2. Materials and Methods
Ni
15Co
15Mn
70, Ni
30Mn
70, and Co
30Mn
70 (at %) ingots were prepared by melting pure Ni, Co, and Mn (purity >99.9 at %) using an Ar-protected arc melting furnace. We had already fabricated NP-Ni in sheet form in a previous study [
12]. Briefly, after annealing Ni
30Mn
70 at 900 °C under Ar to provide microstructure and composition homogenization, the ingots were cold-rolled into ~50-μm-thick sheets at room temperature. For NP-NiCo and NP-Co, alloy ribbons of Ni
15Co
15Mn
70 and Co
30Mn
70 with thicknesses of ~25–30 μm were prepared using an induction melt spinning method. These alloy sheets/ribbons were dealloyed in a 1 M (NH
4)
2SO
4 solution at 50 °C for 5 h. After dealloying, the samples were rinsed thoroughly with water and ethanol and dried under vacuum.
The as-made nanoporous samples were mechanically brittle, and the samples were gently crushed into a powder with particle diameters of 100–200 μm. The crushed samples (100 mg) were loaded into a 4-mm quartz tube and tested in a continuous-flow fixed-bed microreactor in a catalysis system (BELCAT, MicrotracBEL, Osaka, Japan) at atmospheric pressure. The amounts of H2, CH4, CO, and CO2 were monitored and evaluated with an on-line gas analyzer (BELMass, MicrotracBEL, Osaka, Japan), with a reactant gas containing 0.8% CH4, 0.8% CO2, and He for balance being introduced into the reactor at a flow rate of 10 cm3·min−1. The samples were ramped up to 650 °C at a rate of 2 °C·min−1.
The microstructures were characterized using a scanning electron microscope (SEM, JSM-6700, JEOL, Tokyo, Japan) and transmission electron microscope (JEM-2100F, JEOL, Tokyo, Japan) equipped with aberration correctors (CEOS GmbH, Heidelberg, Germany) for the image- and probe-forming lens systems, as well as an energy dispersive X-ray spectrometry (EDS) system (JED-2300T, JEOL, Tokyo, Japan) for chemical composition analysis. A probe convergence angle of 29 mrad and a high-angle annular dark-field (HAADF) detector with an inner angle of greater than 100 mrad were used for HAADF-scanning TEM (STEM). The samples were transferred onto a holey carbon grid on Cu mesh without a uniform carbon support film (JEOL catalog no. 7801 11613, Tokyo, Japan). SEM analysis was conducted at an accelerating voltage of 15.0 kV.
For in-situ TEM, we used a dedicated 1000 kV JEM-1000K RS TEM (JEOL, Tokyo, Japan) equipped with an environmental cell specially designed at Nagoya University, Japan [
13]. The samples were heated up to 600 °C under vacuum and observed in the presence of a CH
4 + CO
2 gas mixture (1 vol % CH
4, 1 vol % CO
2, and 98 vol % Ar) at 600 °C over a wide range of total pressures (1–30 Pa). A furnace-type heating holder (JEOL, EM-Z083171STHH, Tokyo, Japan) was used. It would be relevant to mention that due to the pressure dependence of DRM, using lower pressures results in higher conversion of CH
4 and CO
2 [
14]. The electron beam current flux was 0.23 A·cm
−2, as measured with a Faraday gauge. The nanostructure was very stable under electron beam irradiation both under vacuum and in a pure Ar atmosphere. Detectable changes in the structure were vividly observed from the start of catalysis when the reaction gases were delivered into the environmental cell. In addition, DRM is an endothermic reaction. Therefore, when the sample stage in the TEM is heated up, the sample temperature tended to decrease if we did not increase the output of the heating stage. These factors demonstrate that the self-transformation of the nanostructure was mainly driven by the catalytic reaction.
