The Al Effects of Co-Free and V-Containing High-Entropy Alloys
Abstract
:1. Introduction
2. Experimental Procedures
3. Results
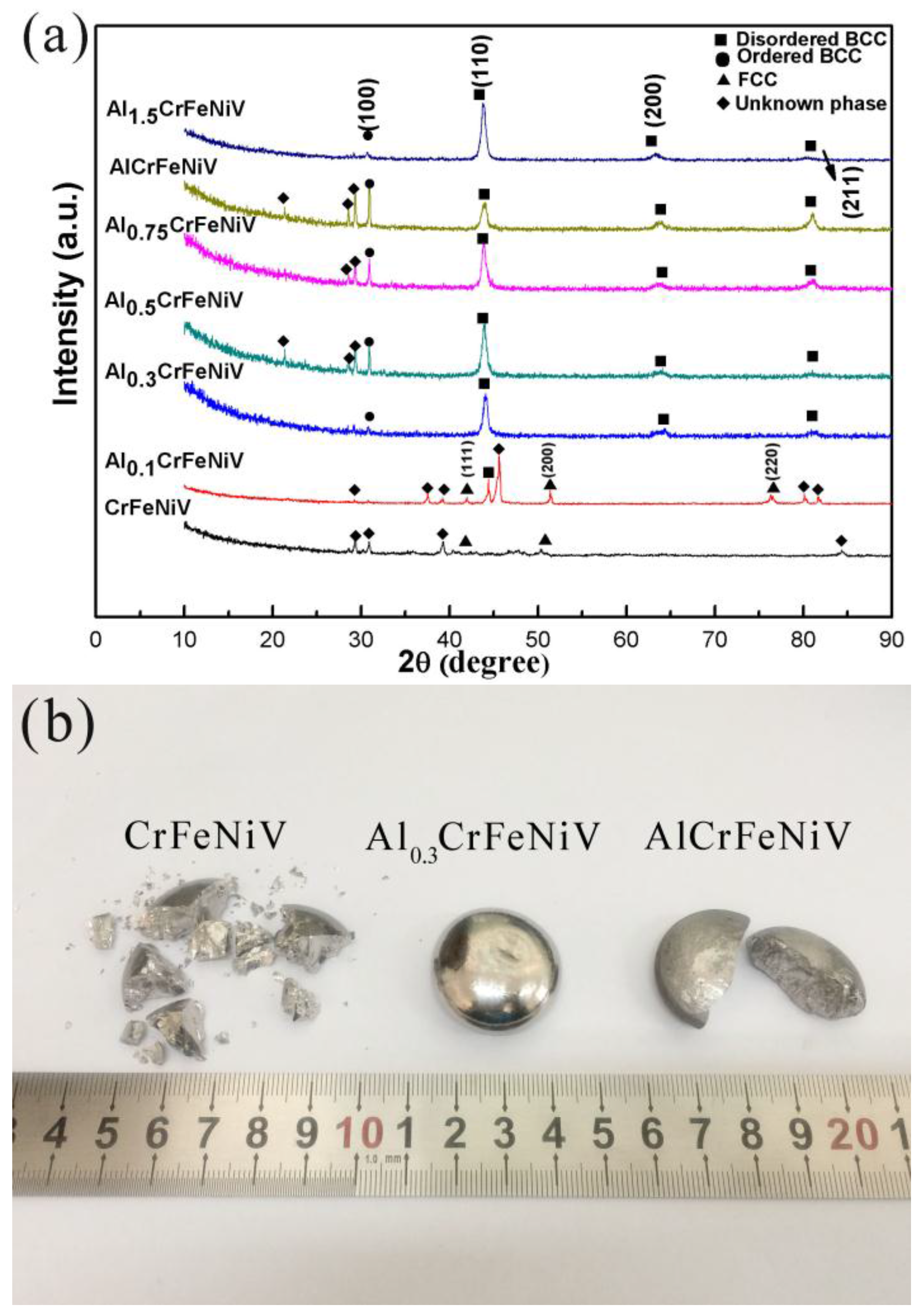
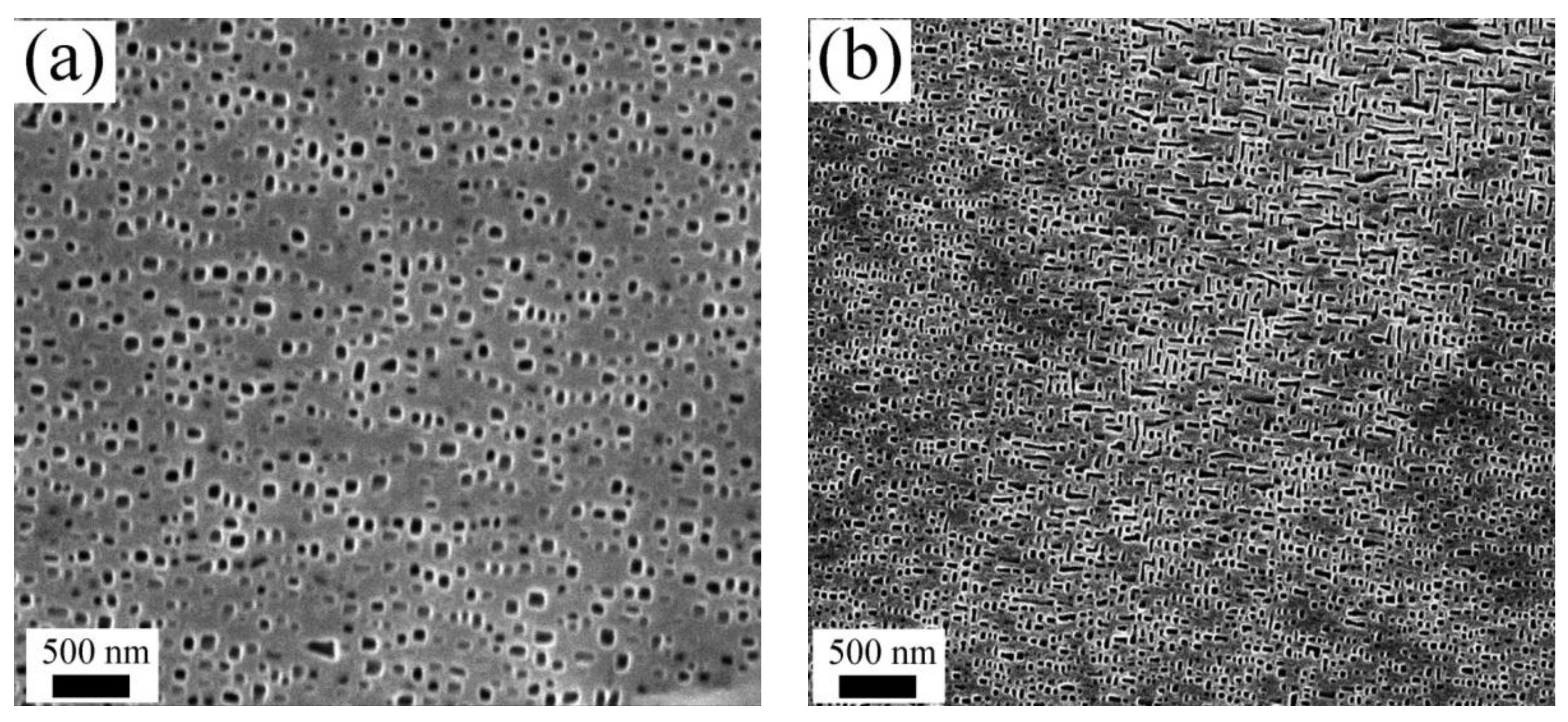
3.1. Results of the XRD and SEM
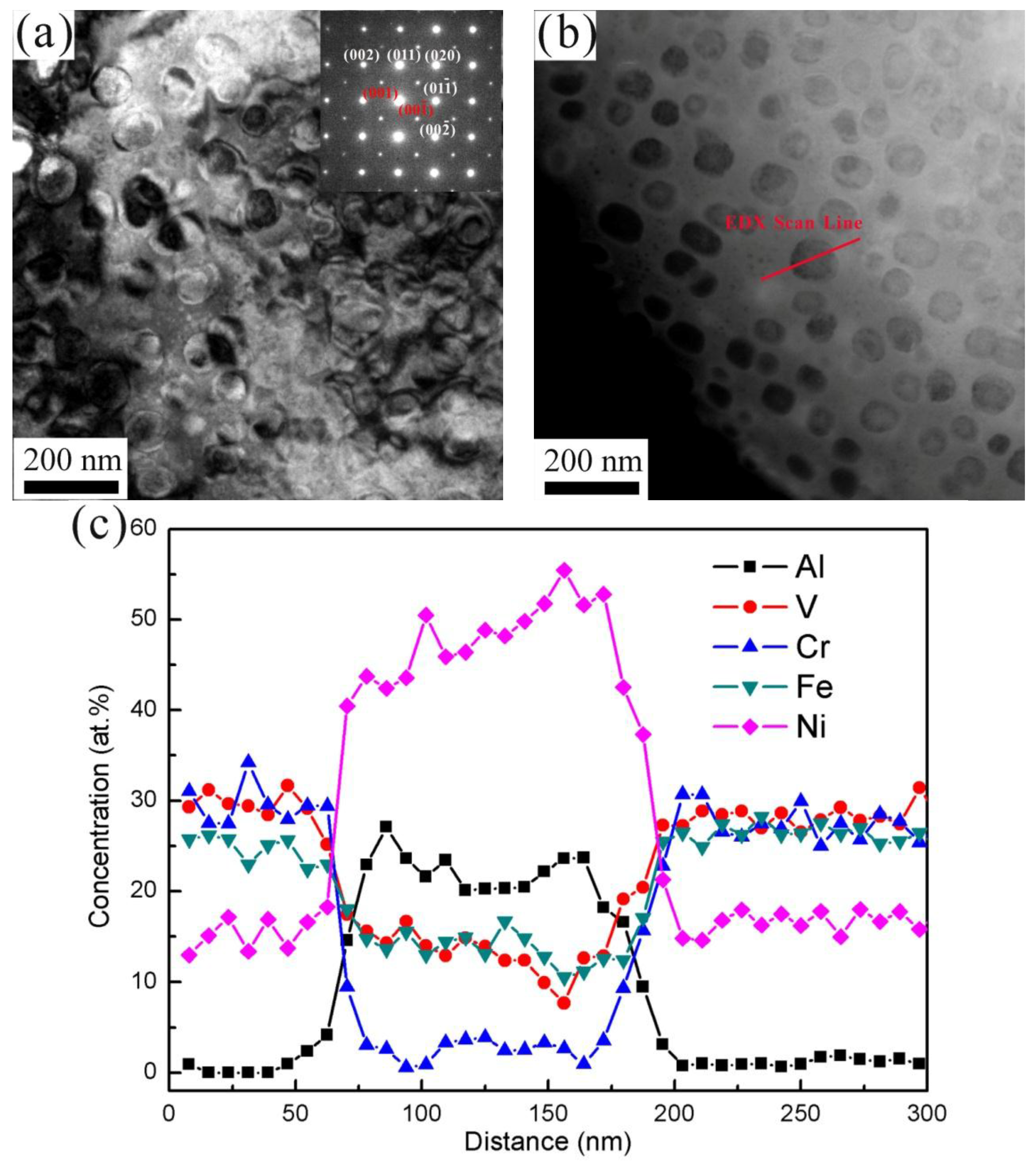
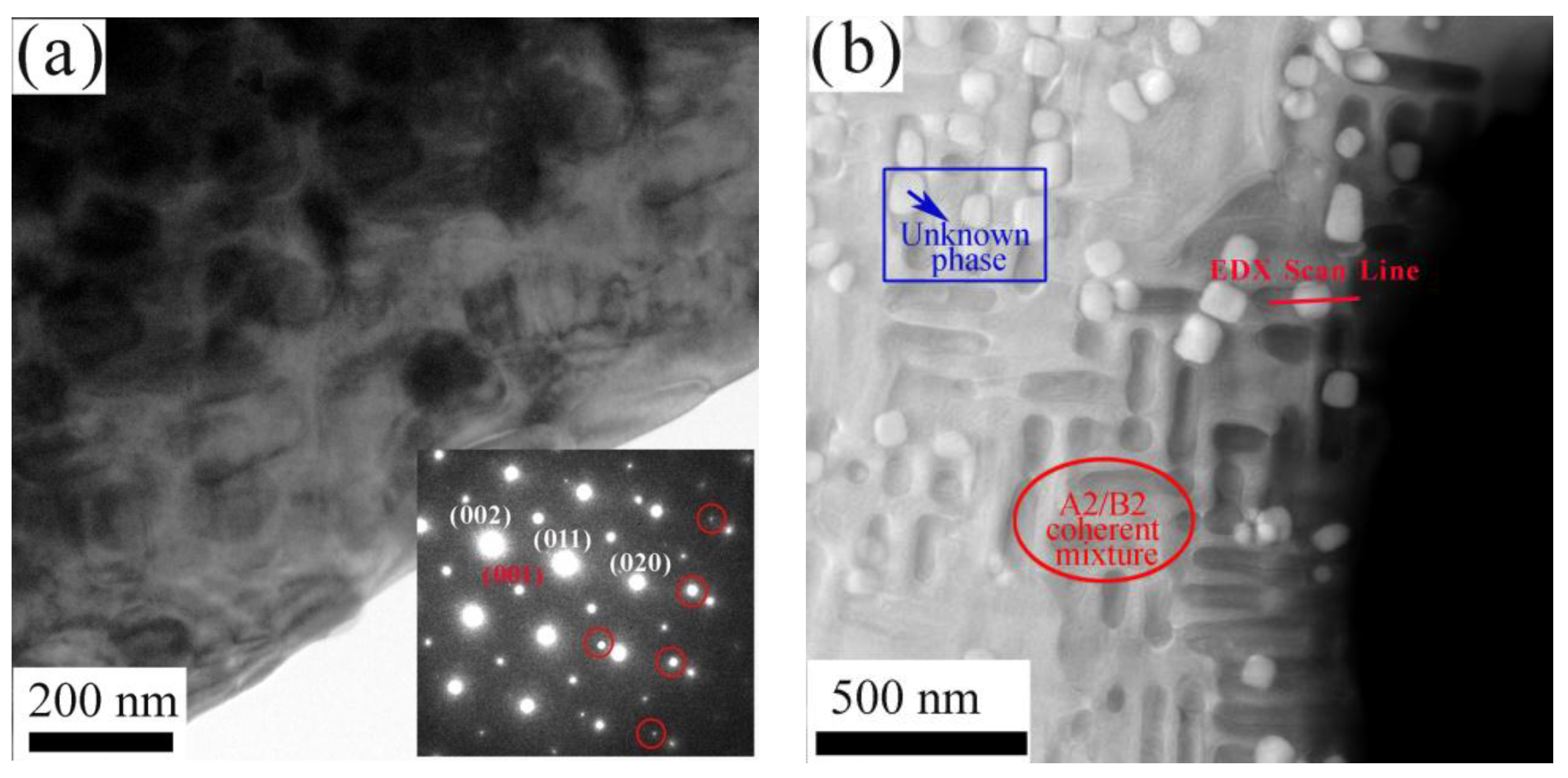
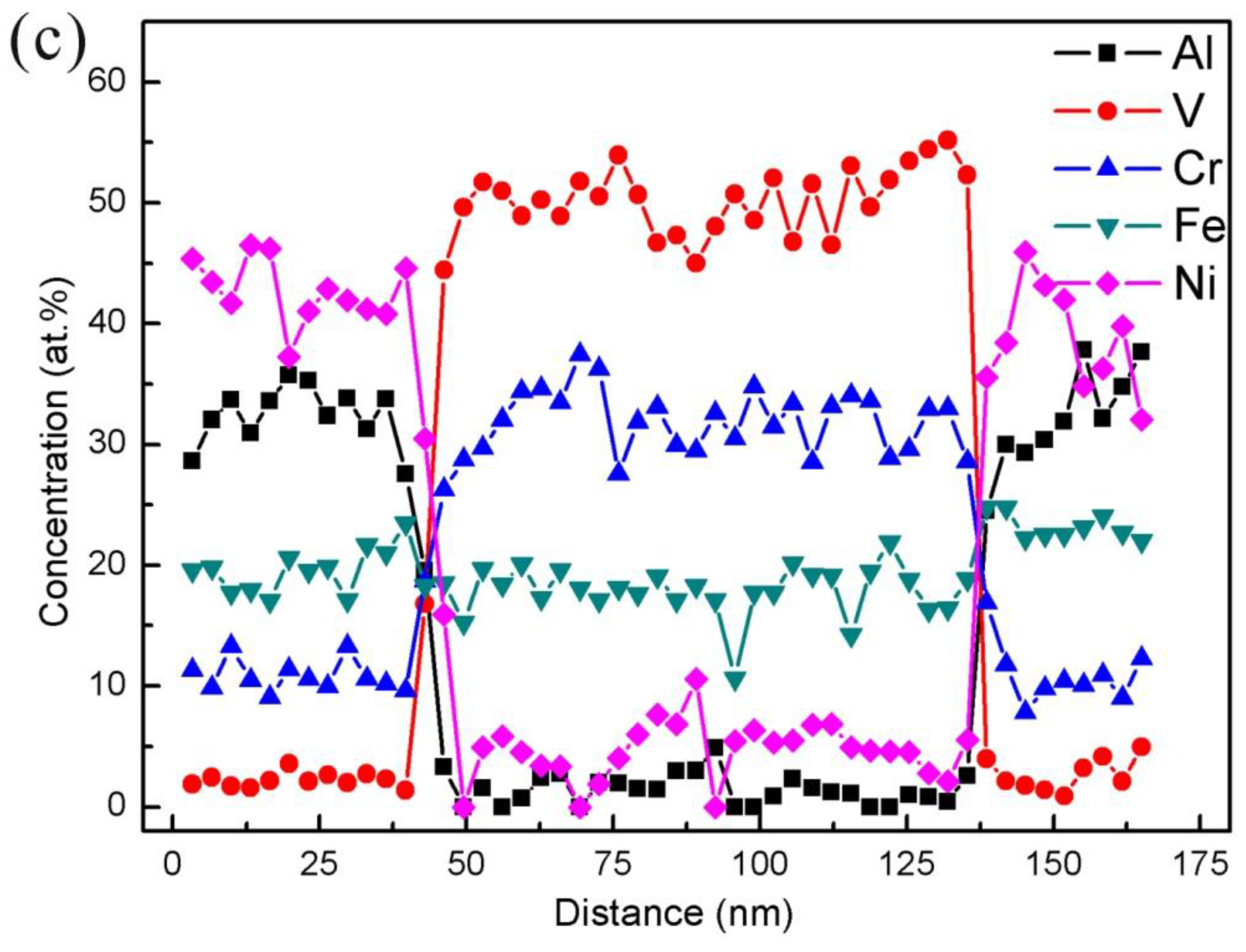
3.2. Results of the TEM and STEM
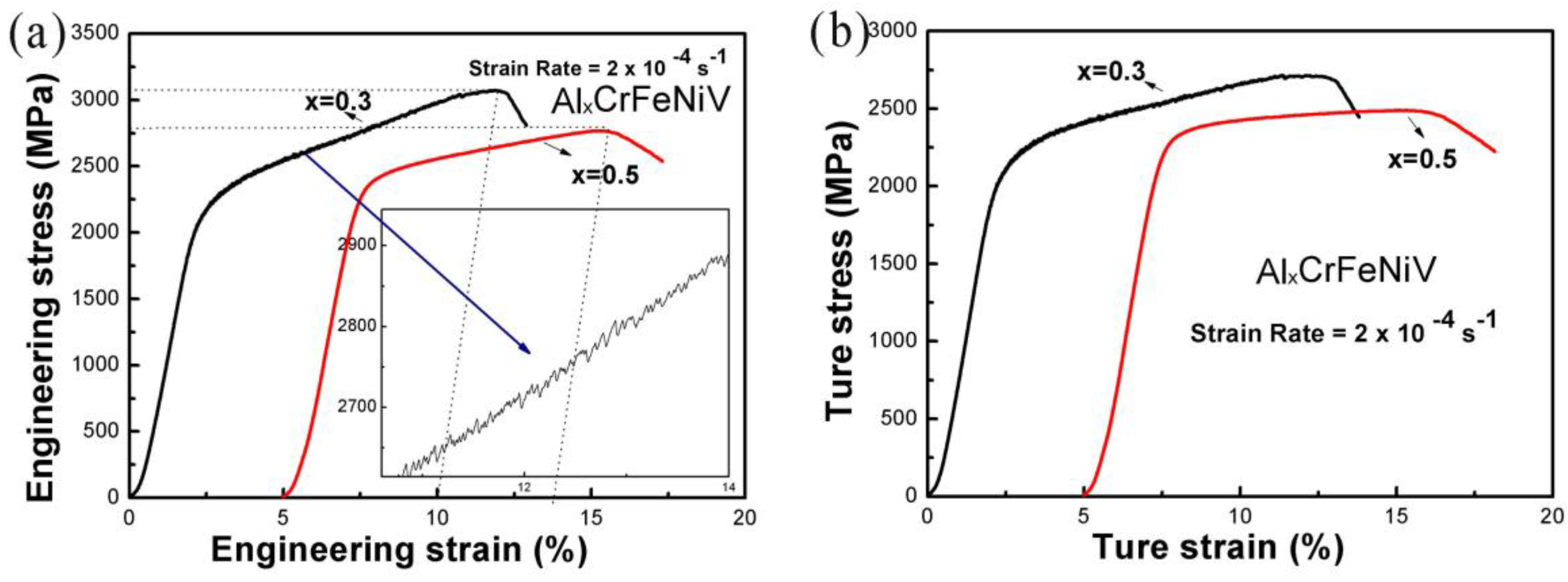
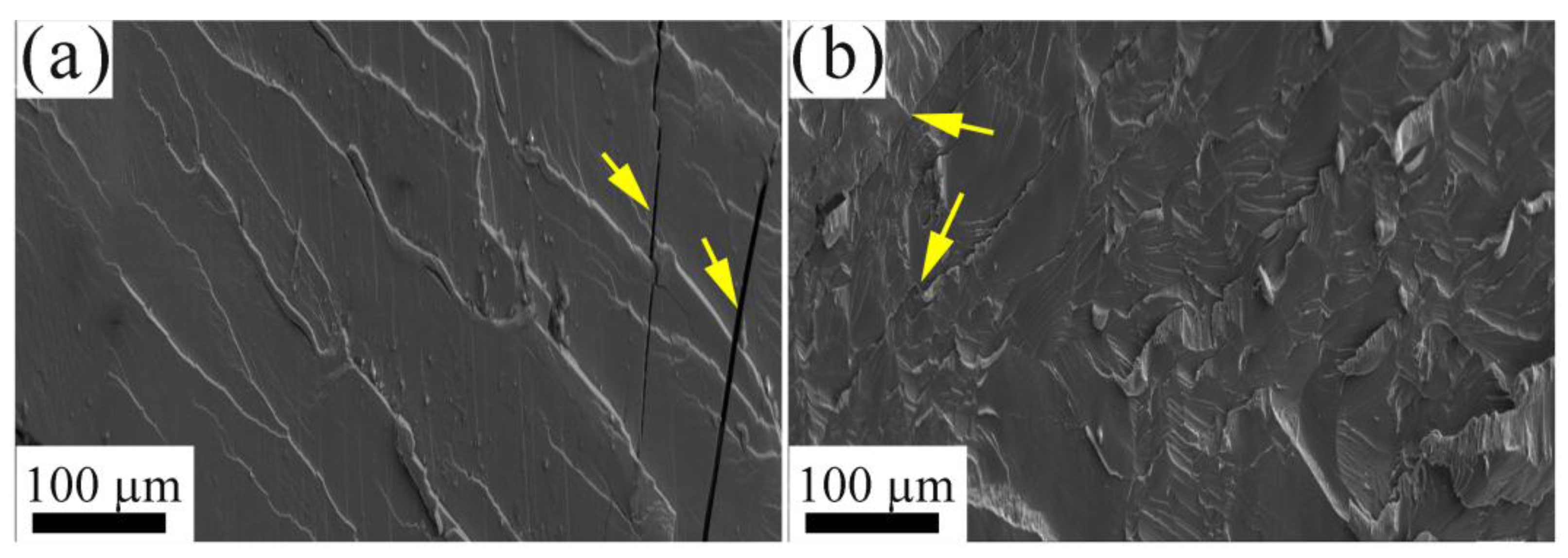
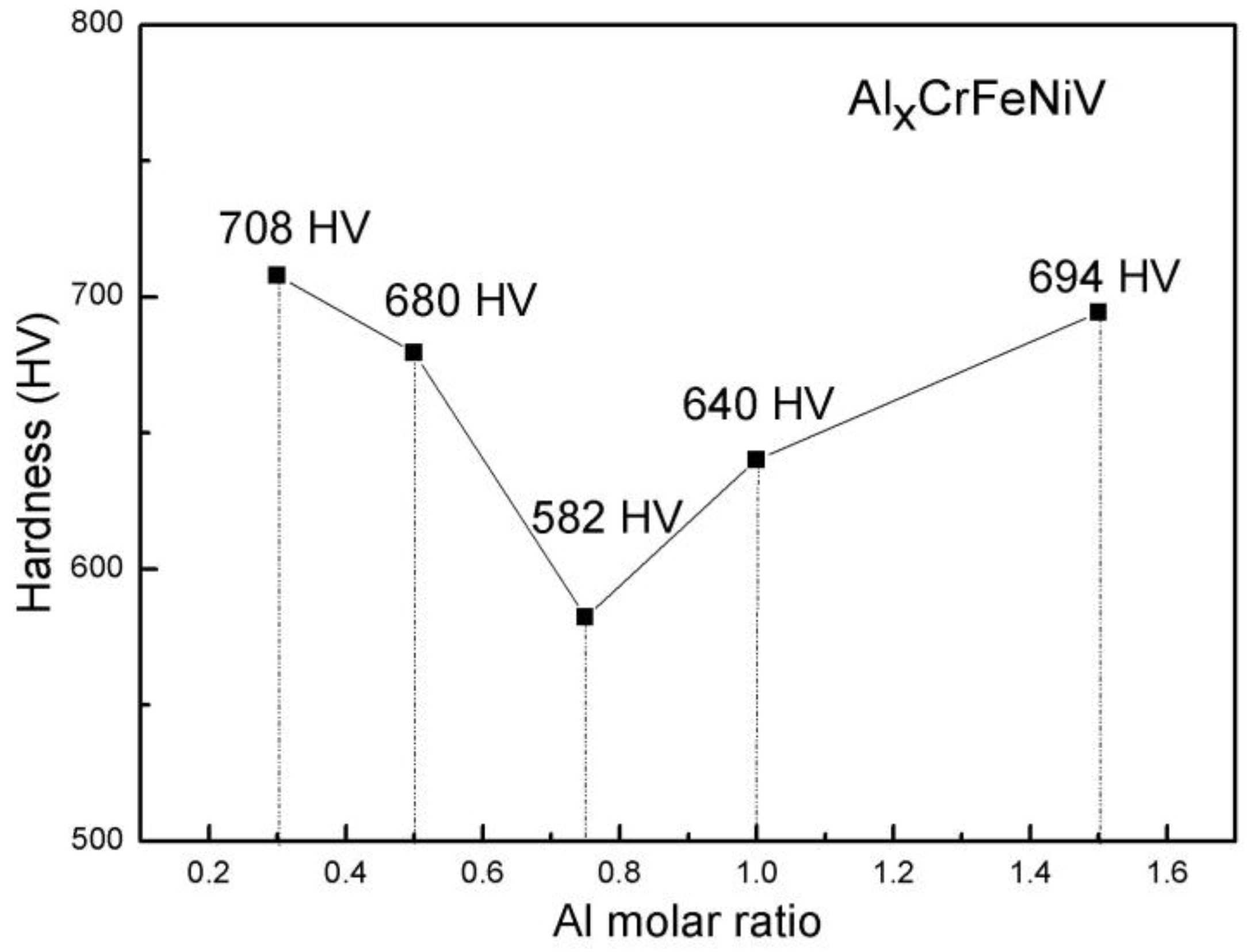
3.3. Mechanical Properties
4. Discussion
5. Conclusions
- (1)
- The crystal structures of the alloys with lower Al content (for example, x = 0 and x = 0.1) were found to be quite different from the other Al contents. In contrast, distinctly similar crystal structures with BCC could be observed with a higher Al content (for example, x ≥ 0.3);
- (2)
- The Al0.3CrFeNiV alloy was comprised of B2 and A2 phases. Meanwhile, the Al0.5CrFeNiV alloy contained a third unknown phase, as well as the coherent A2/B2 phases. Furthermore, the unknown phase in the Al0.5CrFeNiV alloy was found to be significantly enriched in Cr and V;
- (3)
