Electropolishing Behaviour of 73 Brass in a 70 vol % H3PO4 Solution by Using a Rotating Cylinder Electrode (RCE)
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Anodic Polarisation Behaviour
3.2. Potentiostatic Polishing on the Limiting-Current Plateau
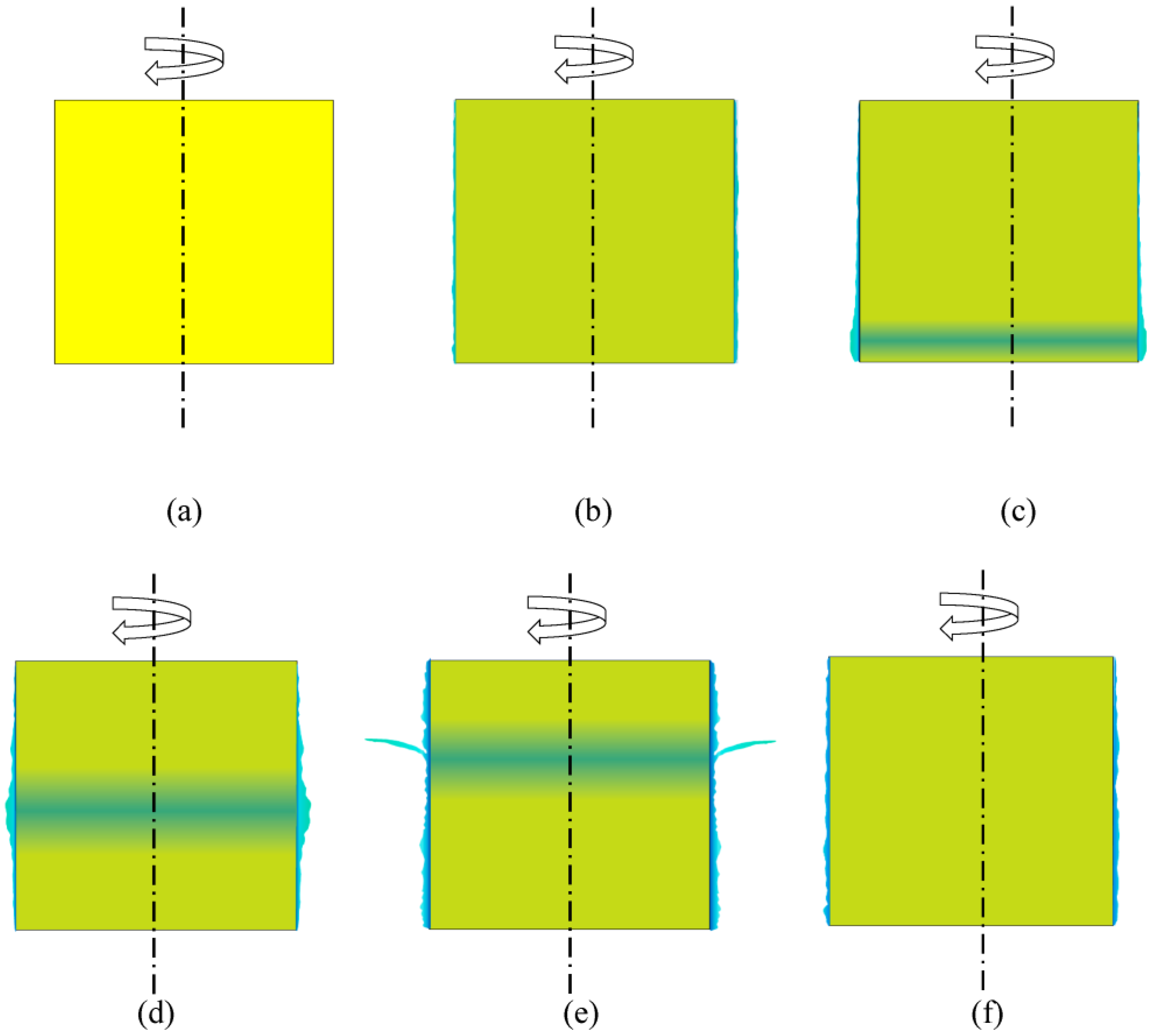
3.3. Effect of the Blue Cu2+-Rich Layer
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Zhang, J.; Tang, N.; Shang, Y.-J.; Xu, F. Effect of alloying elements on corrosion resistance of brass and function mechanisms. Corros. Prot. 2012, 33, 605–609. [Google Scholar]
- Kwon, G.D.; Kim, Y.W.; Moyen, E.; Keum, D.H.; Lee, Y.H.; Baik, S.; Pribat, D. Controlled electropolishing of copper foils at elevated temperature. Appl. Surf. Sci. 2014, 307, 731–735. [Google Scholar] [CrossRef]
- Landolt, D. Fundamental aspects of electropolishing. Electrochim. Acta 1987, 32, 1–11. [Google Scholar] [CrossRef]
- Matlosz, M.; Landolt, D. Shape changes in electrochemical polishing: The effect of temperature on the anodic leveling of Fe-24Cr. J. Electrochem. Soc. 1989, 136, 919–929. [Google Scholar] [CrossRef]
- Gabe, D.R. Electropolishing of copper and copper-based alloys in ortho-phosphoric acid. Corros. Sci. 1972, 12, 113–120. [Google Scholar] [CrossRef]
- Iskander, S.S.; Mansour, I.A.S.; Sedahmed, G.H. Electropolishing of brass alloys in phosphoric acid. Surf. Technol. 1980, 10, 357–361. [Google Scholar] [CrossRef]