3. Results and Discussion
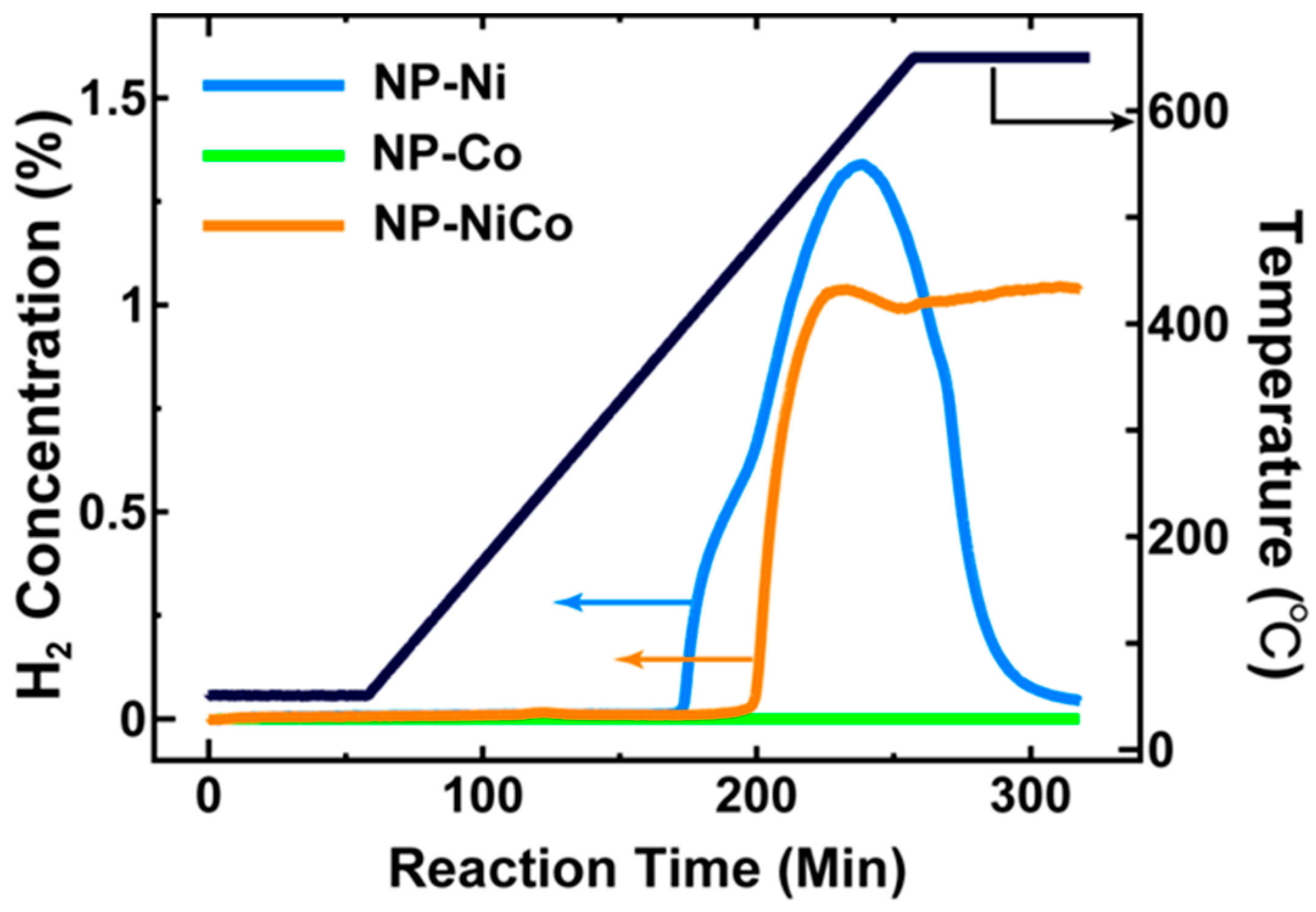
The evolution of H
2 by the three samples (NP-Ni, NP-NiCo, and NP-Co) and the change in temperature over time are shown in
Figure 1. NP-Ni showed a rapid generation of H
2 at ~500 °C, but this began to drop rapidly at 550 °C, indicating poor thermal stability. Unexpectedly, NP-Co showed inert activity, suggesting a possible oxidation of the metal [
15] and reduced activity of Co compared to Ni [
16]. Interestingly, NP-NiCo showed moderate thermal stability and maintained activity up to 650 °C, whereas NP-Ni was unstable.