- Based on the mechanical properties tests, it was determined that both of the AlxCrFeNiV (x = 0.3, and 0.5) alloys displayed very high yield and fracture strengths. However, they were very brittle, with compression fracture strains of less than 10%. Also, the as-cast Al0.3CrFeNiV alloy has the highest microhardness of 708 HV among the as-cast AlxCrFeNiV (x = 0.3, 0.5, 0.75, 1, and 1.5) HEAs. Therefore, efforts should be made to improve the ductility of these alloys. In addition, the fracture mechanisms of the AlxCrFeNiV (x = 0.3, and 0.5) HEAs were determined to be cleavage fractures.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Lu, Z.P.; Wang, H.; Chen, M.W.; Baker, I.; Yeh, J.W.; Liu, C.T.; Nieh, T.G. An assessment on the future development of high-entropy alloys: Summary from a recent workshop. Intermetallics 2015, 66, 67–76. [Google Scholar] [CrossRef]
- Wang, Y.P.; Li, B.S.; Fu, H.Z. Solid solution or intermetallics in a high-entropy alloy. Adv. Eng. Mater. 2009, 11, 641–644. [Google Scholar] [CrossRef]
- Shaysultanov, D.G.; Stepanov, N.D.; Kuznetsov, A.V.; Salishchev, G.A.; Senkov, O.N. Phase composition and superplastic behavior of a wrought alcocrcufeni high-entropy alloy. JOM 2013, 65, 1815–1828. [Google Scholar] [CrossRef]
- Singh, S.; Wanderka, N.; Murty, B.S.; Glatzel, U.; Banhart, J. Decomposition in multi-component AlCoCrCuFeNi high-entropy alloy. Acta Mater. 2011, 59, 182–190. [Google Scholar] [CrossRef]
