1. Introduction
Magnesium alloys present new opportunities for biomedical applications because of their high strength and lightweight properties with favorable biocompatibility [
1]. Most endovascular stents currently available today are permanently implanted and made from corrosion resistant metals such as stainless steel, Nitinol, and cobalt-chromium alloys. A material’s mechanical properties used for such purposes dictate its ability to withstand the device forming processes. Stents are widely used because they can prevent or reduce the tendency for vessel restenosis after an angioplasty leading to shrinkage of the lumen [
2]. Thus, careful material selection is required that allow it to expand narrowed vessels permitting the natural flow of fluids to perfuse an area of arterial damage and to act as a biological scaffold while maintaining the appropriate mechanical integrity to withstand interfacial shear stress induced by intraluminal blood flow. However, the widely used permanent prosthetic devices have several unfavorable clinical shortcomings when implanted in the human body that prevent them from being deemed as ideal devices. Some of these limitations include: long-term endothelial dysfunction, delayed re-endothelialization, thrombogenicity, permanent physical irritation, chronic inflammatory local reactions, mismatches in mechanical behavior between stented and non-stented vessel areas, inability to adapt to growth in young patients, and importantly non-permissive or disadvantageous characteristics for later surgical revascularization [
3,
4].
Ideally, once a stent is implanted within a vessel, the walls of the stents become lined with endothelial cells preventing thrombosis, and after approximately 6–12 months, arterial remodeling and healing is achieved [
3]. After healthy arterial healing and remodeling has been accomplished, there is no longer a functional need for the stent. Recognizing such an idea, the development of biodegradable stents that can degrade once the objectives of permanent stents have been fulfilled is currently being explored [
2,
4,
5,
6]. Although, there are very few metallic materials that can fulfill such physiological criteria; magnesium and its alloys possess the ability to degrade once implanted and minimalize adverse biological effects.
In this study, the mechanical properties and tensile failure mechanism of two novel biodegradable alloys for endovascular medical applications Mg-Zn-Se and Mg-Zn-Cu are characterized. The elastic modulus (E) and surface hardness (H) for both the bare alloys and the air formed oxide layer were characterized by nanoindentation methods. X-ray photoelectron spectroscopy (XPS) was used to determine the chemical composition of the oxide layer. Tensile strength (σmax), and elongation at failure were determined using traditional tensile testing procedures and the poisson ratio (ν) for each alloy was calculated.
Additionally, the fracture mechanisms of the alloys have been investigated by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS). After carefully examining existing literature, this is the first paper to present these alloy compositions with supporting data and the potential application to serve as endovascular medical devices.
4. Discussion
In this paper, the mechanical properties of novel bio-absorbable materials Mg-Zn-Cu and Mg-Zn-Se for endovascular medical device applications are investigated. The composition of these alloys is shown in
Table 3. After comparing the mechanical properties of Mg-Zn-Cu and Mg-Zn-Se alloys to conventionally used materials for endovascular applications such as Nitinol, Pt-10Ir, and magnesium alloys Mg-3A1-1Z, it is evident that these materials possess the qualities suitable for the manufacturing of endovascular medical devices. Both Nitinol and Pt-10Ir alloys possess the elasticity required to conform to vessel geometry and have a high corrosion resistance to serve as permanent implants; while the Mg-3Al-1Z alloy has the appropriate ductility and a reduced corrosion rate to serve as a scaffold for desired wound healing to occur [
8]. Immediately, the density of the Mg-Zn-Se and Mg-Zn-Cu material are very close in value to the density of Magnesium (1.738 g·cm
−3), with values of 1.75 and 1.76 g·cm
−3 respectively [
10]. The increased density for the tested alloys in Mg-Zn-Se and Mg-Zn-Cu indicate that these alloys possess a higher strength to weight ratio and are more favorable to withstand compressive arterial wall stresses in the application of stents than that of pure magnesium stents. More notably, the Mg-Zn-Se alloy possesses a density nearly identical to that of a Mg reinforced alloy with 1.5 wt.% Al
2O
3 (1.756 g·cm
−3) which is currently being explored for stent device applications because of its more favorable strength to weight ratio [
10].
Table 3.
Composition of ternary magnesium alloys.