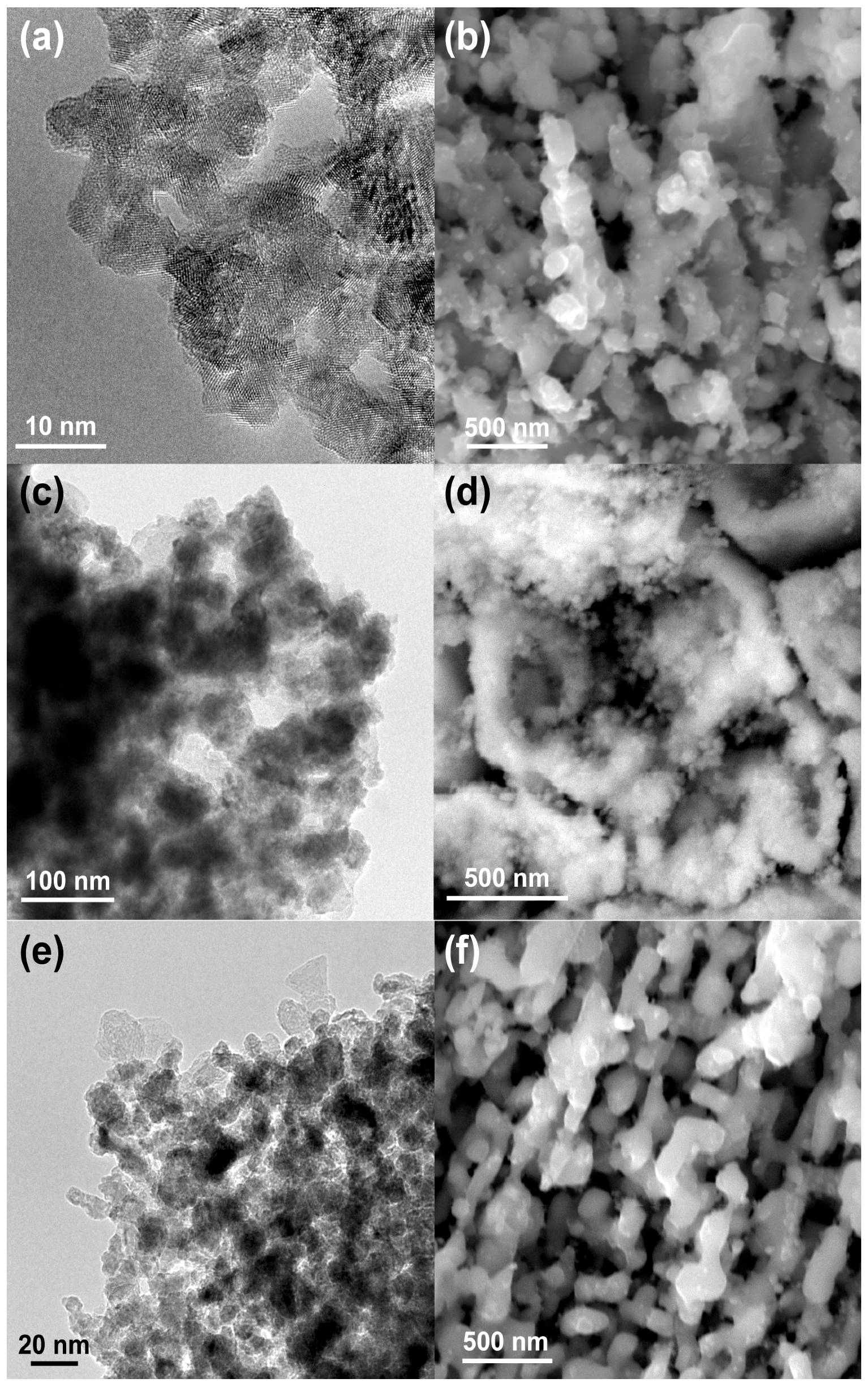
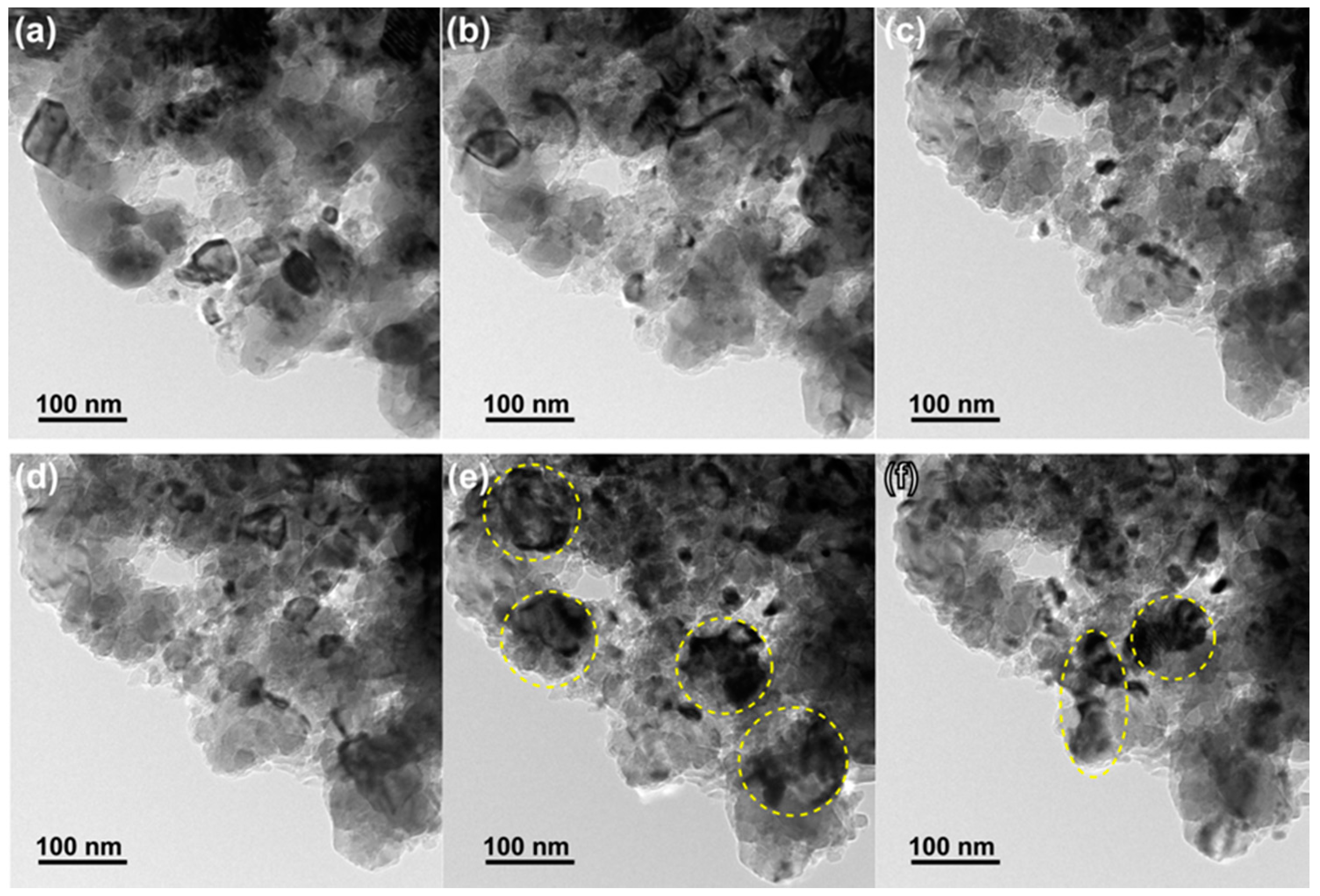
The microstructures of the three samples before and after the catalysis tests are shown in
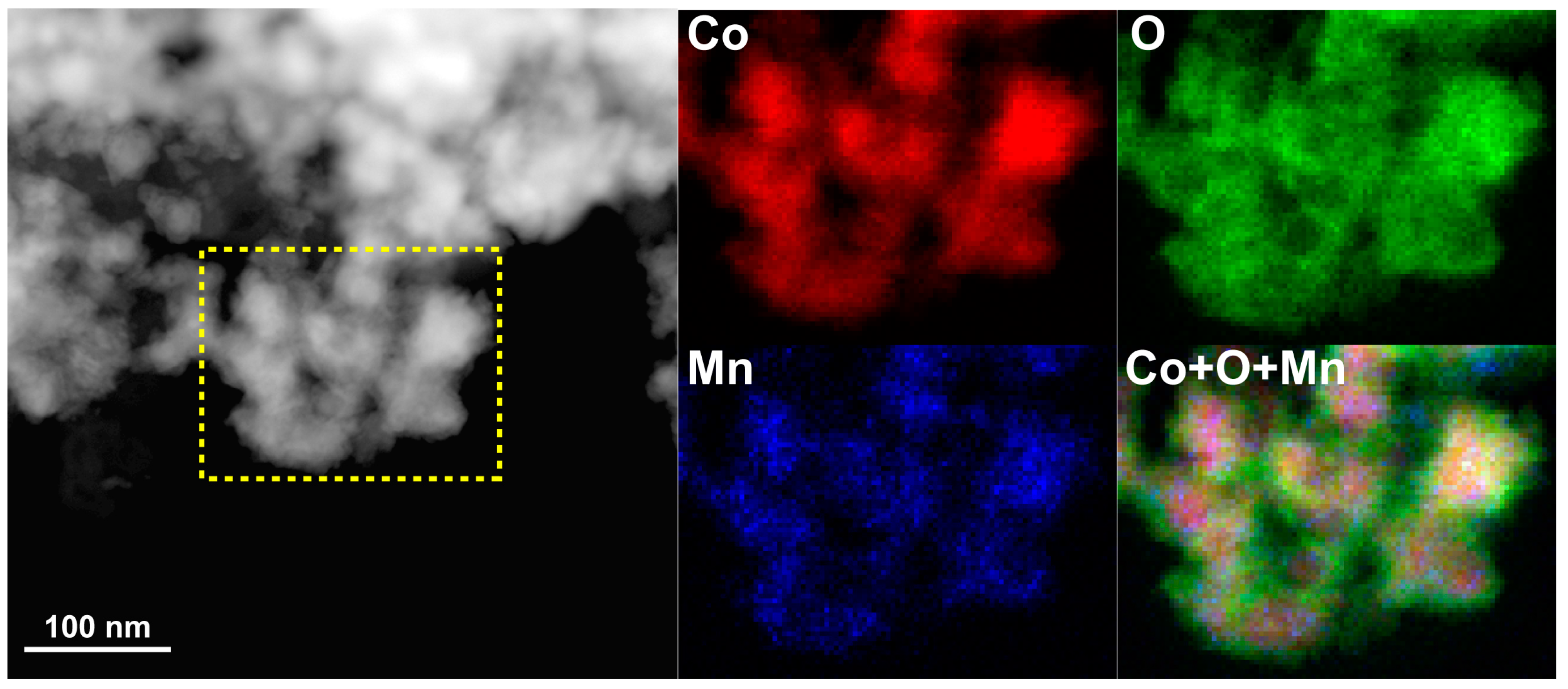
Figure 2. The three samples initially possessed fine pore/ligament microstructures (20–50 nm), but after heat treatment they showed significant pore/ligament coarsening, and the sizes of the pores/ligaments of the three samples after the tests were all 100–200 nm. It is noteworthy that the as-fabricated NP-Co was oxidized according to STEM-EDS mapping (
Figure A1), and the surface of this material was not catalytically active during the reaction. Therefore, surface oxides may hinder the uniform ligament coarsening and cause a bi-modal grain size distribution, as shown in
Figure 2d.