- Otto, F.; Yang, Y.; Bei, H.; George, E.P. Relative effects of enthalpy and entropy on the phase stability of equiatomic high-entropy alloys. Acta Mater. 2013, 61, 2628–2638. [Google Scholar] [CrossRef]
- Yang, T.F.; Xia, S.Q.; Liu, S.; Wang, C.X.; Liu, S.S.; Zhang, Y.; Xue, J.M.; Yan, S.; Wang, Y.G. Effects of Al addition on microstructure and mechanical properties of AlxCoCrFeNi high-entropy alloy. Mater. Sci. Eng. A Struct. 2015, 648, 15–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiao, J.W.; Liaw, P.K. A brief review of high entropy alloys serration behavior and flow units. J. Iron Steel Res. Int. 2016, 23, 2–6. [Google Scholar] [CrossRef]
- Xia, S.Q.; Wang, Z.; Yang, T.F.; Zhang, Y. Irradiation behavior in high entropy alloys. J. Iron Steel Res. Int. 2015, 22, 879–884. [Google Scholar] [CrossRef]
- Xia, S.Q.; Yang, X.; Yang, T.F.; Liu, S.; Zhang, Y. Irradiation resistance in AlxCoCrFeNi high entropy alloys. JOM 2015, 67, 1–5. [Google Scholar] [CrossRef]
- Wang, W.R.; Wang, W.L.; Wang, S.C.; Tsai, Y.C.; Lai, C.H.; Yeh, J.W. Effects of Al addition on the microstructure and mechanical property of AlxCoCrFeNi high-entropy alloys. Intermetallics 2012, 26, 44–51. [Google Scholar] [CrossRef]
- Jantzen, C.M.F.; Herman, H. Spinodal decomposition-phase diagram representation and occurrence. Phase Diagr. 1978, 127–184. [Google Scholar]
