 4200 | 200 | [47] | ||
| La | Al | Cu | Ag | 62.5 | 12.5 | 20 | 5 | 640 | 36 | 201 | [48] | |
| La | Al | Cu | Ag | 55 | 15 | 20 | 10 | 758 | 42 | 208 | [48] | |
| Mg | Cu | Ni | Gd | 65 | 5 | 20 | 10 | 874 | 54 | [49] | ||
| Mg | Y | Zn | Cu | 65 | 10 | 5 | 20 | 860 | 74 | [50] | ||
| Ni | Nb | Ti | Zr | 60 | 15 | 10 | 15 | 2770 | 156 | [51] | ||
| Ni | Si | B | Nb | 72 | 7.68 | 16.32 | 4 | 2510 | 77 | 870 | [52] | |
| Ni | Si | B | Ta | 72 | 7.68 | 16.32 | 4 | 2730 | 75 | 920 | [52] | |
| Ni | Ta | Ti | Zr | 60 | 15 | 15 | 10 | 3180 | 67 | [53] | ||
| Pd | Cu | Ni | P | 40 | 30 | 10 | 20 | 1640 | 515 | [54] | ||
| Pd | Cu | Si | P | 79 | 6 | 10 | 5 | 1475 | 1575 | 82 | [55] | |
| Pd | Pt | Cu | P | 35 | 15 | 30 | 20 | 1410 | 470 | [56] | ||
| Pt | Cu | Ni | P | 57.5 | 14.7 | 5.3 | 22.5 | 1400 | 1470 | [57] | ||
| Ti | Ni | Cu | Sn | 50 | 20 | 25 | 5 | 2050 | 102 | 650 | [58] | |
| Ti | Ni | Cu | Sn | 50 | 20 | 23 | 7 | 2200 | 105 | 670 | [58] | |
| Ti | Ni | Cu | Sn | 50 | 22 | 25 | 3 | 2050 | 98 | 640 | [58] | |
| Zr | Al | Co | Cu | 55 | 20 | 20 | 5 | 2000 | 1960 | 92 | [59] | |
| Zr | Al | Ni | Pd | 65 | 7.5 | 10 | 17.5 | 1340 | 1510 | [60] | ||
| Zr | Cu | Ni | Al | 52 | 32 | 4 | 12 | 1780 | 88 | 501 | [61] | |
| Zr | Cu | Ni | Al | 52 | 30 | 6 | 12 | 1820 | 93 | 506 | [61] | |
| Zr | Cu | Ni | Al | 50 | 26 | 12 | 12 | 1878 | 88 | 498 | [61] | |
| Zr | Cu | Ni | Al | 50 | 34 | 4 | 12 | 1905 | 91 | 517 | [61] | |
| Zr | Cu | Ni | Al | 48 | 32 | 8 | 12 | 1894 | 94 | 513 | [61] | |
| Zr | Cu | Ni | Al | 50 | 32 | 6 | 12 | 1875 | 92 | 521 | [61] | |
| Zr | Cu | Ni | Al | 52 | 28 | 8 | 12 | 1798 | 94 | 512 | [61] | |
| Zr | Cu | Ni | Al | 50 | 30 | 8 | 12 | 1820 | 92 | 526 | [61] | |
| Zr | Cu | Ni | Al | 46 | 34 | 8 | 12 | 1777 | 111 | 562 | [61] | |
| Zr | Cu | Ni | Al | 48 | 28 | 12 | 12 | 1906 | 102 | 530 | [61] | |
| Zr | Cu | Ni | Al | 48 | 34 | 6 | 12 | 1899 | 94 | 529 | [61] | |
| Zr | Cu | Ni | Al | 50 | 28 | 10 | 12 | 1993 | 92 | 517 | [61] | |
| Zr | Cu | Ni | Al | 48 | 30 | 10 | 12 | 1378 | 94 | 520 | [61] | |
| Zr | Cu | Ni | Al | 48 | 30 | 10 | 12 | 1980 | 92 | 528 | [61] | |
| Zr | Cu | Ni | Al | 52 | 26 | 10 | 12 | 1960 | 89 | 509 | [61] | |
| Zr | Cu | Ni | Al | 46 | 30 | 12 | 12 | 1399 | 106 | 552 | [61] | |
| Zr | Cu | Fe | Al | 60 | 25 | 5 | 10 | 1643 | 92 | [62] | ||
| Zr | Cu | Fe | Al | 60 | 20 | 10 | 10 | 1708 | 104 | [62] | ||
| Zr | Fe | Al | Cu | 60 | 10 | 7.5 | 22.5 | 1718 | 100 | [62] | ||
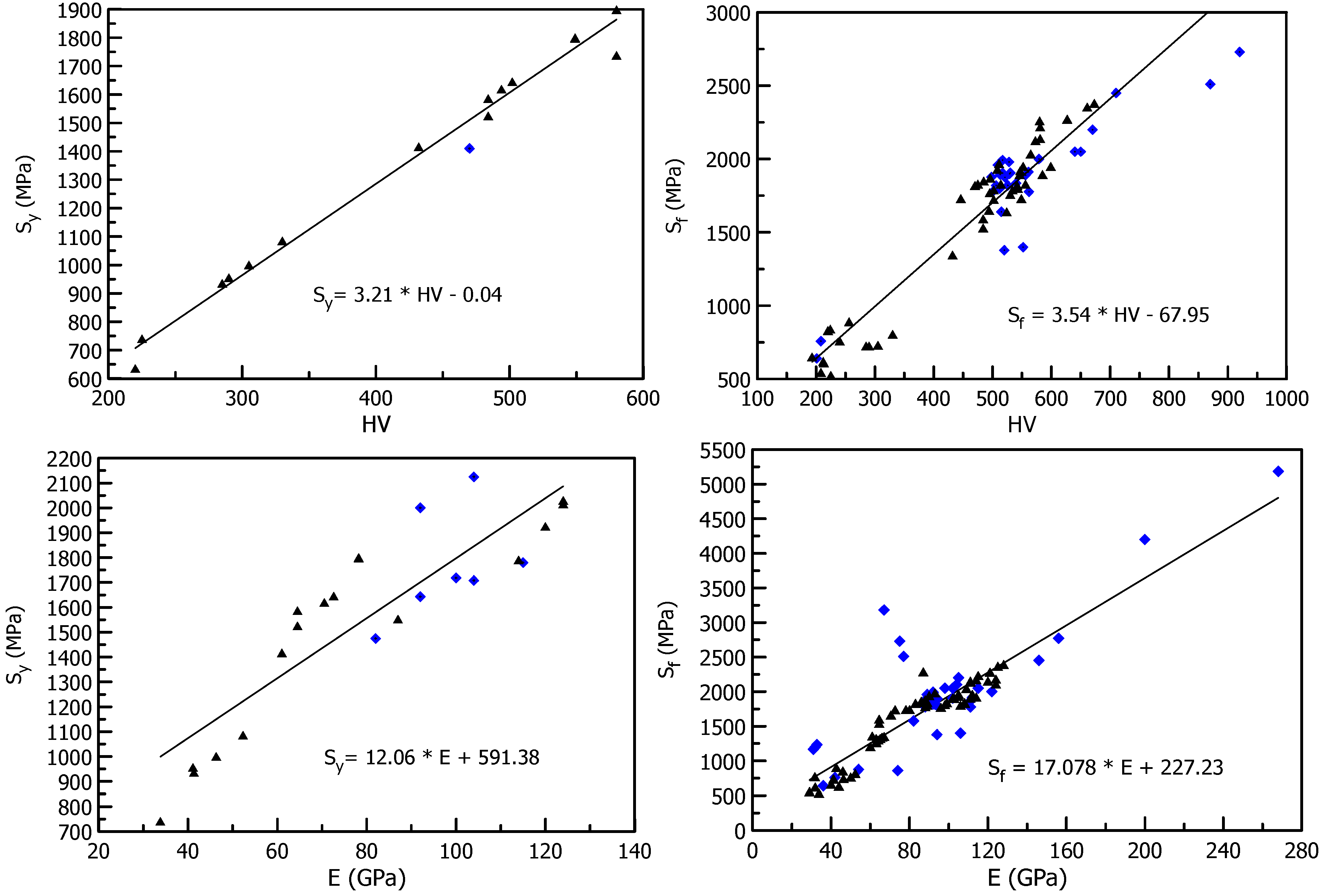
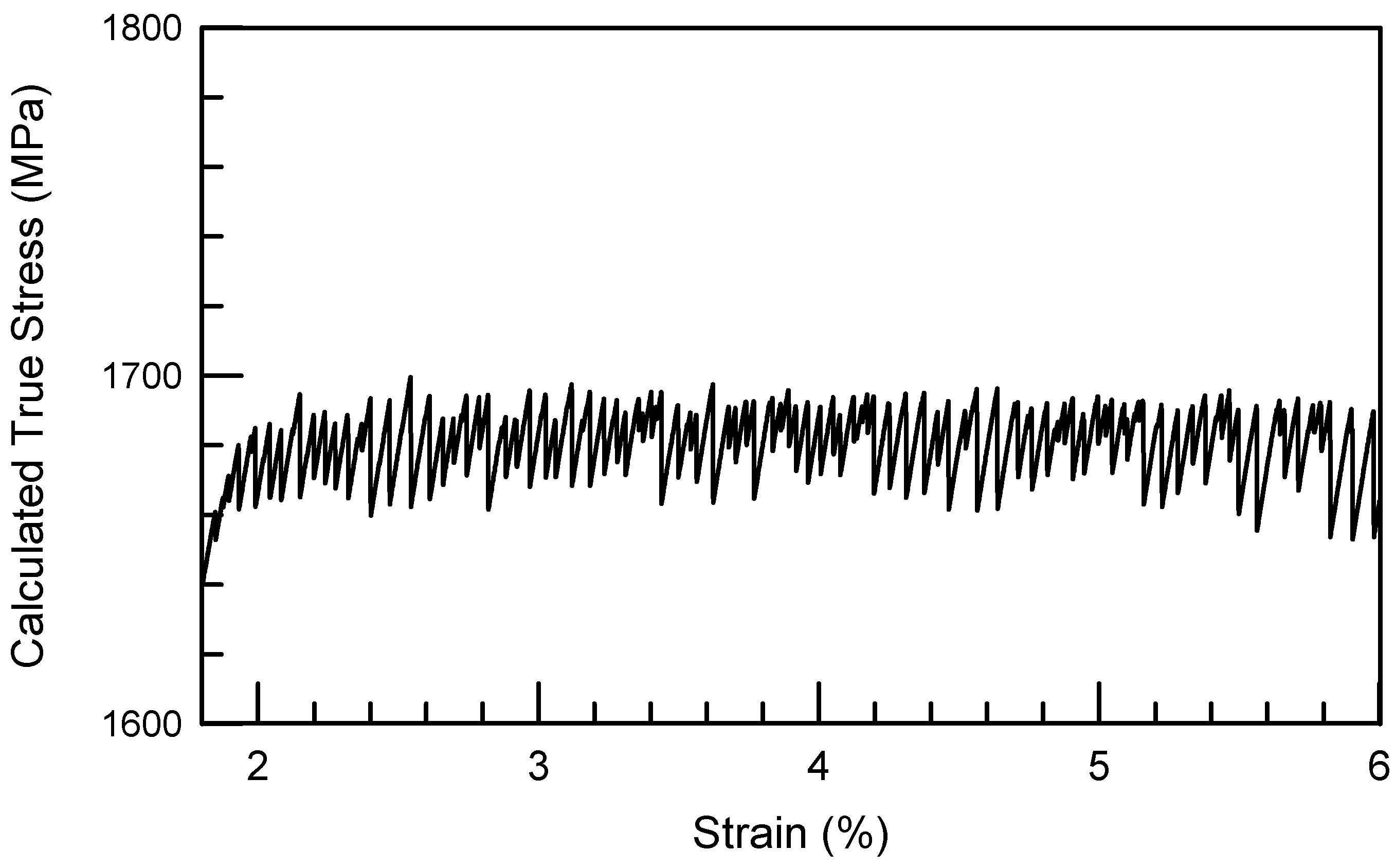
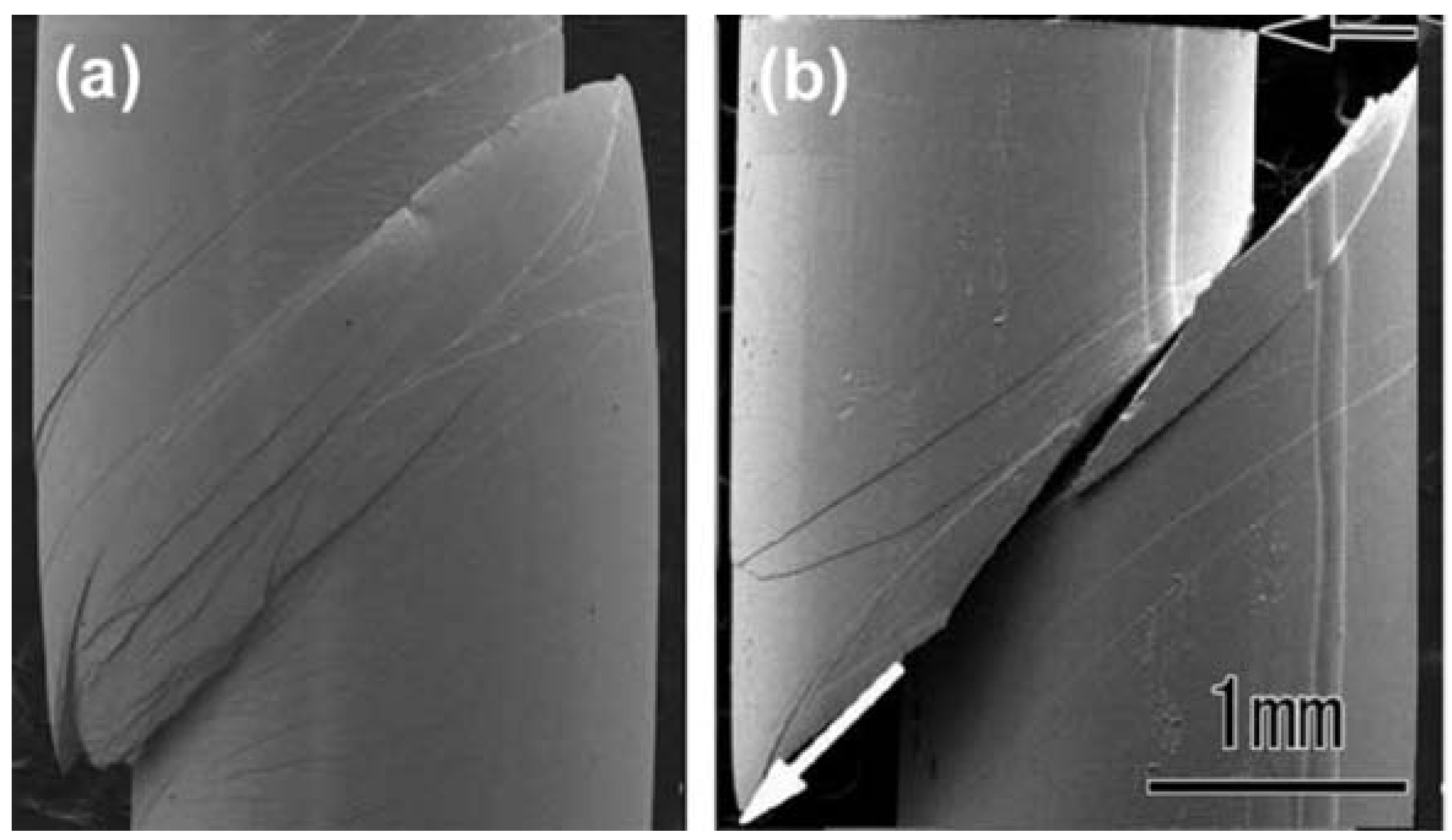
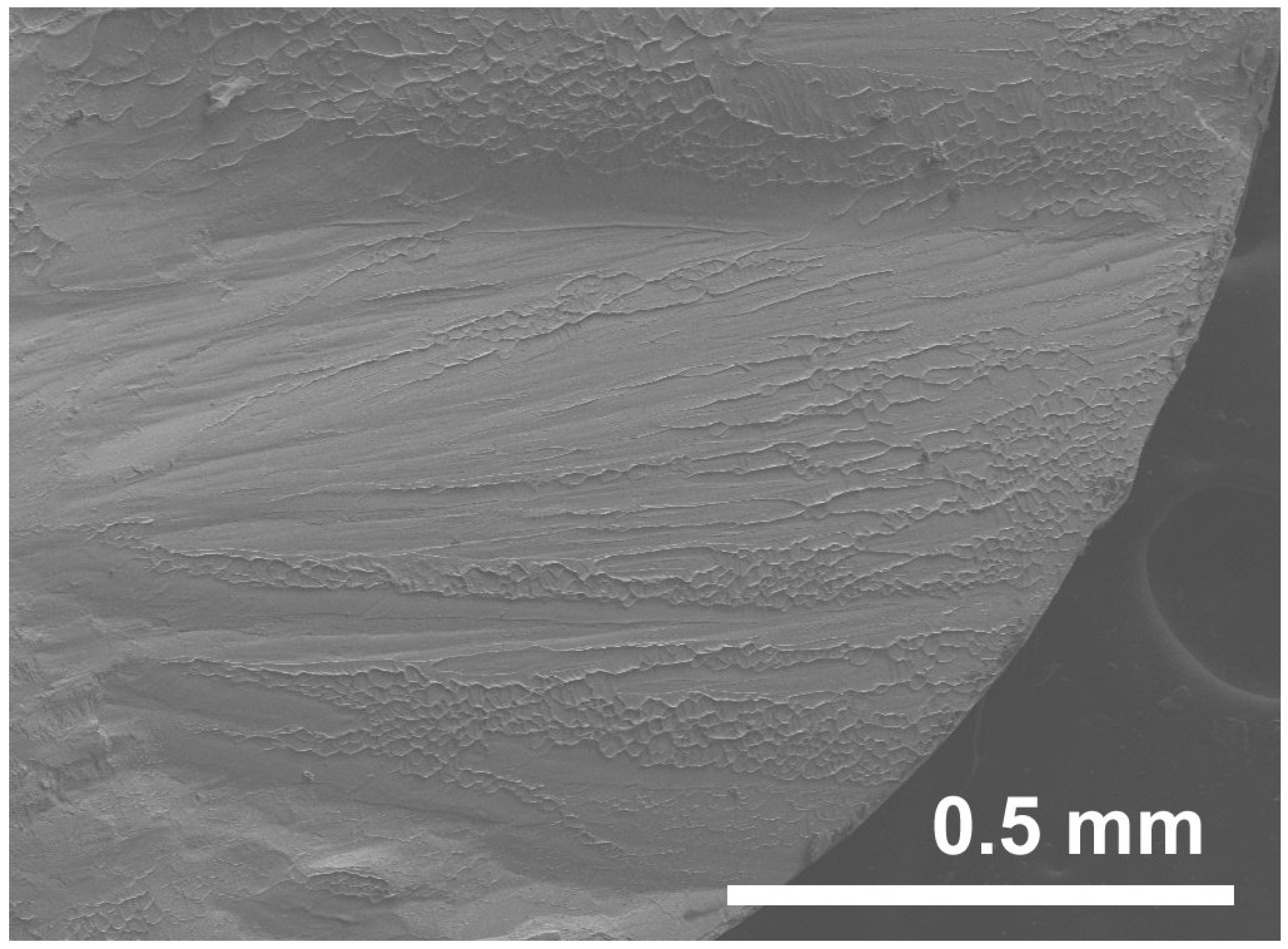
3. Deformation of Bulk Metallic Glasses at Cryogenic Temperature
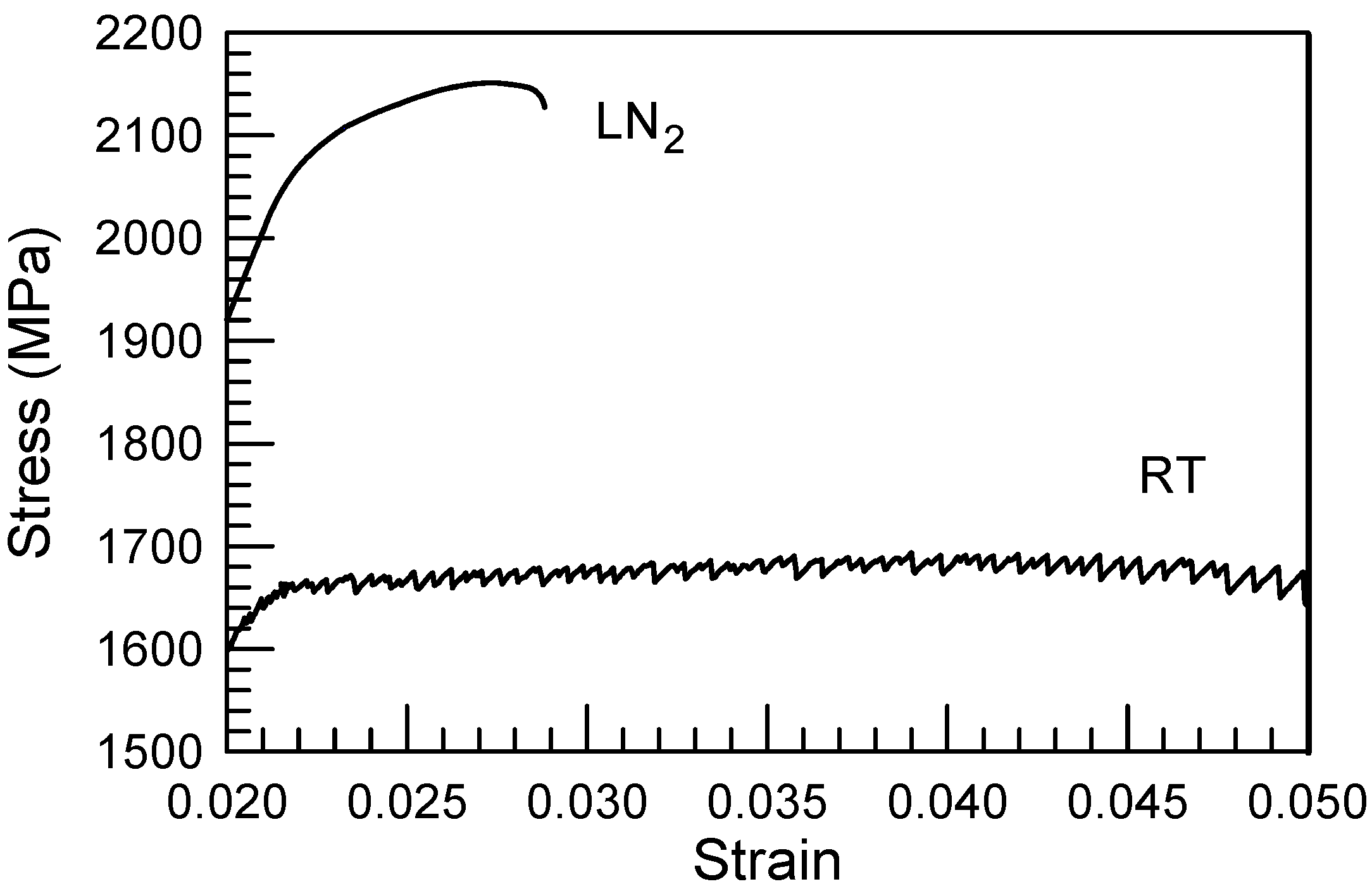
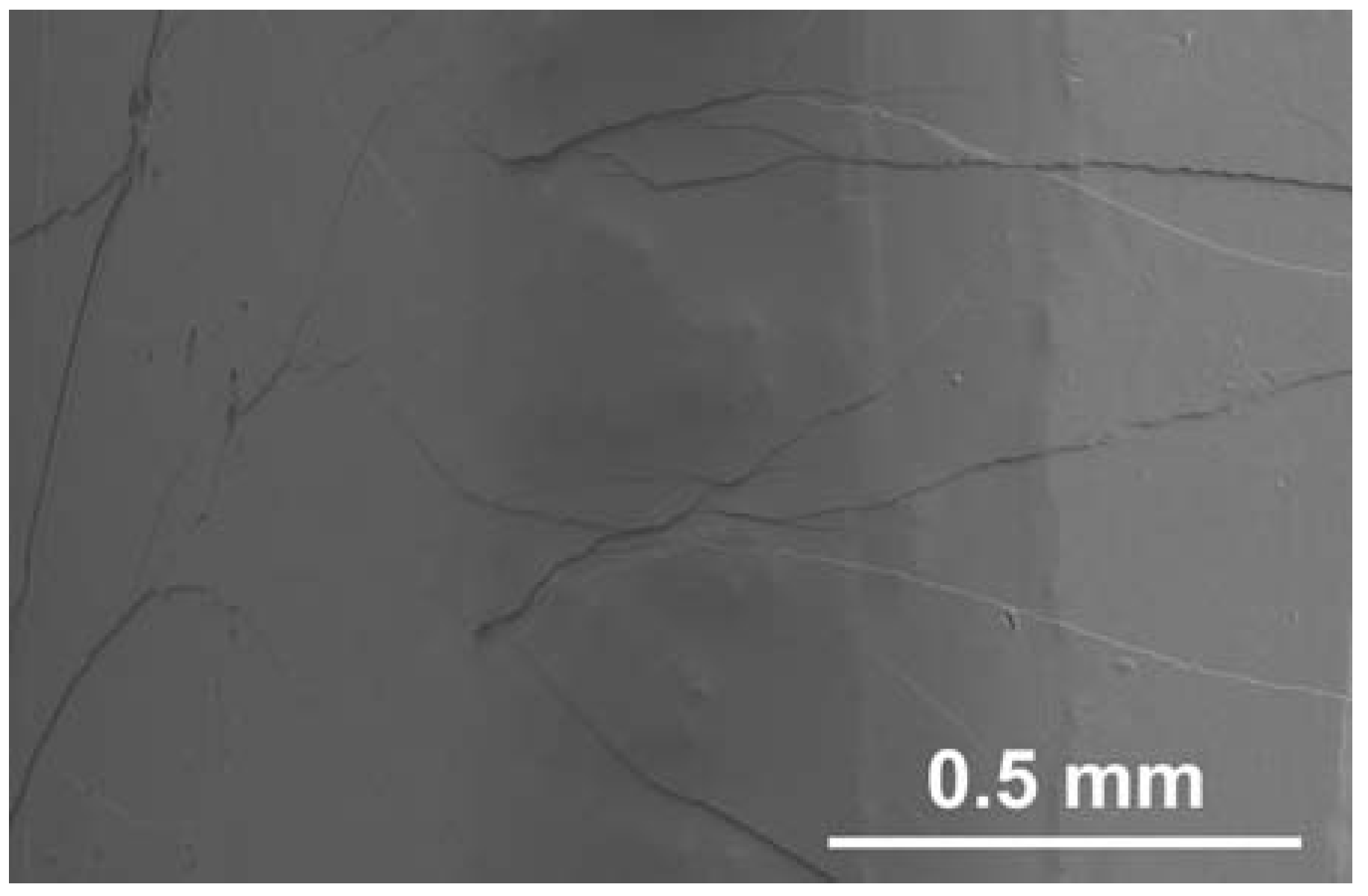
4. Strain-Rate Sensitivity

 is the strain rate. It is important to note that the strain rate changes have been performed in the regions where the stress-strain curves are generally linear which may correspond to the formation of multiple shear bands and macroscopically homogeneous deformation of the sample.
is the strain rate. It is important to note that the strain rate changes have been performed in the regions where the stress-strain curves are generally linear which may correspond to the formation of multiple shear bands and macroscopically homogeneous deformation of the sample.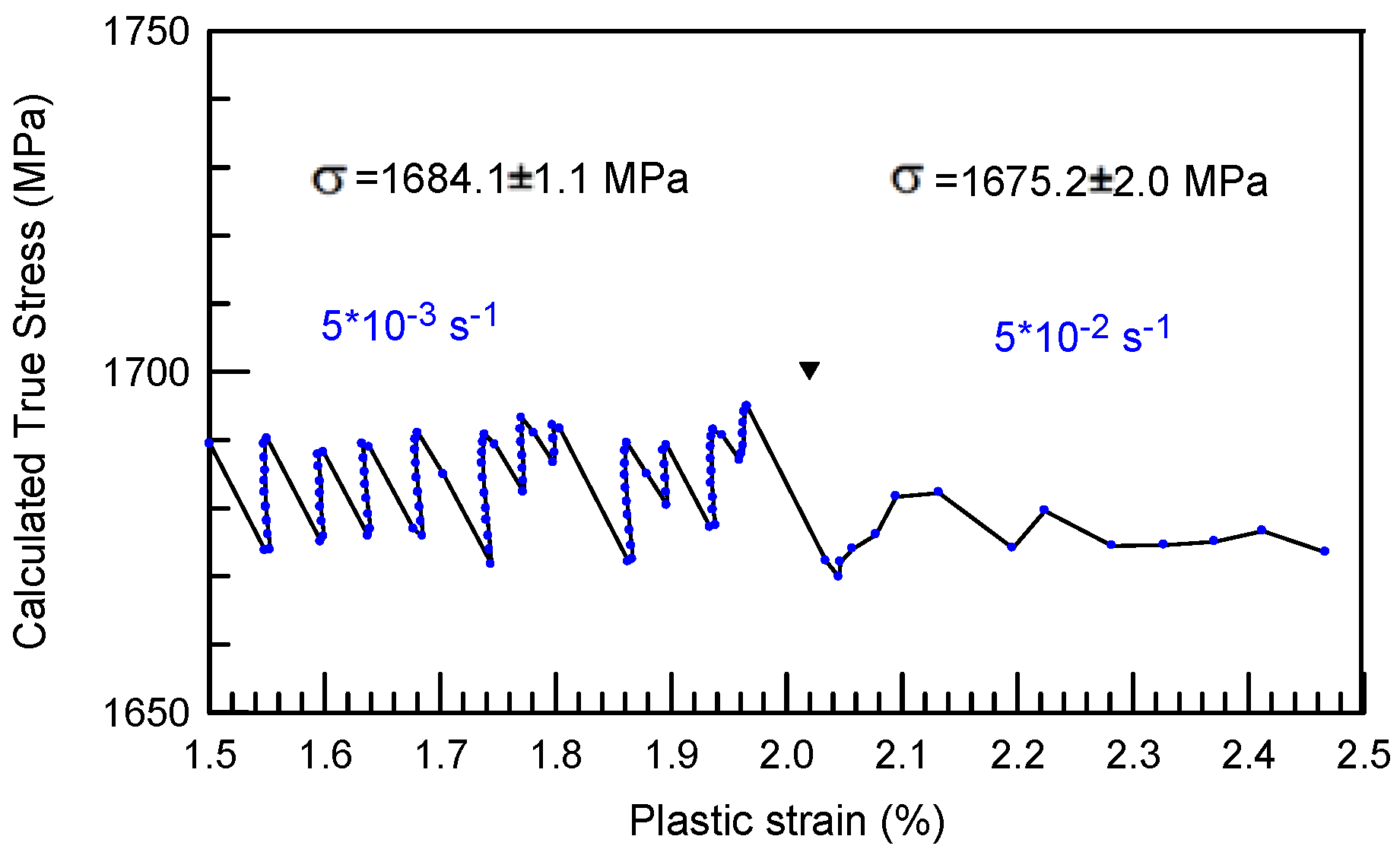
5. In situ Room-Temperature Tensile Deformation in TEM

6. Effect of Cyclic and Long-Term Creep Deformation in the Elastic Region
7. Conclusive Remarks
Acknowledgements
References
- Inoue, A. High strength bulk amorphous alloys with low critical cooling rates. Mater. Trans. JIM 1995, 36, 866–875. [Google Scholar]
- Johnson, W.L. Bulk glass-forming metallic alloys: Science and technology. MRS Bull 1999, 24, 42–56. [Google Scholar]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Argon, A.S. Mechanisms of inelastic deformation in metallic lasses. Phys. Chem. Solids 1982, 43, 945. [Google Scholar] [CrossRef]