- Awad, A.M.; Ghany, N.A.A.; Dahy, T.M. Removal of tarnishing and roughness of copper surface by electropolishing treatment. Appl. Surf. Sci. 2010, 256, 4370–4375. [Google Scholar] [CrossRef]
- Vidal, R.; West, A.C. Copper electropolishing in concentrated phosphoric acid I. Experimental findings. J. Electrochem. Soc. 1995, 142, 2682–2689. [Google Scholar] [CrossRef]
- Varenko, E.S.; Loshkarev, Y.M.; Tarasova, L.P. Surface roughness of brass during electropolishing in orthophosphoric acid solutions. Sov. Electrochem. 1991, 27, 105–107. [Google Scholar]
- Li, D.; Li, N.; Xia, G.; Zheng, Z.; Wang, J.; Xiao, N.; Zhai, W.; Wu, G. An in-situ study of copper electropolishing in phosphoric acid solution. Int. J. Electrochem. Sci. 2013, 8, 1041–1046. [Google Scholar]
- Han, G.H.; Gunes, F.; Bae, J.J.; Kim, E.S.; Chae, S.J.; Shin, H.-J.; Choi, J.-Y.; Pribat, D.; Lee, Y.H. Influence of copper morphology in forming nucleation seeds for graphene growth. Nano Lett. 2011, 11, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.A. On the anodic behavior of copper in aqueous solutions of orthophosphoric acid. J. Electrochem. Soc. 1936, 69, 629–655. [Google Scholar] [CrossRef]
- Jacquet, P.A. Electrolytic method for obtaining bright copper surfaces. Nature 1935, 135, 1076. [Google Scholar] [CrossRef]
- Logie, S.; Chick, J.; Campbell, M.; Bilbao, S. The influence of bore profile on slurred transients in brass instruments. In Proceedings of the Forum Acusticum 2011, Aalborg, Denmark, 26 June–1 July 2011.