- Tong, C.J.; Chen, Y.L.; Chen, S.K.; Yeh, J.W.; Shun, T.T.; Tsau, C.H.; Lin, S.J.; Chang, S.Y. Microstructure characterization of AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2005, 36A, 881–893. [Google Scholar] [CrossRef]
- Hanna, J.A.; Baker, I.; Wittmann, M.W.; Munroe, P.R. A new high-strength spinodal alloy. J. Mater. Res. 2005, 20, 791–795. [Google Scholar] [CrossRef]
- Pike, L.; Chang, Y.; Liu, C. Point defect concentrations and hardening in binary B2 intermetallics. Acta Mater. 1997, 45, 3709–3719. [Google Scholar] [CrossRef]
- Santodonato, L.J.; Zhang, Y.; Feygenson, M.; Parish, C.M.; Gao, M.C.; Weber, R.J.K.; Neuefeind, J.C.; Tang, Z.; Liaw, P.K. Deviation from high-entropy configurations in the atomic distributions of a multi-principal-element alloy. Nat. Commun. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Laktionova, M.A.; Tabchnikova, E.D.; Tang, Z.; Liaw, P.K. Mechanical properties of the high-entropy alloy Al0.5CoCrCuFeNi at temperatures of 4.2–300 K. Low Temp. Phys. 2013, 39, 630–632. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Scott, J.M.; Miracle, D.B. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Liu, S.; Gao, M.C.; Liaw, P.K.; Zhang, Y. Microstructures and mechanical properties of AlxCrFeNiTi0.25. J. Alloy. Compd. 2015, 619, 610–615. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Zhang, Y.; Wang, Y.L.; Chen, G.L. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, Y.; Qiao, Y.; Chen, G.L. Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 2007, 15, 357–362. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; He, J.; Yao, K.; Wei, B.; Chen, G. Metallographic analysis of Cu-Zr-Al bulk amorphous alloys with yttrium addition. Scr. Mater. 2006, 54, 1351–1355. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Cheng, Y.Q.; Liaw, P.K. High-entropy alloys with high saturation magnetization, electrical resistivity, and malleability. Sci. Rep. 2013, 3, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, N.D.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A.; Oleynik, E.E.; Tortika, A.S.; Senkov, O.N. Effect of V content on microstructure and mechanical properties of the CoCrFeMnNiVx high entropy alloys. J. Alloy. Compd. 2015, 628, 170–185. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Shaysultanov, D.G.; Salishchev, G.A.; Tikhonovsky, M.A. Structure and mechanical properties of a light-weight AlNbTiV high entropy alloy. Mater. Lett. 2015, 142, 153–155. [Google Scholar] [CrossRef]
- Li, B.S.; Wang, Y.P.; Ren, M.X.; Yang, C.; Fu, H.Z. Effects of Mn, Ti and V on the microstructure and properties of AlCrFeCoNiCu high entropy alloy. Mater. Sci. Eng. A 2008, 498, 482–486. [Google Scholar] [CrossRef]