- Liu, F.X.; Liaw, P.K.; Wang, G.Y.; Chiang, C.L.; Smith, D.A.; Rack, P.D.; Chu, J.P.; Buchanan, R.A. Specimen-geometry effects on mechanical behavior of metallic glasses. Intermetallics 2006, 14, 1014. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.; Koshiba, H.; Kato, H.; Yavari, A.R. Cobalt-based bulk glassy alloy with ultrahigh strength and soft magnetic properties. Nat. Mater. 2003, 2, 661. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Hua, N.; Zhang, T. Co-based ternary bulk metallic glasses with ultrahigh strength and plasticity. J. Mater. Res. 2011, 26(16), 2072–2079. [Google Scholar] [CrossRef]
- Amiya, K.; Inoue, A. Fe-(Cr, Mo)-(C, B)-Tm bulk metallic glasses with high, strength and high glass-forming ability. Rev. Adv. Mater. Sci. 2008, 18, 27. [Google Scholar]
- Louzguine, D.V.; Inoue, A. Structural and thermal investigations of a high-strength Cu-Zr-Ti-Co bulk metallic glass. Philos. Mag. Lett. 2003, 83, 191–196. [Google Scholar] [CrossRef]
- Inoue, A.; Yamaguchi, H.; Zhang, T.; Masumoto, T. Al-La-Cu amorphous alloys with a wide supercooled liquid region. Mater. Trans. JIM 1990, 31, 104–109. [Google Scholar]
- Zhang, W.; Inoue, A. Synthesis, thermal stability and mechanical properties of Cu-based bulk glassy alloys. World Bulk Met. Glass. Compos. 2007, 201–230. [Google Scholar]
- Inoue, A.; Zhang, W.; Saida, J. Synthesis and fundamental properties of Cu-based bulk glassy alloys in binary and multi-component systems. Mater. Trans. 2004, 4, 1153–1162. [Google Scholar]
- Inoue, A.; Zhang, W.; Zhang, T.; Kurosaka, K. Formation and mechanical properties of Cu-Hf-Ti bulk glassy alloys. J. Mater. Res. 2001, 16, 2836–2844. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, W.; Zhang, T.; Kurosaka, K. High-strength Cu-based bulk glassy alloys in Cu-Zr-Ti and Cu-Hf-Ti ternary systems. Acta Mater. 2001, 49, 2645–2652. [Google Scholar] [CrossRef]
- Zhang, W.; Inoue, A. High glass-forming ability and good mechanical properties of new bulk glassy alloys in Cu-Zr-Ag ternary system. J. Mater. Res. 2006, 21, 234–241. [Google Scholar] [CrossRef]
- Kim, K.B.; Das, J.; Lee, M.H.; Yi, S.; Fleury, E.; Zhang, Z.F.; Wang, W.H.; Eckert, J. Propagation of shear bands in a Cu47.5Zr47.5Al5 bulk metallic glass. J. Mater. Res. 2008, 23, 6–12. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, W. Formation, thermal stability and mechanical properties of Cu-Zr-Al bulk glassy alloys. Mater. Trans. 2002, 43, 2921–2925. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Xie, G.; Inoue, A. Glass-forming ability and mechanical properties of the ternary Cu-Zr-Al and quaternary Cu-Zr-Al-Ag bulk metallic glasses. Mater. Trans. 2007, 48, 1626–1630. [Google Scholar] [CrossRef]
- Zhang, W.; Inoue, A. Formation and mechanical strength of new Cu-based bulk glassy alloys with large supercooled liquid region. Mater. Trans. 2004, 45, 1210–1213. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, W.; Inoue, A. Bulk metallic glass formation near a quaternary Cu-Zr-Ti-Al eutectic point. Mater. Trans. 2006, 47, 2804–2807. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; He, J.P; Yao, K.F.; Wei, B.C.; Chen, G.L. Metallographic analysis of Cu-Zr-Al bulk amorphous alloys with yttrium addition. Scr. Mater. 2006, 54, 1351–1355. [Google Scholar] [CrossRef]
- Bhatt, J.; Wu, J.; Xia, J.; Wand, Q.; Dong, C.; Murty, B.S. Optimization of bulk metallic glass forming compositions in Zr-Cu-Al system by thermodynamic modeling. Intermetallics 2007, 15, 716–721. [Google Scholar] [CrossRef]
- Zhang, W.; Inoue, A. Cu-based bulk glass formation in the Cu-Zr-Ga alloy system and their mechanical properties. Mater. Trans. 2004, 45, 532–535. [Google Scholar] [CrossRef]
