Microstructural and Mechanical Characteristics of Novel 6% Cr Cold-Work Tool Steel
Abstract
:1. Introduction
2. Materials and Methods
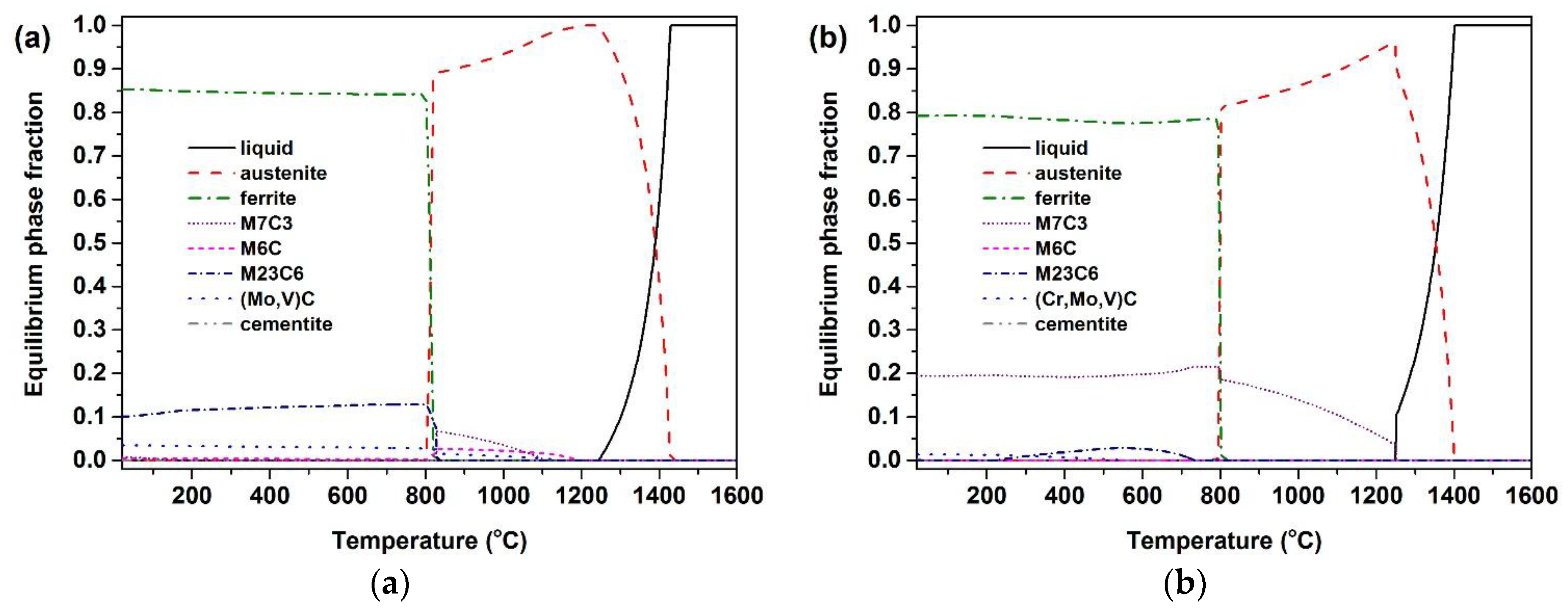
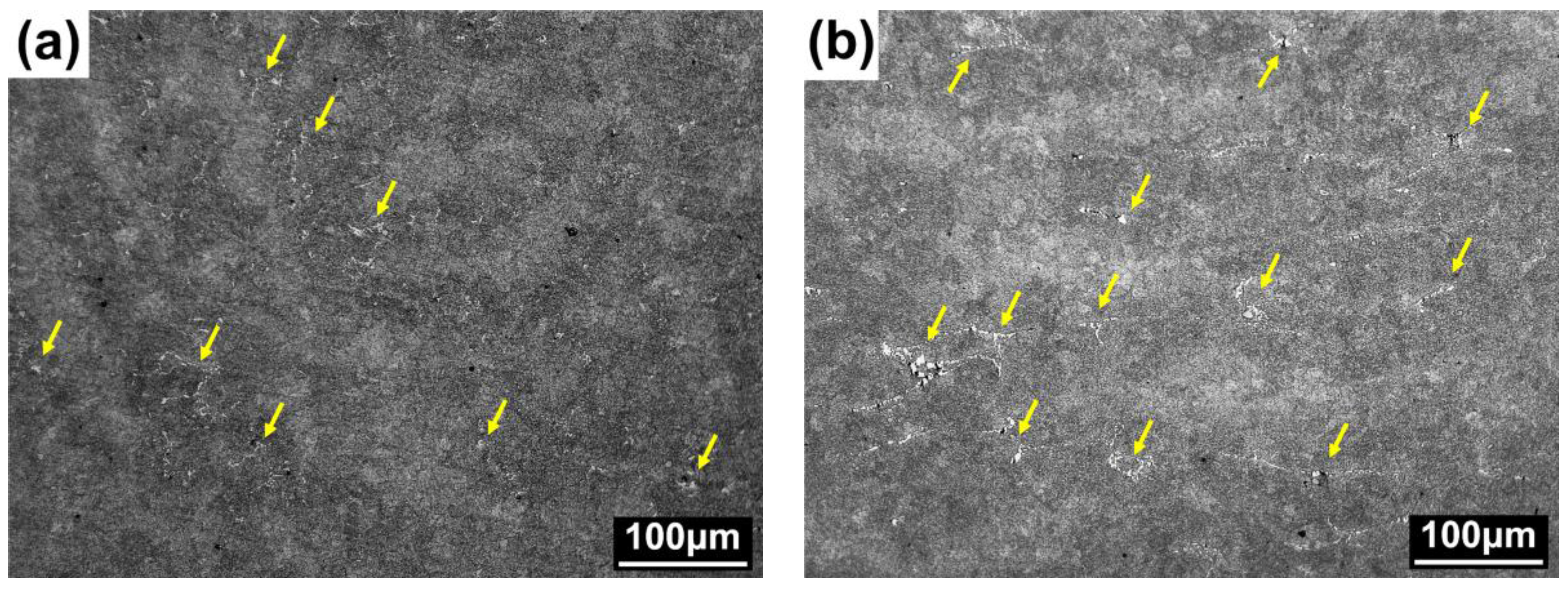
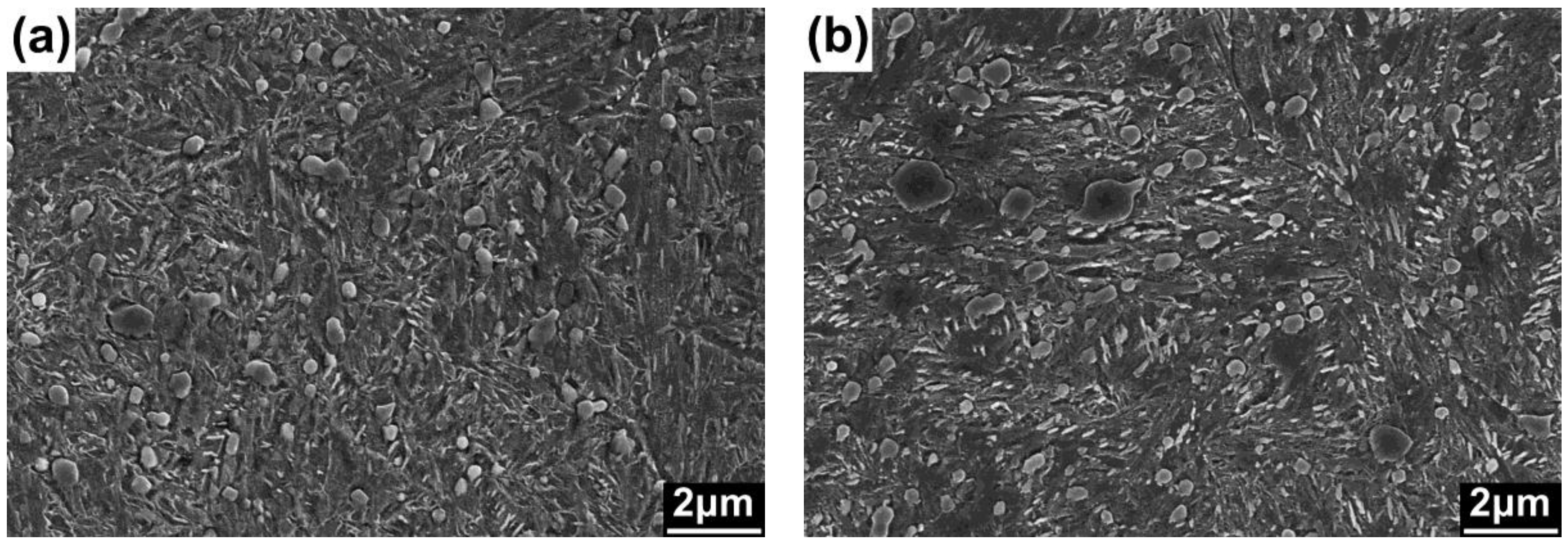
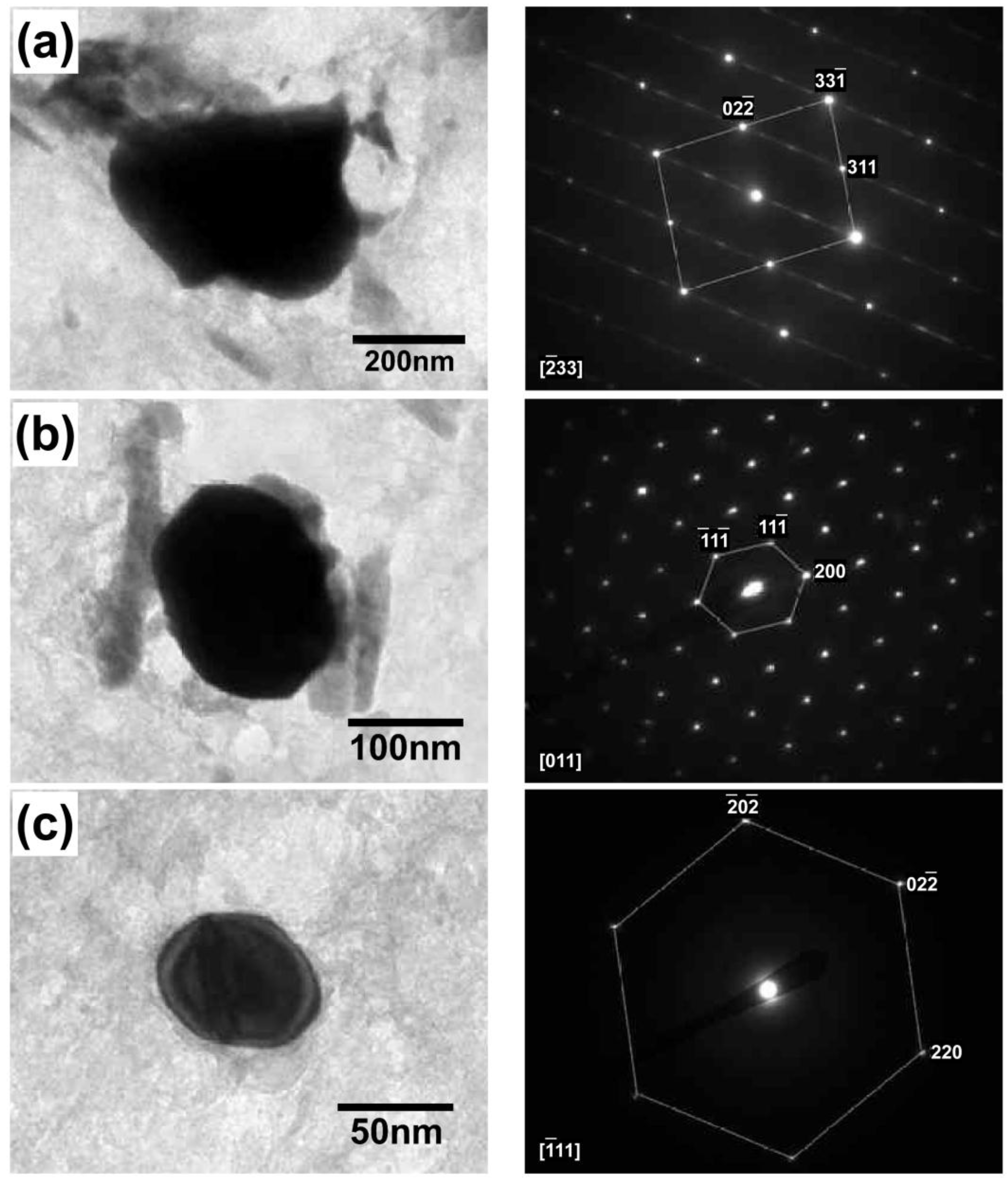
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roberts, G.; Krauss, G.; Kennedy, R. Tool Steel, 5th ed.; ASM International: Materials Park, OH, USA, 1998; pp. 7–28. [Google Scholar]
- Totten, G.E. Steel Heat Treatment: Metallurgy and Technologies, 2nd ed.; CRC Taylor & Francis: New York, NY, USA, 2006; pp. 651–694. [Google Scholar]