- Du, B.; Suni, I.L. Mechanistic studies of cu electropolishing in phosphoric acid electrolytes. J. Electrochem. Soc. 2004, 151, 375–378. [Google Scholar] [CrossRef]
- Huo, J.; Solanki, R.; McAndrew, J. Study of anodic layers and their effects on electropolishing of bulk and electroplated films of copper. J. Appl. Electrochem. 2004, 34, 305–314. [Google Scholar] [CrossRef]
- Glarum, S.H.; Marshall, J.H. The anodic dissolution of copper into phosphoric acid. J. Electrochem. Soc. 1985, 132, 2872–2878. [Google Scholar] [CrossRef]
- West, A.C.; Deligianni, H.; Andricacos, P.C. Electrochemical planarization of interconnect metallization. IBM J. Res. Dev. 2005, 49, 37–48. [Google Scholar] [CrossRef]
- Gentile, M.; Koroleva, E.V.; Skeldom, P.; Thompson, G.E.; Bailey, P.; Noakes, T.C.Q. Influence of pre-treatment on the surface composition of Al-Zn alloys. Corros. Sci. 2010, 52, 688–694. [Google Scholar] [CrossRef]
- Huang, C.A.; Chang, J.H.; Zhao, W.J.; Huang, S.Y. Examination of the electropolishing behaviour of 73 brass in a 70% H3PO4 solution using a rotating disc electrode. Mater. Chem. Phys. 2014, 146, 230–239. [Google Scholar] [CrossRef]
- Pérez, T.; Nava, J.L. Simulation of turbulent flow of a rotating cylinder electrode. Influence of using plates and concentric cylinder as counter electrodes. Int. J. Electrochem. Soc. 2013, 8, 4690–4699. [Google Scholar]