- Men, H.; Fu, J.; Ma, C.; Pang, S.; Zhang, T. Bulk glass formation in ternary Cu-Zr-Ti system. J. Univ. Sci. Technol. Beijing 2007, 14, 19. [Google Scholar] [CrossRef]
- Chen, D.; Takeuchi, A.; Inoue, A. Gd-Co-Al and Gd-Ni-Al bulk metallic glasses with high glass forming ability and good mechanical properties. Mater. Sci. Eng. 2007, 457, 226–230. [Google Scholar] [CrossRef]
- Chen, D.; Takeuchi, A.; Inoue, A. Gd-Ni-Al bulk glasses with great glass-forming ability and better mechanical properties. J. Mater. Sci. 2007, 42, 8662–8666. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Masumoto, T. Al-La-Ni amorphous alloys with a wide supercooled liquid region. Mater. Trans. JIM 1989, 30, 965–972. [Google Scholar]
- Inoue, A.; Kato, A.; Zhang, T.; Kim, S.; Masumoto, T. Mg-Cu-Y amorphous alloys with high mechanical strengths produced by metallic mold casting method. JIM 1991, 32, 609–616. [Google Scholar]
- Park, E.S.; Chang, H.J.; Kim, D.H. Mg-rich Mg-Ni-Gd ternary bulk metallic glasses with high compressive specific strength and ductility. J. Mater. Res. 2007, 22, 334–338. [Google Scholar] [CrossRef]
- Kim, S.G.; Inoue, A.; Masumoto, T. High mechanical strengths of Mg-Ni-Y and Mg-Cu-Y amorphous alloys with significant supercooled liquid region. Mater. Trans. JIM 1990, 31, 929–934. [Google Scholar]
- Inoue, A.; Zhang, T.; Masumoto, T. Zr-Al-Ni amorphous alloys with high class transition temperature and significant supercooled liquid region. Mater. Trans. JIM 1990, 31, 177–183. [Google Scholar]
- Yokoyama, Y.; Yamasaki, T.; Liaw, P.K.; Inoue, A. Relations between the thermal and mechanical properties of cast Zr-TM-Al (TM: Cu, Ni, or Co) bulk glassy alloys. Mater. Trans. 2007, 48, 1846–1849. [Google Scholar] [CrossRef]
- Wada, T.; Zhang, T.; Inoue, A. Formation, thermal stability and mechanical properties in Zr-Al-Co bulk glassy alloys. JIM 2002, 43, 2843–2846. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Kobayashi, A.; Fukaura, K.; Inoue, A. Oxygen embrittlement and effect of the addition of Ni element in a bulk amorphous Zr-Cu-Al alloy. Mater. Trans. 2002, 43, 571–574. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Liaw, P.K.; Nishijima, M.; Hiraga, K.; Buchanan, R.A.; Inoue, A. Fatigue-strength enhancement of cast Zr50Cu40Al10 glassy alloys. Mater. Trans. 2006, 47, 1286–1293. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Yamasaki, T.; Liaw, P.K.; Inoue, A. Relations between the thermal and mechanical properties of cast Zr-TM-Al (TM: Cu, Ni, or Co) bulk glassy alloys. Evolution of mechanical properties of cast Zr50Cu40Al10 glassy alloys by structural relaxation. Mater. Trans. 2005, 46, 2755–2761. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, W. New bulk glassy Ni-based alloys with high strength of 3000 MPa. Mater. Trans. 2002, 43, 708–711. [Google Scholar] [CrossRef]
- Jing, Q.; Zhang, Y.; Wang, D.; Li, Y. A study of the glass forming ability in Zr-Ni-Al alloys. Mater. Sci. Eng. 2006, 441, 106–111. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Masumoto, T. Amorphous Zr-Al-TM (TM = Co,Ni,Cu) alloys with significant supercooled liquid region of over 100 K. Mater. Trans. JIM 1991, 32, 1005–1010. [Google Scholar]
- Yi, S.; Park, T.G.; Kim, D.H. Ni-based bulk amorphous alloys in the Ni-Ti-Zr-(Si, Sn) system. J. Mater. Res. 2000, 15, 11. [Google Scholar]
- Zhang, B.; Zhao, D.Q.; Pan, M.X.; Wang, R.J.; Wang, W.H. Amorphous metallic plastic. Acta Mater. 2006, 54, 3025–3032. [Google Scholar] [CrossRef]
- Koshiba, H.; Inoue, A. Preparation and magnetic properties of Co-based bulk glassy alloys. Mater. Trans. 2001, 42, 2572–2575. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, W.; Kimura, H.; Inoue, A. Excellent mechanical properties of Cu-Hf-Ti-Ta bulk glassy alloys containing in situ dendrite Ta-based BCC phase. Mater. Trans. 2004, 45, 2936–2940. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Q.; Qin, C.; Inoue, A. Synthesis and properties of Cu-Zr-Ag-Al glassy alloys with high glass-forming ability. Mater. Sci. Eng. 2008, 148, 92–96. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, T.; Kurosaka, K.; Zhang, W. High strength Cu-based bulk glassy alloys in Cu-Zr-Ti-Be system. Mater. Trans. 2001, 42, 1800–1804. [Google Scholar] [CrossRef]