- Krauss, G. Steels: Processing, Structure, and Performance; ASM International: Materials Park, OH, USA, 2005; pp. 535–559. [Google Scholar]
- Lee, S.; Kim, S.; Hwang, B.; Lee, B.S.; Lee, C.G. Effect of carbide distribution on the fracture toughness in the transition temperature region of an SA 508 steel. Acta Mater. 2002, 50, 4755–4762. [Google Scholar] [CrossRef]
- Casellas, D.; Caro, J.; Molas, S.; Prado, J.M.; Valls, I. Fracture toughness of carbides in tool steels evaluated by nanoindentation. Acta Mater. 2007, 55, 4277–4286. [Google Scholar] [CrossRef]
- Hong, K.J.; Kang, W.K.; Song, J.H.; Jung, I.S.; Lee, K.A. Study on the anisotropic size change by austenitizing and tempering heat treatment of STD11 tool steel using dilatometry. Korean J. Met. Mater. 2008, 46, 800–808. [Google Scholar]
- Fukaura, K.; Yokoyama, Y.; Yokoi, D.; Tsujii, N.; Ono, K. Fatigue of cold-work tool steels: Effect of heat treatment and carbide morphology on fatigue crack formation, life, and fracture surface observations. Metall. Mater. Trans. A 2004, 35, 1289–1300. [Google Scholar] [CrossRef]
- Kokosza, A.; Pacyna, J. Ewaluation of retained austenite stability in heat treated cold work tool steel. J. Mater. Proc. Technol. 2005, 162–163, 327–331. [Google Scholar] [CrossRef]
- Yaso, M.; Morito, S.; Ohba, T.; Kubota, K. Microstructure of martensite in Fe–C–Cr steel. Mater. Sci. Eng. A 2008, 481–482, 770–773. [Google Scholar] [CrossRef]
- Kang, J.Y.; Heo, Y.U.; Kim, H.Y.; Suh, D.W.; Son, D.M.; Lee, D.H.; Lee, T.H. Effect of copper addition on the characteristics of high-carbon and high-chromium steels. Mater. Sci. Eng. A 2014, 614, 36–44. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kang, J.Y.; Son, D.M.; Lee, D.S.; Lee, T.H.; Jeong, W.C.; Cho, K.M. Microstructure and mechanical properties of cold-wok tool steels: A comparison of 8%Cr steel with STD11. J. Korean Soc. Heat Treat. 2014, 27, 242–252. [Google Scholar] [CrossRef]
- MatCalc Software. Available online: http://www.matcalc.at (accessed on 31 December 2016).