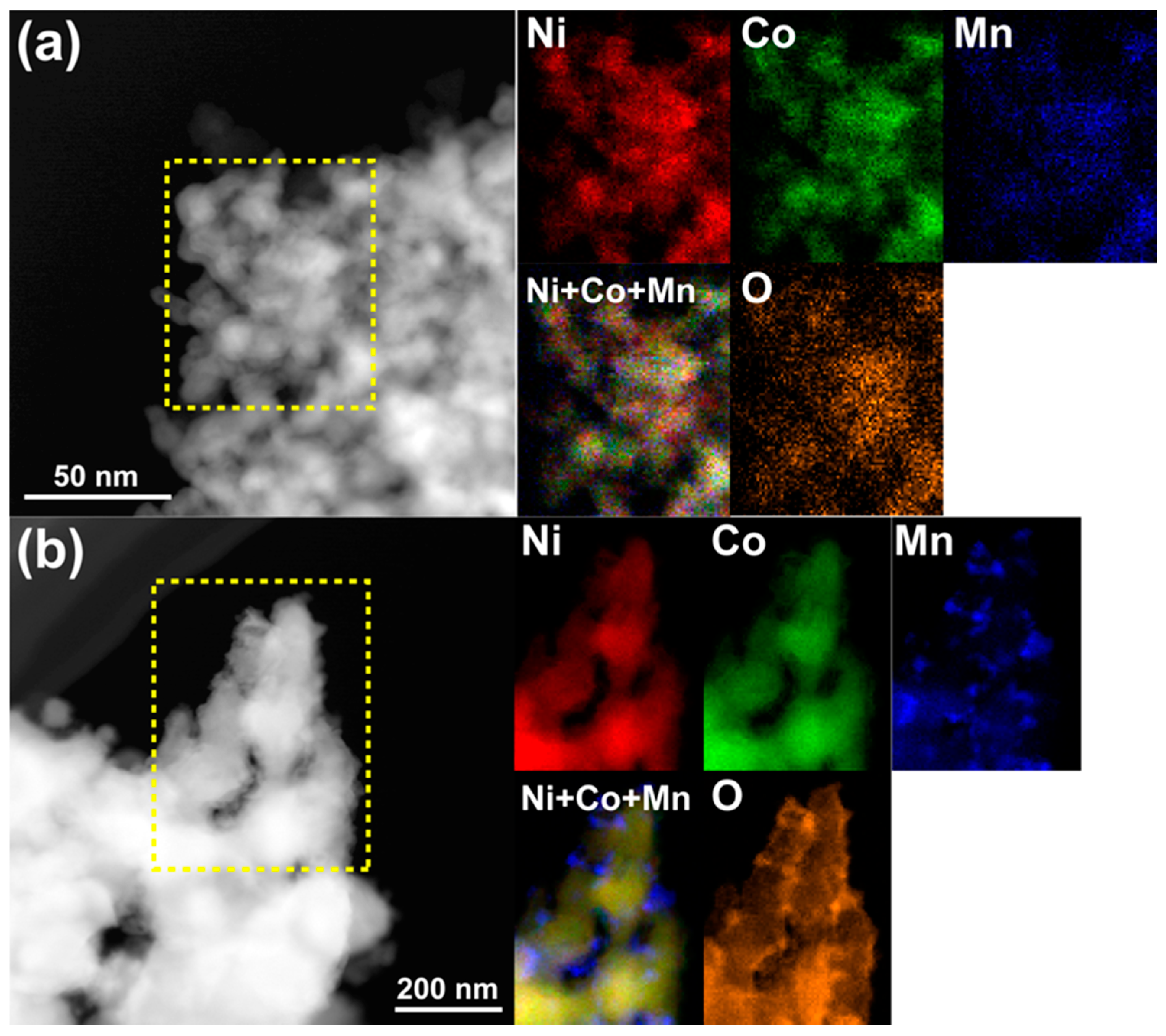
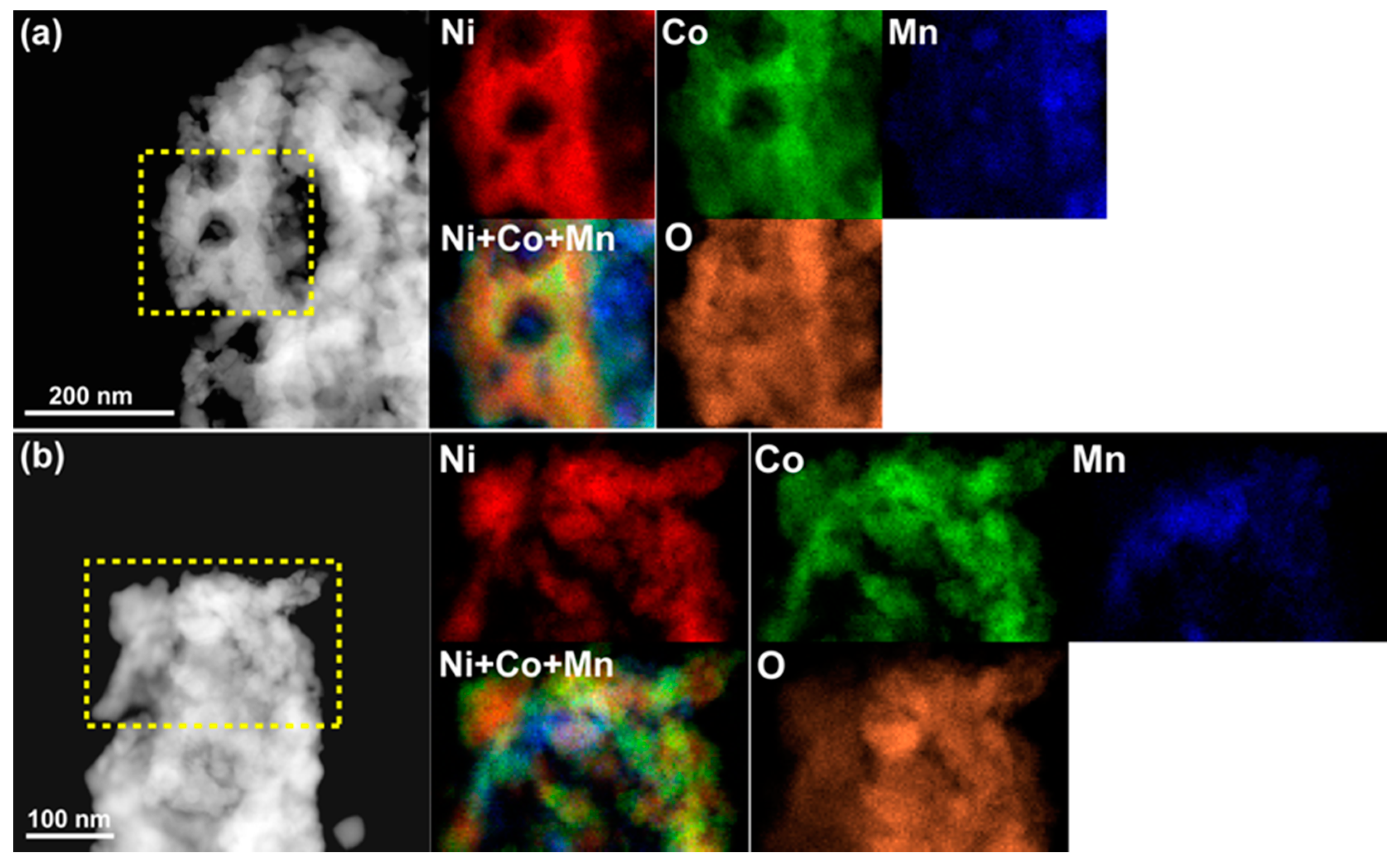
Figure 3 displays the STEM-EDS maps for Ni, Co, Mn, and O in NP-NiCo before and after the catalysis test. Before the test, the four elements were distributed uniformly and well mixed at the nanoscale, but after the test the residual Mn had been converted to Mn oxides, which are not active materials in DRM. These oxides were distributed on uniform NiCo ligaments that displayed almost perfect yellow in the color-mixing map of Ni and Co, regardless of the oxygen distribution. The NiCo ligaments were also oxidized, as shown by the oxygen map. It is worth noting that static observations alone cannot explain why NP-NiCo, which has a similar pore/ligament size to the other materials, possessed improved activity and thermal stability, as indicated by the catalysis test (
Figure 1).