| Alloy composition | Wt.% Mg | Wt.% Zn | Wt.% Cu (X *) | Wt.% Se (X *) |
|---|
| Mg-Zn-Se (98/1/1) | 98 | 1 | - | 1 |
| Mg-Zn-Cu (98/1/1) | 98 | 1 | 1 | - |
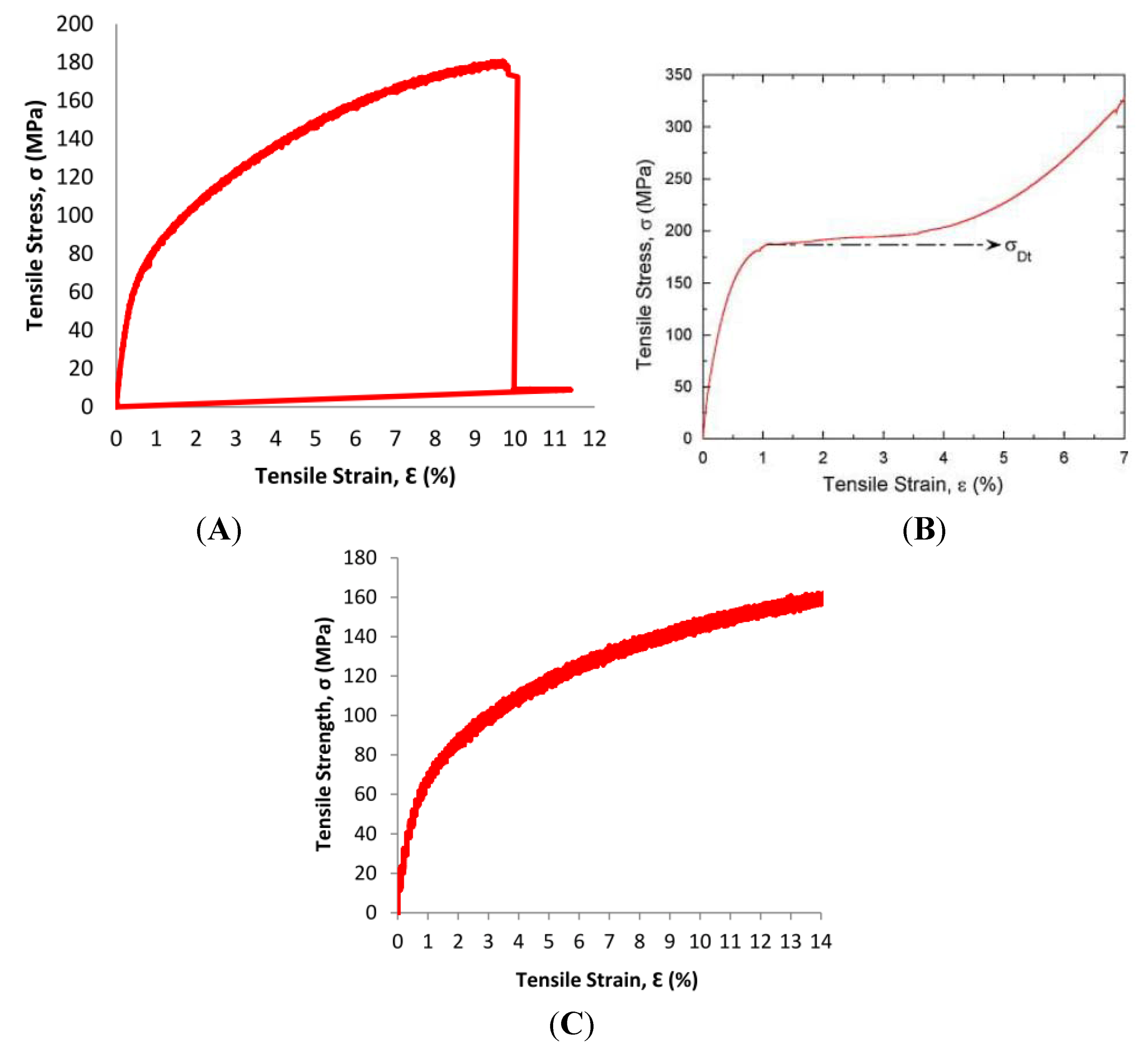
The 0.2% offset values for Mg-Zn-Se and Mg-Zn-Cu were notably lower than any of the values for martensitic Nitinol, Annealed Platinum-10Ir, or the Mg-3A1-1Z alloy. The lower yield strength at the 0.2% offset compared to the other Nitinol, platinum, and magnesium alloys show that the Mg-Zn-Se and Mg-Zn-Cu alloys are more readily permanently deformed by an applied stress when compared to the other aforementioned materials currently used for medical devices. However, the ultimate tensile strength for the Mg-Zn-Se (159 MPa) and Mg-Zn-Cu (152 MPa) alloys obtained from observed stress-strain behavior was higher before material fracture than martensitic Nitinol (70–140 MPa) as seen in
Figure 8.
Figure 8.
Stress-strain behavior from tensile testing of Mg-Zn-Cu and Mg-Zn-Se alloys compared to martensitic nitinol. (
A) Room temperature tensile stress-strain response of the Mg-Zn-Se alloy; (
B) Quasi-static, room temperature tensile stress–strain response of the martensitic Nitinol alloy [
11]. (
C) Room temperature tensile stress-strain response of the Mg-Zn-Cu Alloy.
This suggests, that these Mg-Zn-Se and Mg-Zn-Cu alloys retain their strength and are more stable than NiTi alloys which readily undergo transformation shifts between austenitic and martensitic phases at temperatures ranging from −200 to 110 °C [
12]. This stability is a more desirable quality for endovascular devices, as the strength of the NiTi alloys is greatly reduced during phase transformations of the austenite-martensite phase as evident by its yield strength ranging from 70–140 MPa. However, the super elastic shape memory feature of the NiTi alloy remains highly desirable for medical devices.