- Li, C.; Li, J.C.; Zhao, M.; Jiang, Q. Effect of aluminum contents on microstructure and properties of AlxCoCrFeNi alloys. J. Alloy. Compd. 2010, 504S, S515–S518. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; VÖlkl, R.; Glatzel, U.; Wanderka, N. Phase separation in equiatomic AlCoCrFeNi high-entropy alloy. Ultramicroscopy 2013, 132, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.P.; Chang, Y.S.; Chen, S.K.; Yeh, J.W. Microstructure, thermophysical and electrical properties in AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. Mater. Sci. Eng. B Solid 2009, 163, 184–189. [Google Scholar] [CrossRef]
- Li, C.; Zhao, M.; Li, J.C.; Jiang, Q. B2 structure of high-entropy alloys with addition of Al. J. Appl. Phys. 2008, 104, 113504. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.Y.; Cotton, J.D.; Zhang, Y. Phase Stability of Low-Density, Multiprincipal Component Alloys Containing Aluminum, Magnesium, and Lithium. JOM 2014, 66, 2009–2020. [Google Scholar] [CrossRef]
- Dong, Y.; Lu, Y.; Jiang, L.; Wang, T.; Li, T. Effects of electro-negativity on the stability of topologically close-packed phase in high entropy alloys. Intermetallics 2014, 52, 105–109. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Fang, S.; Xiao, X.; Xia, L.; Li, W.; Dong, Y. Relationship between the widths of supercooled liquid regions and bond parameters of Mg-based bulk metallic glasses. J. Non-Cryst. Solids. 