In order to know the origin of the moderate activity and thermal stability of NP-NiCo, we performed an in-situ TEM study of NP-NiCo during DRM. The changes in the microstructure over time are shown in
Figure 4. When the temperature was increased up to 600 °C under vacuum, the pore/ligaments coarsened, as expected (
Figure 4a). More importantly, the grains in the ligaments became finer when the mixture gas (CH
4 + CO
2) was introduced into the TEM chamber (
Figure 4b,c). After this, the mixture gas was evacuated, and the grains tended to merge and form larger grains over a short amount of time, judging from the diffracted contrasts, meaning that the fine features of the demixed elements were thermodynamically unstable. We cooled down the sample within ~5 min, and then performed STEM-EDS analysis of the sample, as shown in
Figure 5. The chemical demixing of Ni and Co can be clearly observed regardless of surface oxidation, in addition to the dispersion of MnO
2. This chemical analysis suggests that the DRM reaction induced chemical demixing accompanied by the fine grains of Ni and Co working as additional active sites at high temperatures. No carbon deposition was observed in the throughout in-situ observation. However, it should be noted that these unique features could only be observed by in-situ TEM, because the catalysis test usually requires ~30 min to cool down to room temperature in order to protect the reaction system as rapid cooling may break the quartz tube and then expose the residual CO to air. Therefore, chemical demixing was not observed after the conventional catalysis test (
Figure 3). This chemical demixing and grain refinement could also imply “synergic effects” similar to those seen in previous studies, which have shown higher catalytic activity and durability of bimetallic Ni–Co compared to Ni [
17,
18].
The chemical demixing may originate from the different reaction affinities of the metal and reactant species at high energy states. For example, solid-solution Pd-Co alloys show chemical demixing of Pd and Co by microwave H
2 irradiation [
19], indicating the different elemental interactions with H
2. Similarly, Ni and Co have different affinities to DRM: Ni is active but Co is inert; thus, this situation promotes the elemental separation, resulting in grain refinement.
Acknowledgments
This study was mainly supported by the JST-CREST program “Innovative catalysts and creation technologies for the utilization of diverse natural carbon resources” (grant No. JPMJCR15P1) and research funds provided by the “World Premier International (WPI) Research Center Initiative for Atoms, Molecules and Materials”, MEXT (Japan). It was also partially supported by KAKENHI (grant No. JP16H02293). We also acknowledge the support from the Advanced Characterization Nanotechnology Platform of the High Voltage Electron Microscope Laboratory at Nagoya University.
Author Contributions
Takeshi Fujita fabricated samples, conducted TEM/STEM characterizations, and performed catalysis test. Takeshi Fujita, Kimitaka Higuchi, Yuta Yamamoto, Tomoharu Tokunaga, Shigeo Arai and Hideki Abe performed in-situ TEM observations. Takeshi Fujita conducted the study design, data analysis, and data interpretation. Takeshi Fujita wrote the paper. Takeshi Fujita and Hideki Abe contributed to this work through the direction of the research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Muraza, O.; Galadima, A. A review on coke management during dry reforming of methane. Int. J. Energy Res. 2015, 39, 1196–1216. [Google Scholar] [CrossRef]
- Nair, M.M.; Kaliaguine, S. Structured catalysts for dry reforming of methane. New J. Chem. 2016, 40, 4049–4060. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, M.W. Nanoporous metals for catalytic and optical applications. MRS Bull. 2009, 34, 569–576. [Google Scholar] [CrossRef]
- Qiu, H.J.; Li, X.; Xu, H.T.; Zhang, H.J.; Wang, Y. Nanoporous metal as a platform for electrochemical and optical sensing. J. Mater. Chem. C 2014, 2, 9788–9799. [Google Scholar] [CrossRef]
- Qiu, H.J.; Xu, H.T.; Liu, L.; Wang, Y. Correlation of the structure and applications of dealloyed nanoporous metals in catalysis and energy conversion/storage. Nanoscale 2015, 7, 386–400. [Google Scholar] [CrossRef] [PubMed]