The surface properties of metallic medical implants are very important to the stability, biocompatibility, and mechanical integrity of the implant [
13]. An understanding and analysis of the surface oxide film formed on the metallic material is directly related to the ability of metallic ions to be leached from the surface of the implant. The composition of the surface oxide film changes according to reactions between the surfaces of the metallic materials and living tissues and/or biological fluids, therefore it is important to understand the mechanical properties and elemental composition of the surface oxide formations [
14]. In this study, it was shown that the oxide surface layer is primarily composed of oxide coatings most likely including MgO, Mg(OH)
2 and MgCO
3. These coatings generally do not release harmful material components into biological tissues or fluids through diffusion, however, the increased basicity of the Mg(OH)
2 layer may be unsuitable for cell adhesion and growth under static conditions because of the increased basicity of the immediate physiological environment.
The modulus of elasticity (E) and surface hardness (H) for both the bare Mg-Zn-Se and Mg-Zn-Cu alloys and the oxide layer formed on the surface of the alloys were determined by nanoindentation testing. Generally, the (E) and (H) of the air formed oxide layer were both higher in value than the bare metal alloys for both compositions (
Table 2). The higher (E) values for the oxide layers mean that the alloys require more applied stress to deform the materials (strain) than the bare metals. Additionally, the increased (E) of the oxide layers can lead to the development of surface cracks and lead to undesired wear, friction , and material-liquid interfacial interactions [
15]. The increase in (H) of the air formed oxide layer offers a comparative idea of the materials resistance to plastic deformation [
16]. The (H) values for both the bare metals and oxide layers of Mg-Zn-Cu and Mg-Zn-Se are significantly lower than (H) values widely explored for shape-memory NiTi binary and ternary alloys with values ranging from 1–7 GPa [
17]. Given the material properties of the Mg-Zn-Cu and Mg-Zn-Se alloys, it is understood that these materials should possess the ability to be more compliant to elastic deformation than binary and ternary NiTi alloys because of the higher surface hardness values for NiTi alloys with and without surface treatments when measured by nanoindentation. Persaud-Sharma
et al. reported bulk elastic moduli of 32–98 GPa for electro and magneto-electropolished NiTi, NiTiCu, NiTiCr, and NiTiTa alloys with surface hardness values ranging from 1.2 to 6.5 GPa [
17]. Comparably, the bulk elastic moduli for the Mg-Zn-Se and Mg-Zn-Cu alloys are 38 GPa and 41 GPa when calculated by nanoindentation methods, respectively. The lower (E) values for the experimental Mg-Zn-Se and Mg-Zn-Cu alloys indicate that they are softer and less stiff than Nitinol materials which are currently used to manufacture endovascular devices.
A difference in elastic modulus values exists for the Mg-Zn-Se and Mg-Zn-Cu alloys when measured by nanoindentation and MTS tensile testing procedures. The (E) values determined by nanoindentation showed to be greater than the (E) values determined from tensile testing by a factor of 4 (
Table 2). The first possible explanation for this observed difference in values resulting from the two analytical procedures includes the placement of the indenter tip on a non-homogenous sample. In such a sample, areas of separated elemental phases formed within the alloy may possess different mechanical properties as seen with the Mg-Zn-Cu alloy, which would lead to different (E) and (H) values if the indent measurements are collected on or near the grain boundary. Additionally, nanoindentation tests measure a very small portion of the sample surface, almost at the dimension of a single elemental grain. Thus, if the tested sample is non-homogenous, multiple measurements at different areas across the sample surface (
n > 30) are needed to characterize bulk properties of the material. Conventional tensile-testing procedures consider the entire alloy system inclusive of its intergranular interactions, whereas the nanonindentation technique is limited by the depth of the indent, probe tip shape, and restrained by the quality of the sample surface finish. Moreover, this observed difference can be explained by the reduced sensitivity of the MTS equipment when compared to that of the nanoindentation system which operates on a more automated system with software filters to separate critically erroneous unloading data from the remaining load-displacement data. This matter has been extensively studied by Stauss
et al., who attribute differences in microtensile and nanoindentation values to the different size and microstructural levels that are probed [
18].