- Datta, M.; Landolt, D. On the role of mass transport in high rate dissolution of iron and nickel in ECM electrolytes—I. Chloride solutions. Electrochim. Acta 1980, 25, 1255–1262. [Google Scholar] [CrossRef]
- Datta, M.; Landolt, D. On the role of mass transport in high rate dissolution of iron and nickel in ECM electrolytes—II. Chlorate and nitrate solutions. Electrochim. Acta 1980, 25, 1263–1271. [Google Scholar] [CrossRef]
- Datta, M.; Vega, L.F.; Romankiw, L.T.; Duby, P. Mass transport effects during electropolishing of iron in phosphoric-sulfuric acid. Electrochim. Acta 1992, 37, 2469–2475. [Google Scholar] [CrossRef]
- Sun, M.T.; Huang, C.A.; Huang, S.Y. The rod-climbing phenomenon of the viscous film on the surface of brass cylinder during electropolishing in the aqueous phosphoric acids. In Proceedings of the 211th ECS Meeting, Chicago, IL, USA, 6–10 May 2007; p. 552.
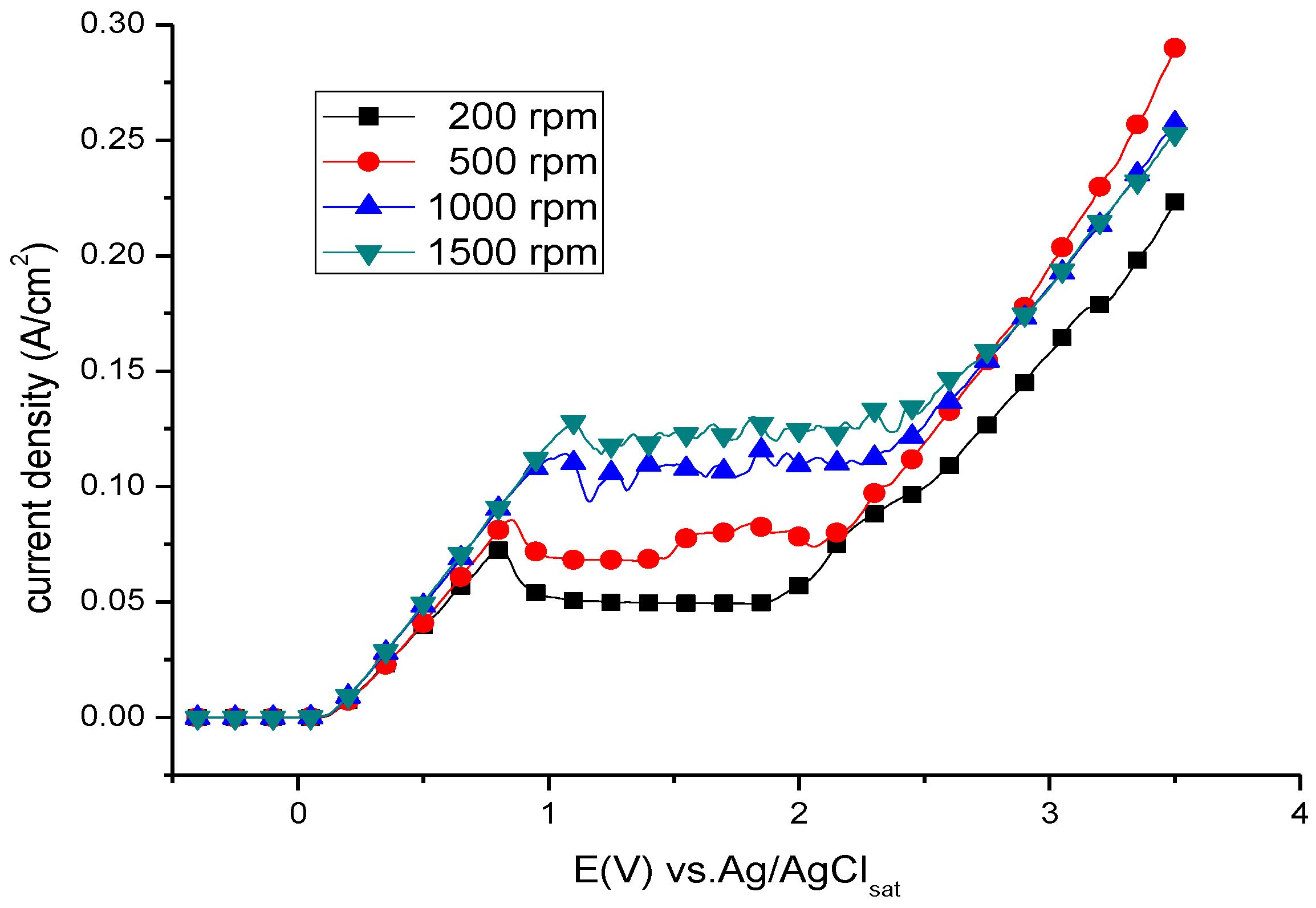


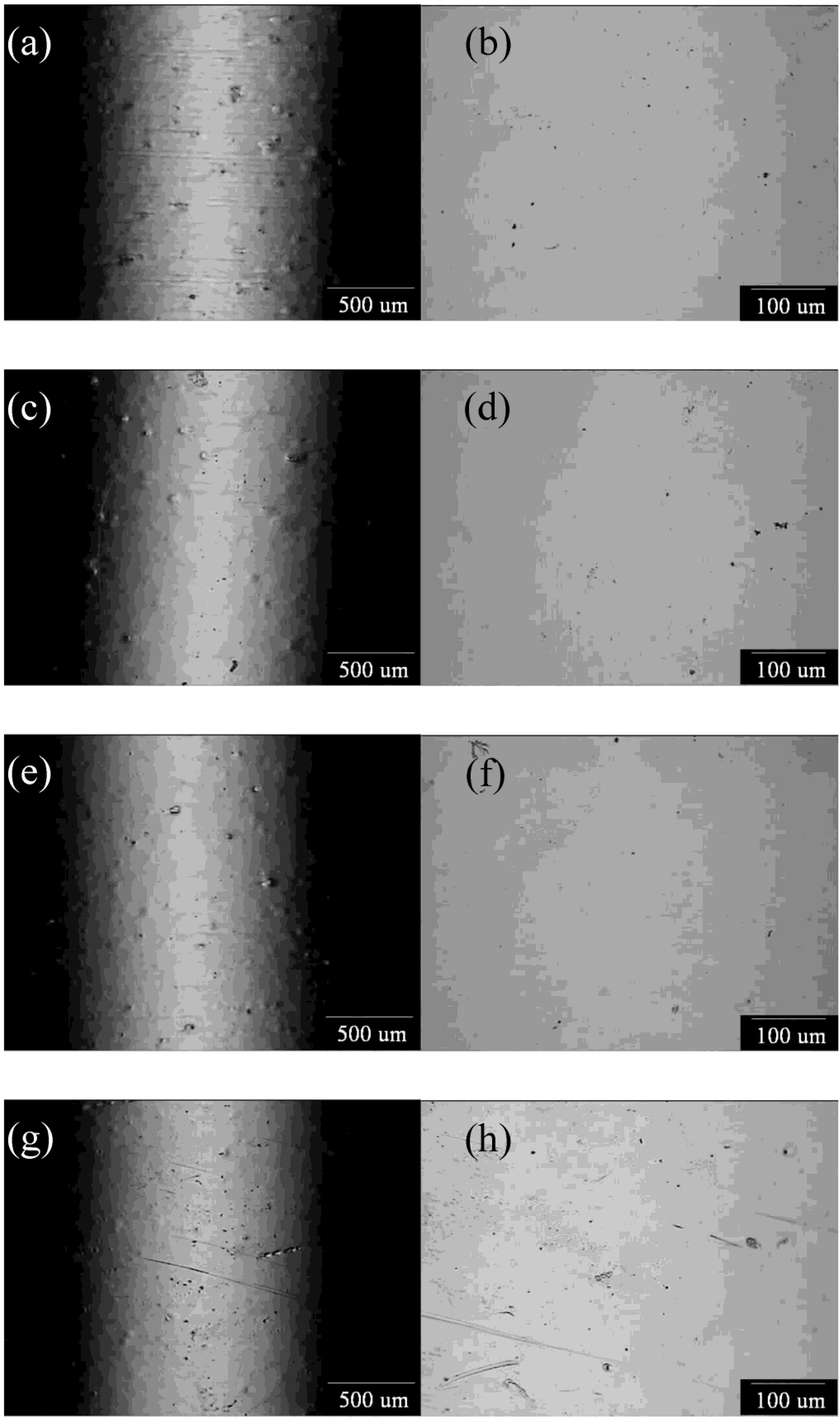
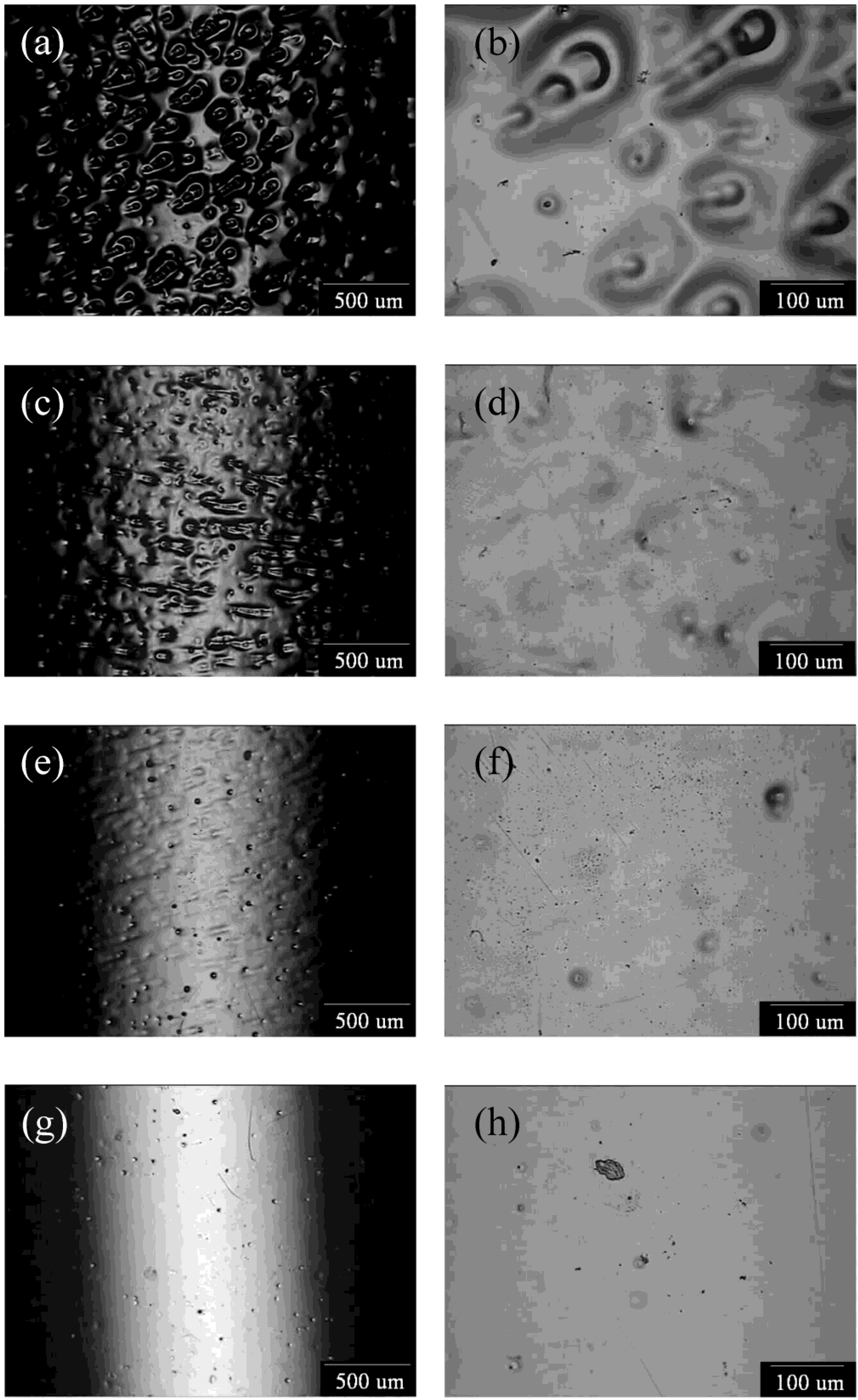
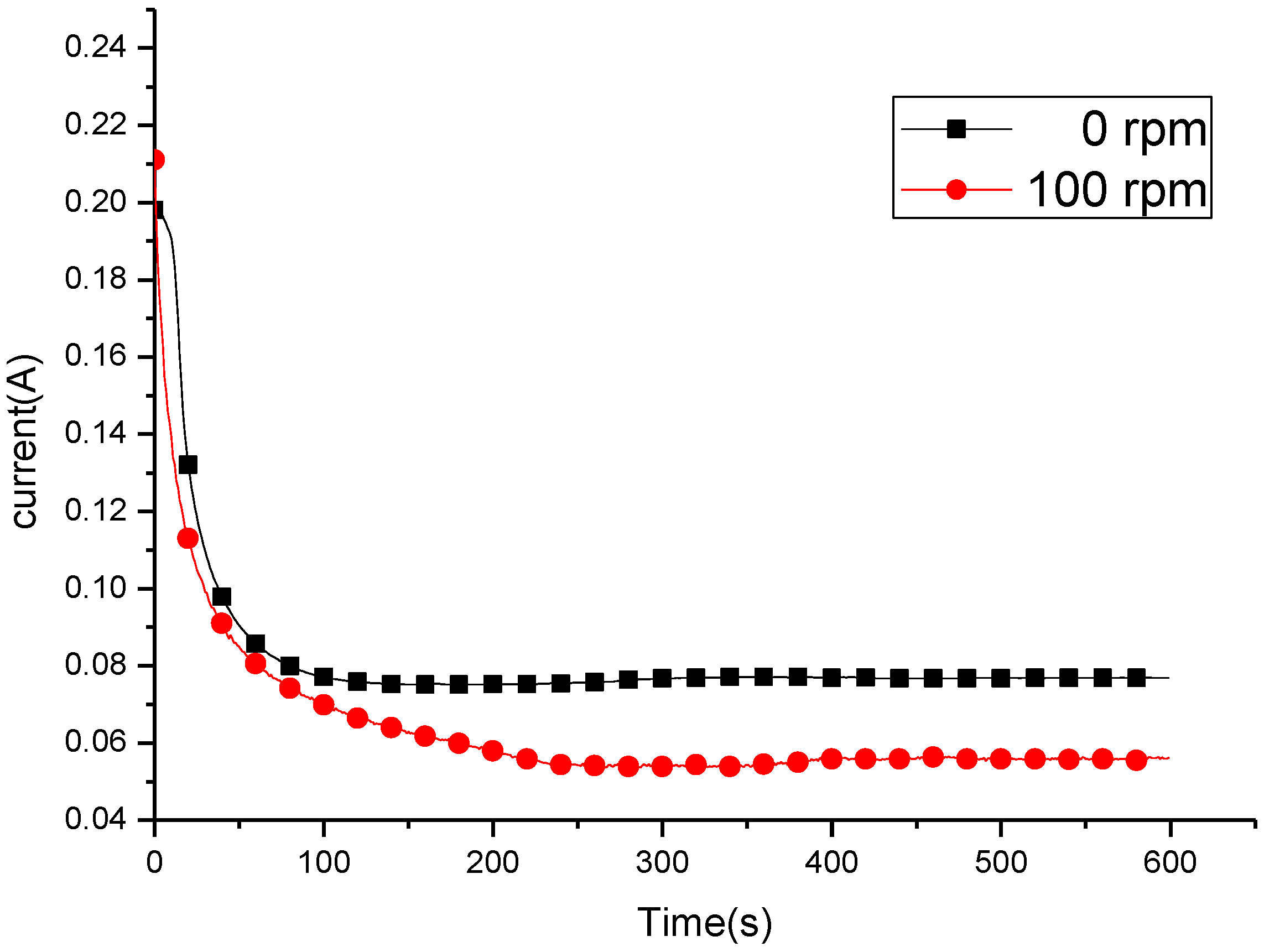


| Rotating Speed (rpm) | Il (A/cm2) | Ip (A/cm2) |
|---|---|---|
| 200 | 0.0494 | 0.0727 |
| 500 | 0.0738 | 0.0855 |
| 1000 | 0.1049 | 0.1142 |
| 1500 | 0.1208 | 0.1278 |
| Rotating Speed (rpm) | Vp (V) | Vs (V) | Vm (V) | Ve (V) |
|---|---|---|---|---|
| 200 | 0.82 | 1.26 | 1.54 | 1.84 |
| 500 | 0.85 | 0.97 | 1.51 | 2.04 |
| 1000 | 1.07 | 1.21 | 1.72 | 2.22 |
| 1500 | 1.1 | 1.22 | 1.72 | 2.22 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.A.; Chen, J.Y.; Sun, M.T. Electropolishing Behaviour of 73 Brass in a 70 vol % H3PO4 Solution by Using a Rotating Cylinder Electrode (RCE). Metals 2017, 7, 30. https://doi.org/10.3390/met7010030
Huang CA, Chen JY, Sun MT. Electropolishing Behaviour of 73 Brass in a 70 vol % H3PO4 Solution by Using a Rotating Cylinder Electrode (RCE). Metals. 2017; 7(1):30. https://doi.org/10.3390/met7010030
Chicago/Turabian StyleHuang, Ching An, Jhih You Chen, and Ming Tsung Sun. 2017. "Electropolishing Behaviour of 73 Brass in a 70 vol % H3PO4 Solution by Using a Rotating Cylinder Electrode (RCE)" Metals 7, no. 1: 30. https://doi.org/10.3390/met7010030