- Zhang, T.; Kurosaka, K.; Inoue, A. Thermal and mechanical properties of Cu-based Cu-Zr- Ti-Y bulk glassy alloys. Mater. Trans. 2001, 42, 2042–2045. [Google Scholar] [CrossRef]
- Makino, A.; Kubota, T.; Chang, C.; Makabe, M.; Inoue, A. FeSiBP bulk metallic glasses with unusual combination of high magnetization and high glass-forming ability. Mater. Trans. 2007, 48, 3024–3027. [Google Scholar] [CrossRef]
- Zhang, W.; Jia, F.; Inoue, A. Formation and properties of new La-based bulk glassy alloys with diameters up to centimeter order. Mater. Trans. 2007, 48, 68–73. [Google Scholar] [CrossRef]
- Yuan, G.; Amiya, K.; Inoue, A. Structural-relaxation, glass-forming ability and mechanical properties of Mg-Cu-Ni-Gd alloys. J. NonCryst. Solids 2005, 351, 729–735. [Google Scholar] [CrossRef]
- Yuan, G.; Zhang, T; Inoue, A. Thermal stability, glass-forming ability and mechanical properties of Mg-Y-Zn-Cu glassy alloys. Mater. Trans 2003, 44, 2271–2275. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, W.; Zhang, T. Thermal stability and mechanical strength of bulk glassy Ni-Nb-Ti-Zr alloys. Mater. Trans. 2002, 43, 1952–1956. [Google Scholar] [CrossRef]
- Shen, B.; Inoue, A. Glass transition behavior and mechanical properties of Ni-Si-B-based glassy alloys. Mater. Trans. 2003, 44, 1425–1428. [Google Scholar] [CrossRef]
- Arai, K.; Zhang, W.; Jia, F.; Inoue, A. Synthesis and thermal stability of new Ni-based bulk glassy alloy with excellent mechanical properties. Mater. Trans. 2006, 47, 2358–2362. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.S.; Lim, H.K.; Kim, W.T.; Kim, D.H. Heating and cooling rate dependence of the parameters representing the glass forming ability in bulk metallic glasses. J. NonCryst. Solids 2005, 351, 1433–1440. [Google Scholar] [CrossRef]
- Liu, L.; Inoue, A.; Zhang, T. Formation of bulk Pd-Cu-Si-P glass with good mechanical properties. Mater. Trans. 2005, 46, 376–378. [Google Scholar] [CrossRef]
- Takenaka, K.; Wada, T.; Nishiyama, N.; Kimura, H.; Inoue, A. New Pd-based bulk glassy alloys with high glass-forming ability and large supercooled liquid region. Mater. Trans. 2005, 46, 1720–1724. [Google Scholar] [CrossRef]
- Inoue, A.; Nishiyama, N.; Kimura, H. Preparation and thermal stability of bulk amorphous Pd40Cu30Ni10P20 alloy cylinder of 72 mm in diameter. Mater. Trans. JIM 1997, 38, 179–183. [Google Scholar]
- Zhang, T.; Inoue, A. Thermal and mechanical properties of Ti-Ni-Cu-Sn amorphous alloys with a wide supercooled liquid region before crystallization. Mater. Trans. JIM 1998, 39, 1001–1006. [Google Scholar]
- Wada, T.; Zhang, T.; Inoue, A. Formation and high mechanical strength of bulk glassy alloys in Zr-Al-Co-Cu system. Mater. Trans. 2003, 44, 1839–1844. [Google Scholar] [CrossRef]
- Saida, J.; Kato, H.; Deny, A.; Setyawan, H.; Yoshimi, K.; Inoue, A. Deformation-induced nanoscale dynamic transformation Studies in Zr-Al-Ni-Pd and Zr-Al-Ni-Cu Bulk Metallic Glasses. Mater. Trans. 2007, 48, 1327–1335. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Inoue, A. Compositional dependence of thermal and mechanical properties of quaternary Zr-Cu-Ni-Al bulk glassy alloys. Mater. Trans. 2007, 48, 1282–1287. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Zhang, W.; Louzguine-Luzgin, D.V.; Inoue, A. High glass-forming ability and unusual deformation behavior of new Zr-Cu-Fe-Al bulk metallic glasses. Mater. Sci. Forum 2010, 654–656, 1042–1045. [Google Scholar]
- Conner, R.D.; Li, Y.; Nix, W.D.; Johnson, W.L. Shear band spacing under bending of Zr-based metallic glass plates. Acta Mater. 2004, 52, 2429–2434. [Google Scholar] [CrossRef]