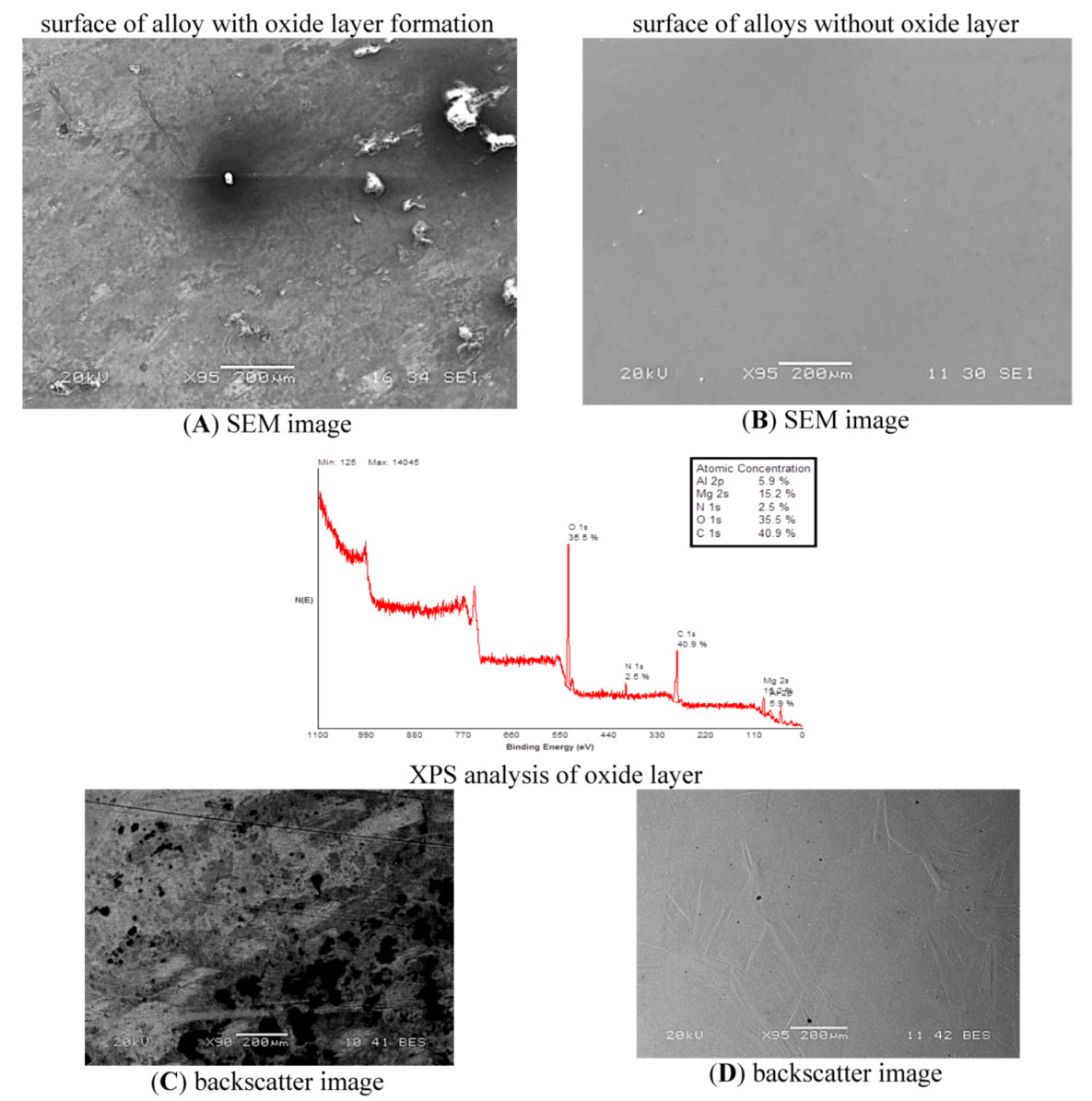
XPS analysis technique was used to determine the elemental composition of the outer 1–10 nm of the material surface for both Mg-Zn-Se and Mg-Zn-Cu oxide layer formation. Mg-Zn-Se showed an oxide layer atomic composition of magnesium (15.2%), oxygen (35.5%), and carbon (40.9%) which would readily form MgO, Mg(OH)
2 and MgCO
3 on the surface of the material (
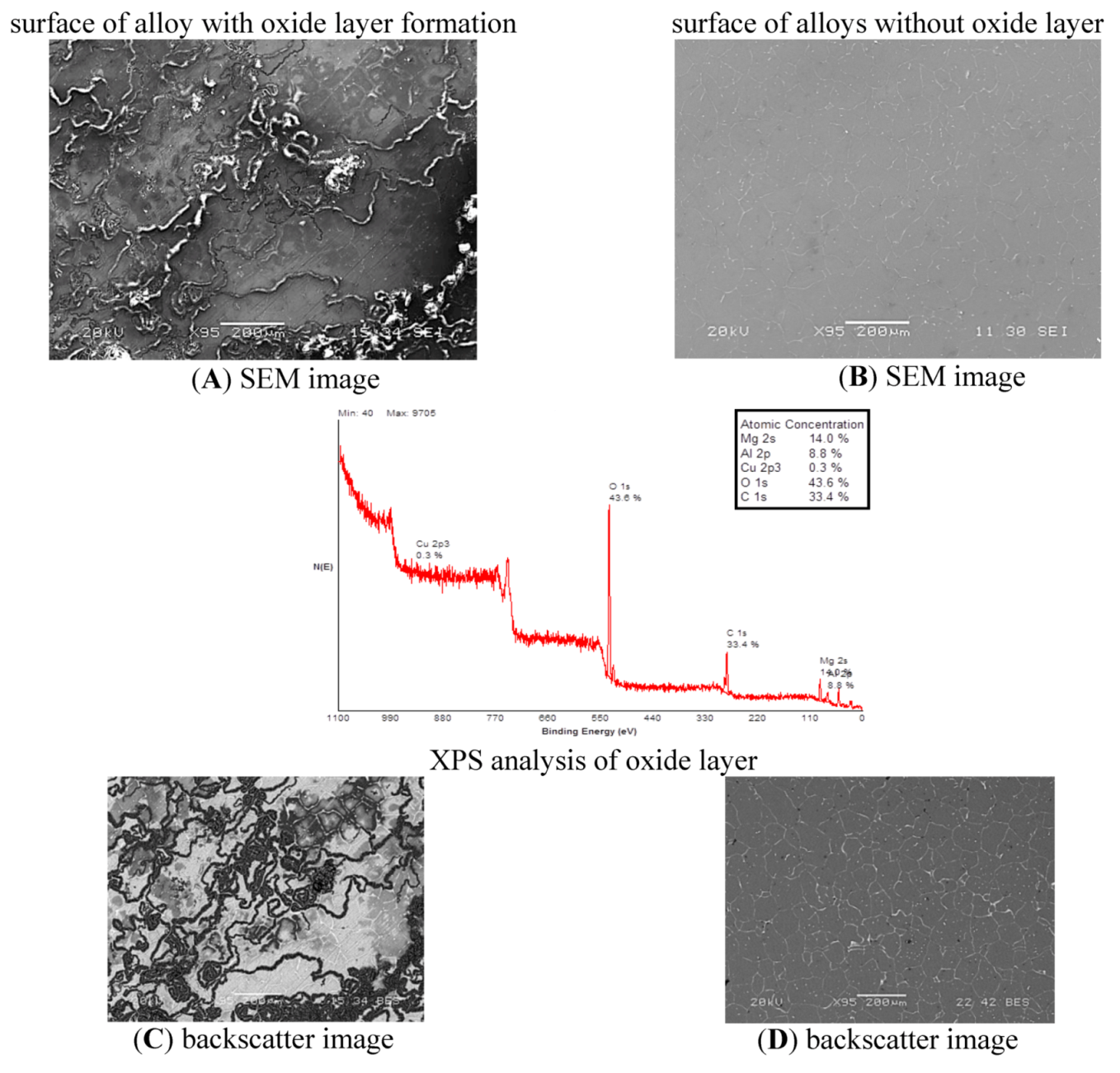
Figure 1). XPS analysis of Mg-Zn-Cu showed oxide layer portions with significant deposition of magnesium (14%), oxygen (43.6%), and carbon (33.4%) (
Figure 2). These are consistent with literature studies on similar magnesium based alloys AZ31 and AZ91, in which XPS analysis revealed the presence of MgO, Mg(OH)
2 and MgCO
3 on the outer surface of both alloys [
19]. It has been concluded that the 5.9% Al and 8.8% Al found on the surface of the Mg-Zn-Se and Mg-Zn-Cu alloys are a result of the Alumina based polishing paste, which was imbedded within the crevices of the alloys that was not removed during the cleaning process (
Figure 1 and
Figure 2). The formation of MgO, Mg(OH)
2 and MgCO
3 on the outer surface of both alloys characterize the majority of the natural oxide layer formation at 25 °C. Larger amounts of oxygen on the surface of the Mg-Zn-Cu alloy shows that this alloy may be susceptible to an increased rate of corrosion by forming pitting sites on its surface as the increased oxygen concentration is a result of electron-transport from the metal being oxidized [