2003, 321, 120–125. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of FCC or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 645–647. [Google Scholar] [CrossRef]
| Alloy No. | Al | Cr | Fe | Ni | V | Atomic Ratio |
|---|---|---|---|---|---|---|
| 1 | 0 | 25 | 25 | 25 | 25 | CrFeNiV |
| 2 | 2.4 | 24.4 | 24.4 | 24.4 | 24.4 | Al0.1CrFeNiV |
| 3 | 7.0 | 23.3 | 23.3 | 23.3 | 23.3 | Al0.3CrFeNiV |
| 4 | 11.1 | 22.2 | 22.2 | 22.2 | 22.2 | Al0.5CrFeNiV |
| 5 | 15.8 | 21.1 | 21.1 | 21.1 | 21.1 | Al0.75CrFeNiV |
| 6 | 20 | 20 | 20 | 20 | 20 | Al1CrFeNiV |
| 7 | 27.3 | 18.2 | 18.2 | 18.2 | 18.2 | Al1.5CrFeNiV |
| No. | Alloy | σ0.2 (MPa) | σp (MPa) | εp (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Al0.3CrFeNiV | 2066.8 | 3072.8 | 9.2 | This work |
| 2 | Al0.5CrFeNiV | 2295.7 | 2766.4 | 8.9 | This work |
| 3 | Al0.5CrFeNiTi0.25 | 1880 | 3475 | 40 | [21] |
| 4 | AlCoCrFeNi | 1500 | 2830 | 26.9 | [22] |
| 5 | AlCoCrFeNiTi0.5 | 2260 | 3140 | 23.3 | [22] |
| 6 | AlCoCrFeNiTi | 1860 | 2580 | 8.8 | [22] |
| 7 | CoCrCuFeNi | 230 | 888 | 50.2 | [23] |
| 8 | CoCrCuFeNiTi0.5 | 700 | 1650 | 21.6 | [23] |
| 8 | CoCrCuFeNiTi0.8 | 1042 | 1848 | 2.11 | [23] |
| 10 | CoCrCuFeNiTi | 1227 | 1272 | 0 | [23] |
| 11 | Al4Cu48Zr48 | 1199 | 1882 | 5.3 | [24] |