- Donovan, P.E.; Stobbs, W.M. The structure of shear bands in metallic glasses. Acta Metall. 1981, 29, 1419–1436. [Google Scholar] [CrossRef]
- Chen, H.S. Plastic flow in metallic glasses under compression. Scr. Metar. 1973, 7, 931–935. [Google Scholar] [CrossRef]
- Mear, F.O.; Wada, T.; Louzguine-Luzgin, D.V.; Inoue, A. Highly inhomogeneous compressive plasticity in nanocrystal-toughened Zr-Cu-Ni-Al bulk metallic glass. Philos. Mag. Lett. 2009, 89, 276–281. [Google Scholar] [CrossRef]
- Yavari, A.R.; Lewandowski, J.J.; Eckert, J. Mechanical properties of bulk metallic glasses. MRS Bull. 2007, 32, 635–638. [Google Scholar] [CrossRef]
- Pan, J.; Chan, K.C.; Chena, Q.; Liu, L. Enhanced plasticity by introducing icosahedral medium-range order in ZrCuNiAl metallic glass. Intermetallics 2012, 24, 79–83. [Google Scholar] [CrossRef]
- Chen, N.; Louzguine-Luzgin, D.V.; Xie, G.Q.; Wada, T.; Inoue, A. Influence of minor Si addition on glass forming ability and mechanical properties of Pd40Ni40P20 alloy. Acta Mater. 2009, 57, 2775–2780. [Google Scholar] [CrossRef]
- Chen, N.; Pan, D.; Louzguine-Luzgin, D.V.; Xie, G.Q.; Chen, M.W.; Inoue, A. Improved thermal stability and ductility of flux-treated Pd40Ni40Si4P16 BMG. Scr. Mater. 2010, 62, 17–20. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Zhang, W.; Xie, G.Q.; Louzguine-Luzgin, D.V.; Inoue, A. Stable flowing of localized shear bands in soft bulk metallic glasses. Acta Mater. 2010, 58, 904–909. [Google Scholar] [CrossRef]
- Kato, H.; Saida, J.; Inoue, A. Influence of hydrostatic pressure during casting on as cast structure and mechanical properties in Zr65Al7.5Ni10Cu17.5 − xPdx (x= 0, 17.5) alloys. Scr. Mater. 2004, 51, 1063–1068. [Google Scholar] [CrossRef]
- Schroers, J.; Johnson, W.L. Ductile bulk metallic glass. Phys. Rev. Lett. 2004, 93, 255506–255510. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Wang, W.H.; Greer, A.L. Intrinsic plasticity or brittleness of metallic glasses. Philos. Mag. Lett. 2005, 85, 77. [Google Scholar] [CrossRef]
- Madge, S.V.; Louzguine-Luzgin, D.V.; Lewandowski, J.J.; Greer, A.L. Toughness, extrinsic effects and Poisson’s ratio of bulk metallic glasses. Acta Mate. 2012, 60, 4800–4809. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Xie, G.; Zhang, Q.; Inoue, A. Effect of Fe on the glass-forming ability, structure and devitrification behavior of Zr-Cu-Al bulk glass-forming alloys. Philos. Mag. 2010, 90, 1955–1968. [Google Scholar] [CrossRef]
- Wright, W.J.; Schwarz, R.B.; Nix, W.D. Localized heating during serrated plastic flow in bulk metallic glasses. Mater. Sci. Eng. 2001, 319–321, 229–232. [Google Scholar]
- Dalla Torre, F.H.; Dubach, A.; Siegrist, M.E.; Löffler, J.F. Negative strain rate sensitivity in bulk metallic glass and its similarities with dynamic strain aging effect during deformation. Appl. Phys. Lett. 2006, 89, 091918. [Google Scholar] [CrossRef]
- Yu, H.B.; Wang, W.H.; Zhang, J.L.; Shek, C.H.; Bai, H.Y. Statistic analysis of the mechanical behavior of bulk metallic glasses. Adv. Eng. Mater. 2009, 11, 370–375. [Google Scholar] [CrossRef]
- Liu, F.X.; Liaw, P.K.; Wang, G.Y.; Chiang, C.L.; Smith, D.A.; Rack, P.P.D; Chu, P.P.; Buchanan, R.A. Specimen-geometry effects on mechanical behavior of metallic glasses. Intermetallics 2006, 14, 1014–1018. [Google Scholar] [CrossRef]
- Uchic, M.D.; Dimiduk, D.M.; Florando, N.; Nix, W.D. Sample dimensions influence strength and crystal plasticity. Science 2004, 305, 986–989. [Google Scholar] [CrossRef]
- Wang, G.; Feng, Q.; Yang, B.; Jiang, W.; Liaw, P.K.; Liu, C.T. Thermographic studies of temperature evolutions in bulk metallic glasses. Intermetallics 2012, 30, 1–11. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Sun, B.B.; Sui, M.L.; Lu, K.; Ma, E. Bulk metallic glass formation in the binary Cu-Zr system. Appl. Phys. Lett. 2004, 84, 4029. [Google Scholar]