20].
The tensile strength of as-cast Mg-Zn-Cu (152 MPa) and Mg-Zn-Se (159 MPa) were lower than annealed Pt-10Ir (380 MPa), and Mg-3A1-1Z (255 MPa), but most comparable to martensitic Nitinol (70–140 MPa). The elongation at failure for Mg-Zn-Cu (13%) and Mg-Zn-Se (12%) were most comparable to the Mg-3A1-1Z (10%–25%), but lower than annealed Pt-10Ir (20%). However, the mean 13% and 12% elongation for Mg-Zn-Cu and Mg-Zn-Se are significantly higher than the elongation values for currently available cast magnesium alloys which range from 2%–8% tensile elongation for non-high pressured diecast methods [
21]. Previous literature suggests that the relative uniformity of critical surface tension for endovascular devices is a primary determinant for the thrombogenicity of implanted stent materials and that tensile elongation itself does not have a significant effect on surface tension [
22]. Thus, the high percentages of elongation for Mg-Zn-Se and Mg-Zn-Cu are likely the result of a uniformly distributed surface tension that would be unlikely to result in a thrombogenic surface once implanted. The poisson ratio (
ν) of Mg-Zn-Se (0.39) and Mg-Zn-Cu (0.27) are comparable to both martensitic Nitinol (0.33) and annealed Pt-10Ir (0.38) (
Table 3). The ratio of both Mg-Zn-Se and Mg-Zn-Cu are below the incompressibility limit of 0.5, thus the materials retain the longitudinal and transverse elastic flexibility [
23].