- Juarez, T.; Biener, J.; Weissmüller, J.; Hodge, A.M.C. Nanoporous metals with structural hierarchy: A review. Adv. Eng. Mater. 2017, in press. [Google Scholar] [CrossRef]
- Fujita, T. Hierarchical nanoporous metals as a path toward the ultimate three-dimensional functionality. Sci. Technol. Adv. Mater. 2017, in press. [Google Scholar]
- Biener, J.; Biener, M.M.; Madix, R.J.; Friend, C.M. Nanoporous gold: Understanding the origin of the reactivity of a 21st century catalyst made by pre-columbian technology. ACS Catal. 2015, 5, 6263–6270. [Google Scholar] [CrossRef]
- Fujita, T.; Tokunaga, T.; Zhang, L.; Li, D.W.; Chen, L.Y.; Arai, S.; Yamamoto, Y.; Hirata, A.; Tanaka, N.; Ding, Y.; et al. Atomic observation of catalysis-induced nanopore coarsening of nanoporous gold. Nano Lett. 2014, 14, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Abe, H.; Tanabe, T.; Ito, Y.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Hirata, A.; Chen, M.W. Earth-abundant and durable nanoporous catalyst for exhaust-gas conversion. Adv. Funct. Mater. 2016, 26, 1609–1616. [Google Scholar] [CrossRef]
- Qiu, H.J.; Kang, J.L.; Liu, P.; Hirata, A.; Fujita, T.; Chen, M.W. Fabrication of large-scale nanoporous nickel with a tunable pore size for energy storage. J. Power Sources 2014, 247, 896–905. [Google Scholar] [CrossRef]
- Tanaka, N.; Usukura, J.; Kusunoki, M.; Saito, Y.; Sasaki, K.; Tanji, T.; Muto, S.; Arai, S. Development of an environmental high-voltage electron microscope for reaction science. Microscopy 2013, 62, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Oyama, S.T.; Hacarlioglu, P.; Gu, Y.; Lee, D. Dry reforming of methane has no future for hydrogen production: Comparison with steam reforming at high pressure in standard and membrane reactors. Int. J. Hydrogen Energy 2012, 37, 10444–10450. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Wang, H.Y. Carbon deposition and catalytic deactivation during CO2 reforming of CH4 over CO2/γ-Al2O3 catalysts. J. Catal. 2002, 205, 289–293. [Google Scholar] [CrossRef]
- Xu, J.K.; Zhou, W.; Li, Z.J.; Wang, J.H.; Ma, J.X. Biogas reforming for hydrogen production over nickel and cobalt bimetallic catalysts. Int. J. Hydrogen Energy 2009, 34, 6646–6654. [Google Scholar] [CrossRef]
- Ay, H.; Uner, D. Dry reforming of methane over CeO2 supported Ni, Co and Ni-Co catalysts. Appl. Catal. B Environ. 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Li, L.D.; Anjum, D.H.; Zhu, H.B.; Saih, Y.; Laveille, P.V.; D’Souza, L.; Basset, J.M. Synergetic effects leading to coke-resistant NiCo bimetallic catalysts for dry reforming of methane. ChemCatChem 2015, 7, 427–433. [Google Scholar] [CrossRef]
- Tokunaga, T.; Hayashi, Y.; Fujita, T.; Silva, S.R.P.; Amaratunga, G.A.J. Demixing of solid-soluted Co-Pd binary alloy induced by microwave plasma hydrogen irradiation technique. Jpn. J. Appl. Phys. 2006, 45, L860–L863. [Google Scholar] [CrossRef]
Figure 1.
Concentrations of H2 in outlet gases for NP-Ni, NP-Co, and NP-NiCo samples, as well as the change in temperature over time.
Figure 2.
Microstructure observations taken by SEM or TEM before (a,c,e) and after (b,d,f) the catalysis tests; (a,b) NP-Ni; (c,d) NP-Co; and (e,f) NP-NiCo.
Figure 3.
STEM images and energy dispersive X-ray spectrometry (EDS) chemical maps of the highlighted areas showing the distributions of Ni (red), Co (green), Mn (blue), and O (orange) from Ni-K, Co-K, Mn-K, and O-K, respectively, in NP-NiCo (a) before the catalysis test and (b) after the test. Combined images of Ni, Co, and Mn are also shown.
Figure 4.
Microstructure evolution as observed by in-situ TEM. Images taken: (a) after temperature reached 600 °C under vacuum; (b,c) after 5 and 10 min of exposure to the gas mixture, respectively; (d) just after evacuation of the gas mixture; (e,f) during cooling to room temperature under vacuum. The selected areas indicate coarsened grains during cooling.
Figure 5.
STEM images and EDS chemical maps of NP-NiCo after the in-situ TEM study. The selected areas in two different places (a,b) show the heterogeneous distributions of Ni (red), Co (green), Mn (blue), and O (orange) from Ni-K, Co-K, Mn-K, and O-K, respectively.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).