- Yokoyama, Y.; Fujita, K.; Yavari, A.R.; Inoue, A. Correlation between structural relaxation and shear transformation zone volume of a bulk metallic glass. Philos. Mag. Lett. 2009, 89, 322. [Google Scholar] [CrossRef]
- Inoue, A.; Zhang, W.; Tsurui, T.; Yavari, A.R.; Greer, A.L. Unusual room-temperature compressive plasticity in nanocrystal-toughened bulk copper-zirconium glass. Philos. Mag. Lett. 2005, 85, 221–229. [Google Scholar] [CrossRef]
- Louzguine, D.V.; Kato, H.; Inoue, A. High-strength Cu-based crystal-glassy composite with enhanced ductility. Appl. Phys. Lett. 2004, 84, 1088–1089. [Google Scholar] [CrossRef]
- Das, J.; Tang, M.B.; Kim, K.B.; Theissmann, R.; Baier, F.; Wang, W.H.; Eckert, J. Work-hardenable ductile bulk metallic glass. Phys. Rev. Lett. 2005, 94, 205501. [Google Scholar] [CrossRef]
- Mear, F.O.; Wada, T.; Louzguine-Luzgin, D.V.; Inoue, A. Structural investigations of rapidly solidified Mg–Cu–Y alloys. J. Alloy. Compd. 2010, 496, 149–154. [Google Scholar] [CrossRef]
- Hajlaoui, K.; Yavari, A.R.; LeMoulec, A.; Botta, W.J.; Vaughan, F.G.; Das, J.; Greer, A.L.; Kvick, A. Plasticity induced by nanoparticle dispersions in bulk metallic glasses. J. NonCryst. Solids 2007, 353, 327–331. [Google Scholar]
- Saida, J.; Kato, H.; Setyawan, A.D.H.; Inoue, A. Characterization and properties of nanocrystal-forming Zr-based bulk metallic glasses. Rev. Adv. Mater. Sci. 2005, 10, 34–38. [Google Scholar]
- Louzguine-Luzgin, D.V.; Zeng, Y.; Setyawan, A.D.H.; Nishiyama, N.; Kato, H.; Saida, J.; Inoue, A. Corrosion resistance and XPS studies of Ni-rich Ni-Pd-P-B bulk glassy alloys. J. Mater. Res 2007, 22, 1087–1090. [Google Scholar] [CrossRef]
- Gao, M.C.; Hackenberg, R.E.; Shiflet, G.J. Deformation-induced nanocrystal precipitation in Al-base metallic glasses. Mater. Trans. 2001, 42, 1741–1747. [Google Scholar] [CrossRef]
- Jiang, W.H.; Atzmon, M. Plastic flow of a nanocrystalline/amorphous Al90Fe5Gd5 composite formed by rolling. Intermetallics 2006, 14, 962–965. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Inoue, A. Comparative study of the effect of cold rolling on the structure of Al-RE-Ni-Co (RE = rare-earth metals) amorphous and glassy alloys. J. NonCryst. Solids 2006, 352, 3903–3909. [Google Scholar] [CrossRef]
- Zhang, Z.F.; He, G.; Zhang, H.; Eckert, J. Rotation mechanism of shear fracture induced by high plasticity in Ti-based nano-structured composites containing ductile dendrites. Scri. Mater. 2005, 52, 945–949. [Google Scholar] [CrossRef]
- Dalla Torre, F.H.; Dubach, A.; Schallibaum, J.; Loffler, J.F. Shear striations and deformation kinetics in highly deformed Zr-based bulk metallic glasses. Acta Mater. 2008, 56, 4635–4646. [Google Scholar] [CrossRef]
- Sergueeva, A.V.; Mara, N.A.; Branagan, D.J.; Mukherjee, A.K. Strain rate effect on metallic glass ductility. Scr. Mater. 2004, 50, 1303–1307. [Google Scholar] [CrossRef]
- Hajlaoui, K.; Stoica, M.; LeMoulec, A.; Charlot, F.; Yavari, A.R. Fe-Nb-B bulk metallic glass with high boron content. Rev. Adv. Mater. Sci. 2008, 18, 23–26. [Google Scholar]
- Louzguine-Luzgin, D.V.; Vinogradov, A.; Yavari, A.R.; Li, S.; Xie, G.; Inoue, A. On the deformation and fracture behaviour of a Zr-based glassy alloy. Philos. Mag. 2008, 88, 2979–2987. [Google Scholar] [CrossRef]
- Pampillo, C.A. Flow and fracture in amorphous alloys. J. Mater. Sci 1975, 10, 1194–1227. [Google Scholar] [CrossRef]
- Lewandowski, J.J.; Greer, A.L. Temperature rise at shear bands in metallic glasses. Nat. Mater. 2006, 5, 15–18. [Google Scholar] [CrossRef]
- Yang, B.; Morrison, M.L.; Liaw, P.P.K.; Buchanan, R.A.; Wang, G.; Liu, C.T.; Denda, M. Dynamic evolution of nanoscale shear bands in a bulk-metallic glass. Appl. Phys. Lett 2005, 86, 141904–141907. [Google Scholar] [CrossRef]