| 12 | Al0.3CoFeNiSi0.3 | 938 | 2857 | 33 | [25] |
| 13 | MoNbTaW | 1058 | 1211 | 1.5 | [20] |
| 14 | MoNbTaVW | 1246 | 1270 | 0.5 | [20] |
| 15 | CoCrFeMnNiV0.75 | 740 | 1325 | 7.8 | [26] |
| 16 | CoCrFeMnNiV | 1660 | 1845 | 0.5 | [26] |
| 17 | AlNbTiV | 1020 | 1318 | 5 | [27] |
| 18 | AlCrCoCuFeNiV | 1469 | 1970 | 16 | [28] |
| 19 | AlCrCoCuFeNi | 1303 | 2081 | 24 | [28] |
| 20 | AlCrCoCuFeMnNi | 1005 | 1480 | 15 | [28] |
| 21 | AlCrCoCuFeNiTi | 1234 | 1356 | 9 | [28] |
| Alloys | δ (%) | ΔHmix (kJ/mol) | ΔSmix (J/mol·K) | Tm (K) | Ω | VEC | ∆χ (%) |
|---|---|---|---|---|---|---|---|
| Al0.3CrFeNiV | 3.96 | −11.83 | 12.83 | 1896.29 | 2.06 | 6.95 | 11.85 |
| Al0.5CrFeNiV | 4.39 | −13.14 | 13.15 | 1853.50 | 1.85 | 6.78 | 11.92 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, S.; Yang, X.; Chen, M.; Yang, T.; Zhang, Y. The Al Effects of Co-Free and V-Containing High-Entropy Alloys. Metals 2017, 7, 18. https://doi.org/10.3390/met7010018
Xia S, Yang X, Chen M, Yang T, Zhang Y. The Al Effects of Co-Free and V-Containing High-Entropy Alloys. Metals. 2017; 7(1):18. https://doi.org/10.3390/met7010018
Chicago/Turabian StyleXia, Songqin, Xiao Yang, Mingbiao Chen, Tengfei Yang, and Yong Zhang. 2017. "The Al Effects of Co-Free and V-Containing High-Entropy Alloys" Metals 7, no. 1: 18. https://doi.org/10.3390/met7010018