- Hwang, K.C.; Lee, S.H.; Lee, H.C. Effects of alloying elements on microstructure and fracture properties of cast high speed steel rolls: Part I: Microstructural analysis. Mater. Sci. Eng. A 1998, 254, 282–295. [Google Scholar] [CrossRef]
- Hong, K.J.; Song, J.H.; Chung, I.S. Carbide behavior in STD11 tool steel during heat treatment. J. Korean Soc. Heat Treat. 2011, 24, 262–270. [Google Scholar]
- Shim, J.J.; Kim, Y.H.; Lee, S.Y. A study on the growth characteristics of complex carbides precipitated during spheroidizing in medium chromium steels. Korean J. Met. Mater. 1990, 28, 409–415. [Google Scholar]
- Yamasaki, S. Modelling Precipitation of Carbides in Martensitic Steels. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2004. [Google Scholar]
- Dieter, G.E. Mechanical Metallurgy, 3rd ed.; McGraw-Hill: New York, NY, USA, 1990; pp. 95–104. [Google Scholar]
- Fukaura, K.; Yokoyama, Y.; Yokoi, D.; Tsuji, N.; Ono, K. Fatigue of cold work tool steels: Effect of heat treatment and carbide morphology on fatigue crack formation, life and fracture surface observations. Metall. Mater. Trans. A 2004, 35A, 1289–1300. [Google Scholar] [CrossRef]