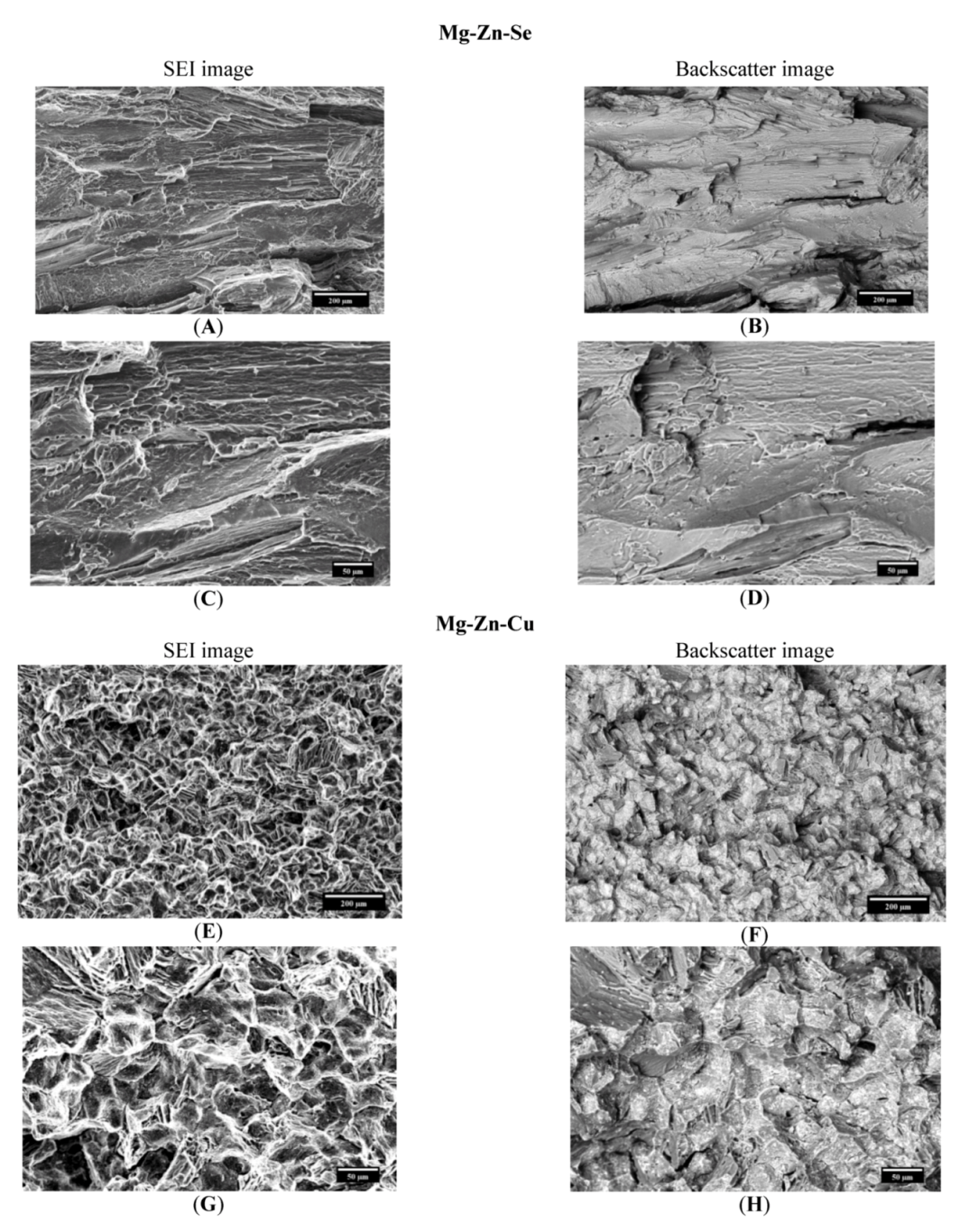
The fracture analysis of the Mg-Zn-Se alloy and the Mg-Zn-Cu alloy was performed visually by SEM imaging and by energy dispersive X-ray spectroscopy (EDS). Analysis revealed that the Mg-Zn-Se alloy failed transgranularly as evident by the smooth faced fractured surfaces (
Figure 3A–D). Mg-Zn-Cu predominantly failed by intergranular failure as seen by the outlined grain structures on the fractured surfaces (
Figure 3E–H). The transgranular failure of Mg-Zn-Se is comparable to the transgranular fracture mechanism of the AZ31B alloys which is currently used for stenting devices [
24]. This mode of failure indicates that the Mg-Zn-Se alloy is more brittle like the AZ31B alloy. Alternatively, the Mg-Zn-Cu alloy undergoes intergranular failure indicating that it is more ductile, which is a more favorable quality in the medical device forming process. Backscatter imaging revealed no separate phases in the fractured surfaces of the Mg-Zn-Se alloy, whereas two visibly separate phases were evident for Mg-Zn-Cu alloy (