- Chen, N.; Louzguine-Luzgin, D.V.; Xie, G.Q.; Inoue, A. Nanoscale wavy fracture surface of a Pd-based bulk metallic glass. Appl. Phys. Lett. 2009, 94, 131906. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Suh, J.Y.; Wiest, A.; Duan, G.; Lind, M.L.; Demetriou, M.D.; Johnson, W.L. Designing metallic glass matrix composites with high toughness and tensile ductility. Nature 2008, 451, 1085. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Suh, J.Y.; Wiest, A.; Lind, M.L.; Demetriou, M.D.; Johnson, W.L. Development of tough, low-density titanium-based bulk metallic glass matrix composites with tensile ductility. PNAS 2008, 105, 20136. [Google Scholar]
- Louzguine-Luzgin, D.V.; Vinogradov, A.; Xie, G.; Li, S.; Lazarev, A.; Hashimoto, S.; Inoue, A. High-strength and ductile glassy-crystal Ni-Cu-Zr-Ti composite exhibiting stress-induced martensitic transformation. Philos. Mag. 2009, 89, 2887–2901. [Google Scholar] [CrossRef]
- Otsuka, K.; Wayman, C.M. Shape Memory Materials; Otsuka, K., Wayman, C.M., Eds.; Cambridge University Press: Cambridge, UK, 1998; pp. 27–48. [Google Scholar]
- Fukuda, T.; Saburi, T.; Chihara, T.; Tsuzuki, Y. Mechanism of B2-B19-B19’ transformation in shape memory Ti-Ni-Cu alloys. Mater. Trans. JIM 1995, 36, 1244–1248. [Google Scholar]
- Kawashima, A.; Zeng, Y.; Fukuhara, M.; Kurishita, H.; Nishiyama, N.; Miki, H.; Inoue, A. Mechanical properties of a Ni60Pd20P17B3 bulk glassy alloy at cryogenic temperatures. Mater. Sci. Eng. 2008, 498, 475–481. [Google Scholar] [CrossRef]
- Tabachnikova, E.D.; Podol’ski, A.V.; Bengus, V.Z.; Smirnov, S.N.; Luzgin, D.V.; Inoue, A. Low-temperature plasticity anomaly in the bulk metallic glass Zr64.13Cu15.75Ni10.12Al10. Low Temp. Phys. 2008, 34, 675–677. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Vinogradov, A.; Li, S.; Kawashima, A.; Xie, G.; Yavari, A.R.; Inoue, A. Deformation and fracture behavior of metallic glassy alloys and glassy-crystal composites. Metall. Mater. Trans. 2011, 42A, 1504–1510. [Google Scholar]
- Vinogradov, A.; Lazarev, A.; Louzguine-Luzgin, D.V.; Yokoyama, Y.; Li, S.; Yavari, A.R.; Inoue, A. Propagation of shear bands in metallic glasses and transition from serrated to non-serrated plastic flow at low temperatures. Acta Mater. 2010, 58, 6736. [Google Scholar] [CrossRef]
- Woodford, D.A. Strain-rate sensitivity as a measure ductility. Trans. Am. Soc. Met. 1969, 62, 291–293. [Google Scholar]
- Hufnagel, T.; Jiao, C.; Li, T.; Xing, Y.; Ramesh, L.Q. Deformation and failure of Zr57Ti5Cu20Ni8Al10 bulk metallic glass under quasi-static and dynamic compression. J. Mater. Res. 2002, 17, 1441. [Google Scholar] [CrossRef]
- Dalla Torre, F.H.; Dubach, A.; Siegrist, M.; Löffler, J.F. Shear striations and deformation kinetics in highly deformed Zr-based bulk metallic glasses. Appl. Phys. Lett. 2006, 89, 091918. [Google Scholar] [CrossRef]
- Liu, F.X.; Gao, Y.F.; Liaw, P.K. Rate-dependent deformation behavior of Zr-based metallic-glass coatings examined by nanoindentation. Metall. Mater. Trans. 2008, 8, 1862–1867. [Google Scholar]
- Pan, D.; Chen, M.W. Rate-change instrumented indentation for measuring strain rate sensitivity. J. Mater. Res. 2009, 4, 1466–1470. [Google Scholar]
- González, S.; Xie, G.Q.; Louzguine-Luzgin, D.V.; Perepezko, J.H.; Inoue, A. Deformation and strain rate sensitivity of a Zr-Cu-Fe-Al metallic glass. Mater. Sci. Eng. 2011, 528, 3506–3512. [Google Scholar] [CrossRef]
- Song, S.X.; Bei, H.; Wadsworth, J.; Nieh, T.G. Flow serration in a Zr-based bulk metallic glass in compression at low strain rates. Intermetallics 2008, 16, 813. [Google Scholar] [CrossRef]
- Dalla Torre, F.H.; Dubach, A.; Nelson, A.; Löffler, J.F. Temperature, strain and strain rate dependence of serrated flow in bulk metallic glasses. Mater. Trans. 2007, 48, 1774. [Google Scholar] [CrossRef]
- Dalla Torre, F.H.; Dubach, A.; Siegrist, M.; Löffler, J.F. Negative strain-rate sensitivity in bulk metallic glass and its similarities with the dynamic strain-aging effect during deformation. Appl. Phys. Lett. 2006, 89, 091918. [Google Scholar] [CrossRef]