- Kubota, K.; Ohba, T.; Morito, S. Frictional properties of new developed cold work tool steel for high tensile strength steel forming die. Wear 2011, 271, 2884–2889. [Google Scholar] [CrossRef]
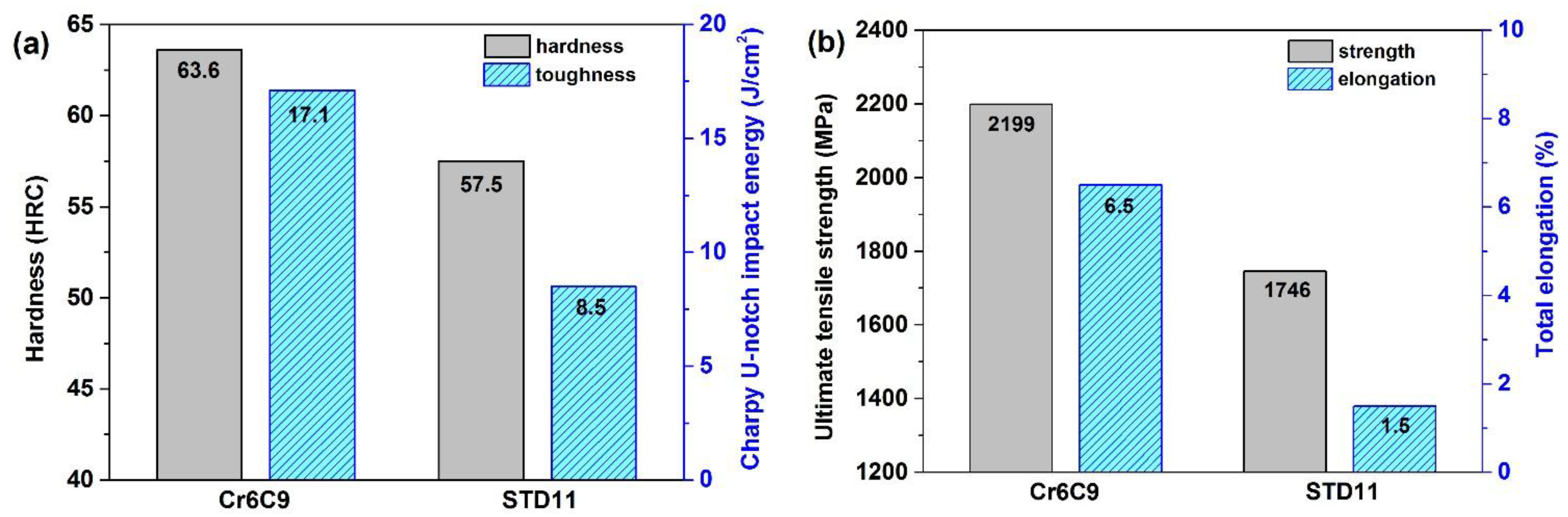
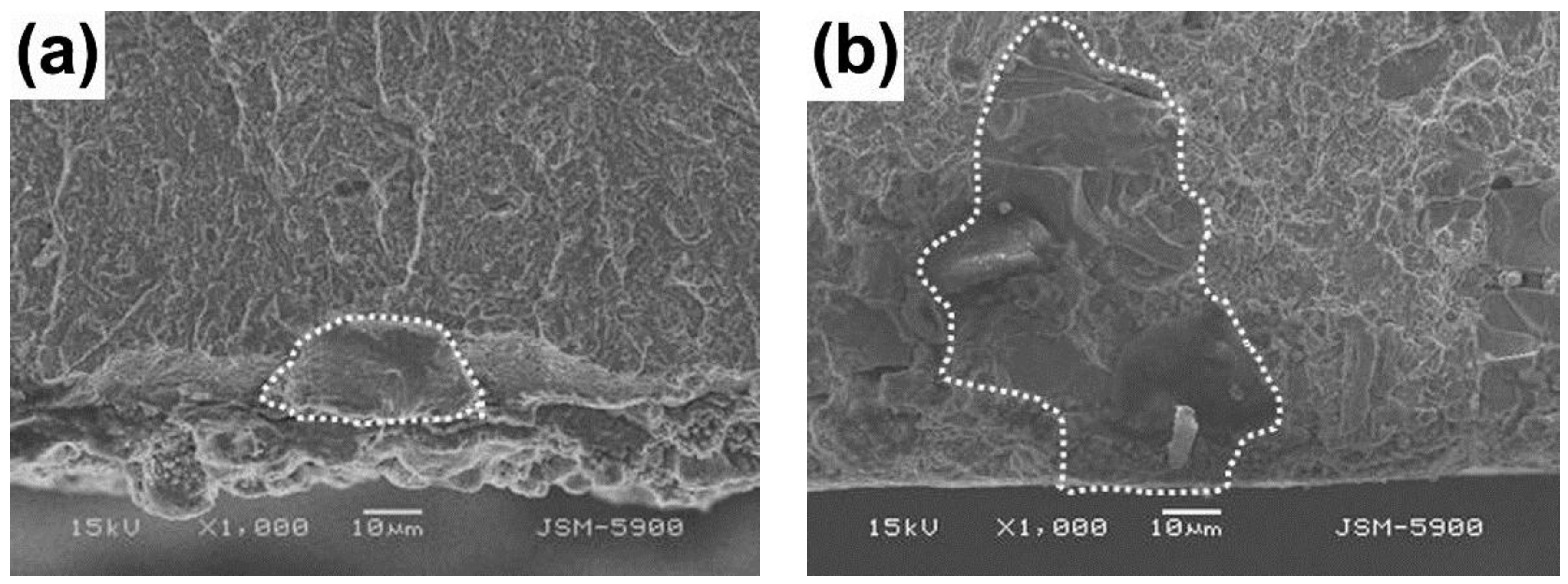
| Steels | C | Si | Mn | Cr | Mo | W | V | Fe |
|---|---|---|---|---|---|---|---|---|
| Cr6C9 | 0.88 | 0.56 | 0.56 | 6.08 | 3.27 | 0.52 | 0.99 | Bal. |
| STD11 | 1.48 | 0.23 | 0.26 | 11.17 | 0.74 | - | 0.23 | Bal. |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.; Kim, M.; Lee, S.-J. Microstructural and Mechanical Characteristics of Novel 6% Cr Cold-Work Tool Steel. Metals 2017, 7, 12. https://doi.org/10.3390/met7010012
Kang S, Kim M, Lee S-J. Microstructural and Mechanical Characteristics of Novel 6% Cr Cold-Work Tool Steel. Metals. 2017; 7(1):12. https://doi.org/10.3390/met7010012
Chicago/Turabian StyleKang, Singon, Minwook Kim, and Seok-Jae Lee. 2017. "Microstructural and Mechanical Characteristics of Novel 6% Cr Cold-Work Tool Steel" Metals 7, no. 1: 12. https://doi.org/10.3390/met7010012