Figure 3F,H). Further EDS analysis on the phases identified them as being primarily composed of Cu on the faces of the fractured surfaces and along the grain boundaries (
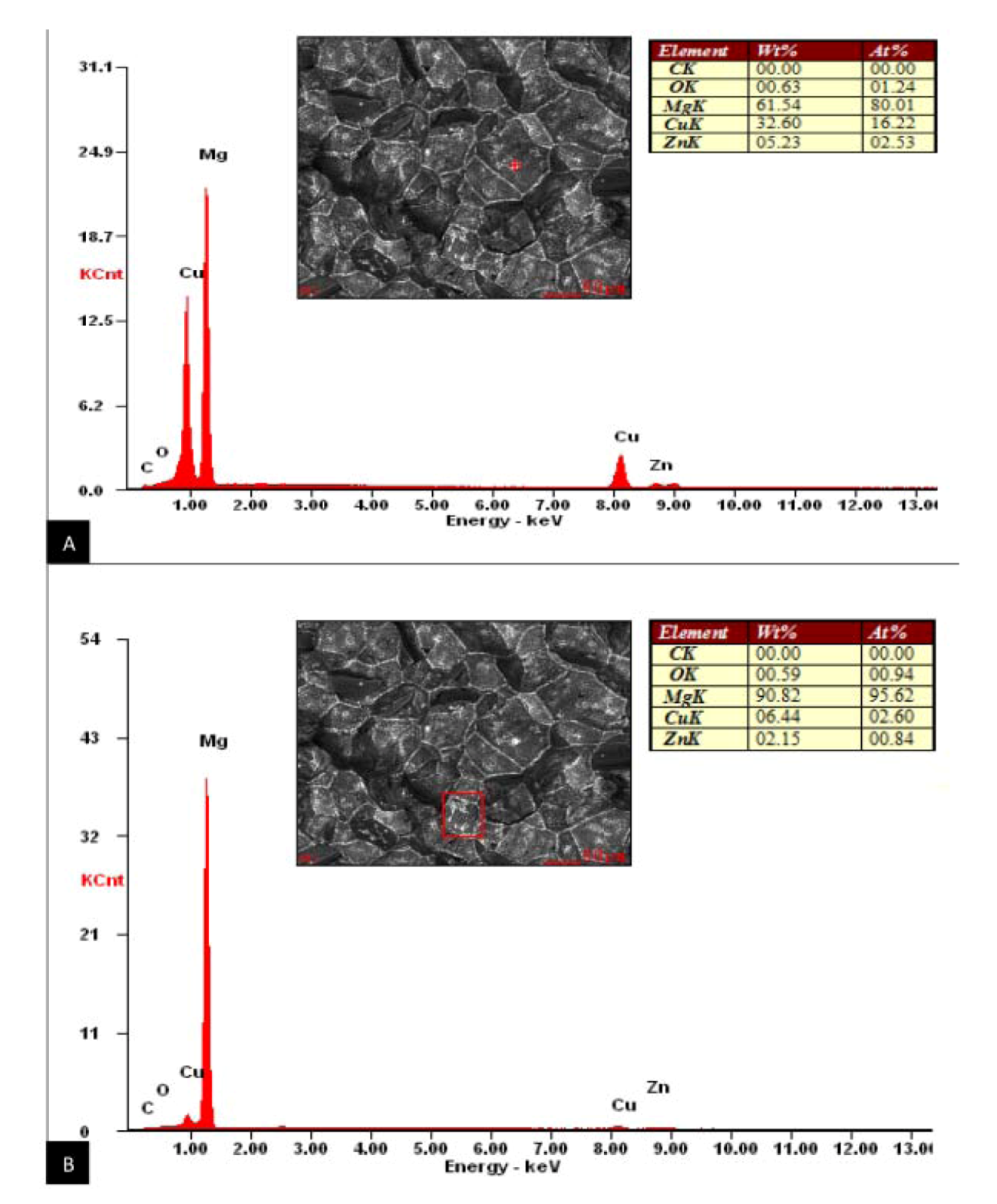
Figure 5). This is consistent with Zhiyong
et al., who proposed that this accumulation of Cu along the grain boundary of a high zinc magnesium alloy with copper additions (Mg-10Zn-5Al-0.1Sb) is actually the formation of a new Mg
2Cu phase. The localization of the thermally stable Mg
2Cu phase along the grain boundaries can strengthen the alloy by dispersive strengthening through pinning the movement of dislocations and the slip of the Mg-Zn-Cu matrix [
25,
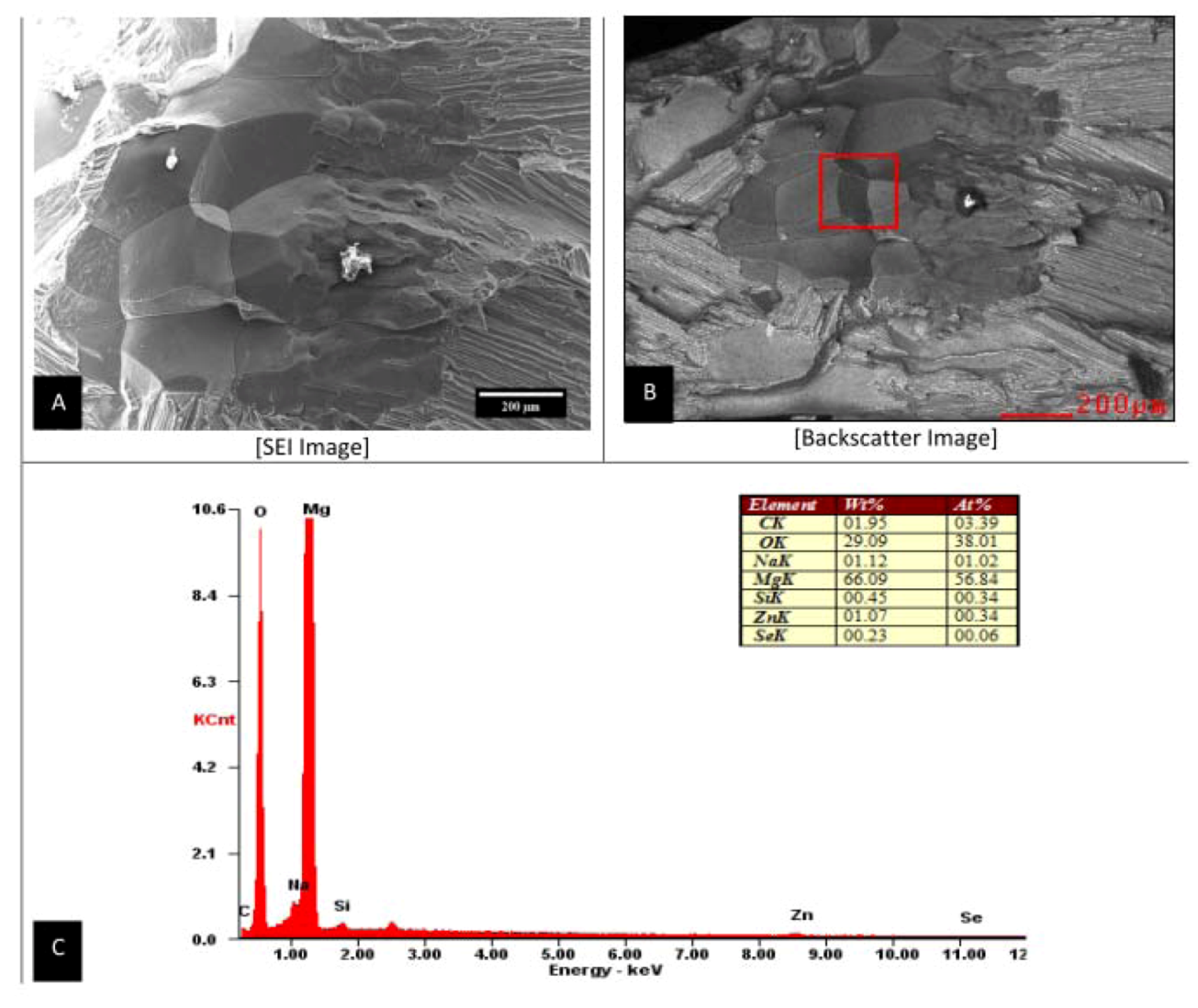
26]. Material manufacturing defects common to occur within the bulk material of the Mg-Zn-Cu and Mg-Zn-Se alloys were apparent in some tensile specimens, which led to premature mechanical failure. MgO and Mg(OH)
2 concentrated regions were detected in Mg-Zn-Se defective specimens which led to premature tensile failure (
Figure 6). This is in accordance with previous literature which found similar oxide formations of MgO, Mg(OH)
2, and MgCO
3 within the inner layer of the AZ31 and AZ91 alloy [
19]. The casting defect for the Mg-Zn-Cu alloy showed a region with higher concentrations of MgO, but more deplete of Zn and Cu as compared to grain boundary concentrations (
Figure 7). Regions of such a homogenous magnesium concentration and the depletion of Cu from within the matrix are believed to have led to the deteriorated tensile strength of the Mg-Zn-Cu alloy. The poisson ratio of both alloys are below the incompressibility limit of 0.5, hence the alloys possess the longitudinal and transverse elastic flexibility required for manufacturing.