- Trichy, G.R.; Scattergood, R.O.; Koch, C.C.; Murty, K.L. Influence of experimental parameters on the plastic flow curve obtained by ball indentation testing. Intermetallics 2005, 53, 1461. [Google Scholar]
- Hajlaoui, K.; Yavari, A.R.; Doisneau, B.; LeMoulec, A.; Botta, W.J.F.; Vaughan, G.; Greer, A.L.; Inoue, A.; Zhang, W.; Kvick, A. Shear delocalization and crack blunting of a metallic glass containing nanoparticles: In situ deformation in TEM analysis. Scr. Mater. 2006, 54, 1829–1834. [Google Scholar] [CrossRef]
- Guo, H.; Yan, P.F.; Wang, Y.B.; Tan, J.; Zhang, Z.F.; Sui, M.L.; Ma, E. Tensile ductility and necking of metallic glass. Nat. Mater. 2007, 6, 735. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Yavari, A.R.; Xie, G.Q.; Madge, S.; Li, S.; Saida, J.; Greer, A.; Inoue, A. Tensile deformation behaviour of Zr-based glassy alloys. Philos. Mag. Lett. 2010, 90, 139. [Google Scholar] [CrossRef]
- Guo, H.; Yan, P.P.F.; Wang, Y.B.; Tan, J.; Zhang, Z.F.; Sui, M.L.; Ma, E. Tensile ductility and necking of metallic glass. Nat. Mater. 2007, 6, 735–739. [Google Scholar] [CrossRef]
- Georgarakis, K.; Aljerf, M.; Li, Y.; Lemoulec, A.; Charlot, F.; Yavari, A.R.; Chornokhvostenko, K.; Tabachnikova, E.; Evangelakis, G.A.; Miracle, D.B.; Greer, A.L.; Zhang, T. Shear band melting and serrated flow in metallic glasses. Appl. Phys. Lett. 2008, 93, 031907. [Google Scholar]
- Schuh, C.A.; Hufnagel, T.C.; Ramamurty, U. Mechanical behavior of amorphous alloys. Acta Mater. 2007, 5, 4067–4109. [Google Scholar]
- Lewandowski, J.J.; Shazly, M.; Nouri, A.S. Intrinsic and extrinsic toughening of metallic glasses. Scr. Mater. 2008, 54, 337–341. [Google Scholar]
- Caron, A.; Kawashima, A.; Fecht, H.J.; Louzguine-Luzguin, D.V.; Inoue, A. On the anelasticity and strain induced structural changes in a Zr-based bulk metallic glass. Appl. Phys. Lett. 2011, 99, 171907. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Xie, G.; Zhang, Q.; Inoue, A. Cooling rate, structure, thermal stability and crystallization behaviour of Cu-based bulk glass-forming alloys. J. Phys. 2009, 144, 012047. [Google Scholar]
- Cavaille, J.Y.; David, L.; Perez, J. Relaxation phenomena in non crystalline solids: Case of polymeric materials. Mater. Sci. Forum 2001, 366–368, 499–545. [Google Scholar]
- Pelletier, J.M.; Louzguine-Luzgin, D.V.; Li, S.; Inoue, A. Elastic and viscoelastic properties of glassy, quasicrystalline and crystalline phases in Zr65Cu5Ni10Al7.5Pd12.5 alloys. Acta Mater. 2011, 59, 2797–2806. [Google Scholar] [CrossRef]
- Ke, H.B.; Wen, P.; Peng, H.L.; Wang, W.H.; Greer, A.L. Homogeneous deformation of metallic glass at room temperature reveals large dilatation. Scr. Mater. 2011, 64, 966–969. [Google Scholar] [CrossRef]
- Lee, S.C.; Lee, C.M.; Yang, J.W.; Lee, J.C. Microstructural evolution of an elastostatically compressed amorphous alloy and its influence on the mechanical properties. Scr. Mater. 2008, 58, 591. [Google Scholar] [CrossRef]
- Park, K.W.; Lee, C.M.; Wakeda, M.; Shibutani, Y.; Falk, M.L.; Lee, J.C. Elastostatically induced structural disordering in amorphous alloys. Acta Mater. 2008, 56, 5440. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Louzguine-Luzgin, D.V.; Louzguina-Luzgina, L.V.; Churyumov, A.Y. Mechanical Properties and Deformation Behavior of Bulk Metallic Glasses. Metals 2013, 3, 1-22. https://doi.org/10.3390/met3010001
Louzguine-Luzgin DV, Louzguina-Luzgina LV, Churyumov AY. Mechanical Properties and Deformation Behavior of Bulk Metallic Glasses. Metals. 2013; 3(1):1-22. https://doi.org/10.3390/met3010001
Chicago/Turabian StyleLouzguine-Luzgin, Dmitri V., Larissa V. Louzguina-Luzgina, and Alexander Yu. Churyumov. 2013. "Mechanical Properties and Deformation Behavior of Bulk Metallic Glasses" Metals 3, no. 1: 1-22. https://doi.org/10.3390/met3010001






