1. Introduction
Magnesium and its alloys are modern lightweight materials applicable in a wide range of industrial fields from aerospace and automotive to biomedical applications. Its main advantages are a good strength to weight ratio and biocompatibility in combination with biodegradability. However, due to the high reactivity of pure Mg and the mechanical properties, not really sufficient for engineering applications, mainly magnesium alloys are used [
1,
2,
3,
4].
Good mechanical properties of magnesium and its alloys can be furthermore significantly upgraded by decreasing the grain size, nowadays performed mainly via severe plastic deformation (SPD) techniques [
3,
5], powder metallurgy (PM) processing [
1,
2,
6,
7] or by a combination of both methods. Decreasing the metallic grain size within the material volume, either by alloying elements, SPD methods, such as extrusion, equal channel angular pressing (ECAP) and high-pressure torsion (HPT) or PM, leads to increase of hardness, tensile and yield strength, but the plasticity of the material was shown to be decreased [
6,
7,
8,
9,
10].
PM processing of magnesium is influenced by its high affinity to oxygen, which results in the formation of a thermodynamically-stable layer of corrosion products on the magnesium powder particles’ surface. The created layer dramatically inhibits the diffusion processes required for material densification during PM processing [
4]. Because of the high affinity of magnesium to oxygen, a protective atmosphere (usually argon or nitrogen) has to be used for handling of magnesium powders and specimens, as well as for subsequent sintering [
4].
Applying high pressures at elevated temperatures (hot pressing) to process magnesium results in high plastic deformation of powder particles. Powder particles’ deformation leads to cracking of the layer of corrosion products normally present on the powder particles’ surface [
11,
12,
13]. The applied plastic deformation results in an increase of the contact area of powder particles and, in combination with the applied temperature, enhances the diffusion processes. Increasing compacting pressures usually also lead to a decrease in the porosity of the processed bulk material [
11,
12,
13]. The porosity of the processed bulk material is usually considered as a disadvantage of the PM processing techniques. However, the porous biocompatible material can be incorporated well into the tissue and can degrade at a specific rate, which provides a tool for the tailoring properties of PM processed materials for biomedical applications [
14,
15]. The functional porosity of a PM processed magnesium-based implant would support the primary fixation and the degradation of the implant by enabling the ingrowth of bone cells (osteointegration) into the degrading implant. Furthermore, the corrosion products of magnesium, created during implant biodegradation, support osteoconductivity of the bone [
4].
Changes in porosity also have a significant effect on corrosion resistance and corrosion attack progression within the material volume. Highly porous materials corrode very rapidly, as a larger area of the material surface is exposed to the corrosion environment. Corrosion resistance of either pure magnesium or magnesium alloys is seldom suitable for technical applications or even biomedical applications [
3,
9,
16,
17,
18,
19,
20]. Magnesium corrosion resistance can be improved by alloying the metal with aluminum, zinc or rare earth metal elements; however, for significantly better corrosion resistance, another way of reducing the degradation rate must be considered. Conversion coatings are widely studied as corrosion protection for magnesium and its alloys. Fluoride and calcium phosphate-based conversion coatings have a great potential of reducing the corrosion rate of biomedical magnesium implants [
1,
2,
3,
4,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24].
Electrochemical characterization of PM magnesium materials is commonly carried out either in NaCl solution or in Hank's Balanced Salt Solution (HBSS) or enriched HBSS [
21,
25,
26,
27,
28,
29]. Electrochemical impedance spectroscopy (EIS) measurements provide complex information of the material degradation characteristics during exposure in corrosion medium in time. From the chemical point of view, several specific reactions can occur in HBSS and enriched HBSS medium due to the additional content of several ions when compared to NaCl solution [
30].
The properties of pure magnesium materials prepared via PM methods are seldom studied [
15,
27,
31,
32,
33] or the studies refer to wrought pure magnesium materials [
5]. Most of the available studies characterize the PM processed pure magnesium and magnesium-based materials in terms of mechanical and corrosion properties; however, the analyses are focused on the influence of the material porosity due to its possible use for biomedical applications in the form of the porous implants.
Porous magnesium samples with different porosity (due to the addition of an ammonium bicarbonate powder) structures and mechanical properties were analyzed in [
31]. Magnesium powder samples with different porosities, due to the spacer addition, were prepared by uniaxial cold pressing (265 MPa) with additional heat treatment (for removal of hexane and sintering at 550 °C for 6 h). In the case of pure magnesium compacts, the porosity of 12 vol % corresponded to a flexural strength of 38 MPa. The increase of the compacts’ porosity was observed to be accompanied by the mechanical properties’ decrease (the porosity of 38 vol % corresponded to the flexural strength of 4.4 MPa). The negative influence of the porosity was observed also in the case of material immersion into 9 g/L NaCl solution.
The same authors studied also the influence of the purity of the argon atmosphere on final PM processed magnesium-based material properties in [
32], varying the sintering times as 0 h, 3 h, 6 h, 12 h and 24 h. While the samples prepared using technical Ar reached similar porosities in the sintering time range, the porosity of the samples prepared using gettered Ar was observed to decrease with increasing sintering time. Under a gettered Ar atmosphere, a prolonged sintering time enhanced diffusion connections between magnesium particles and improved the mechanical properties of the samples (flexural strength of 9 MPa for 3 h and 115.4 MPa for 24 h), whereas under a technical Ar atmosphere (flexural strength of 5 MPa for 3 h and 3.4 MPa for 24 h), oxidation at the particle surfaces caused deterioration in the mechanical properties of the samples.
Mechanical properties and corrosion resistance of cold pressed magnesium (310 MPa) and extruded at 420 °C employing an extrusion ratio of 16:1 were studied in [
24]. Processed material reached the ultimate tensile strength of 320 MPa, a yield stress of 280 MPa and 2% ductility. The reached values were higher when compared to a coarse-grained cast AZ31 magnesium alloy, except material ductility. PM processed pure magnesium mechanical properties were superior to those of cast pure magnesium as provided by the authors. Silane film and anticorrosive paint were shown to enhance the corrosion behavior of PM magnesium during the first hours of immersion, but their protection effectiveness completely disappears after two days. For longer immersion times, the fluoride conversion coating prepared in HF solution was shown to be an effective barrier to protect PM magnesium from degradation.
Pre-rolling of magnesium powder prior to spark plasma sintering and hot extrusion was used for material grain refinement in [
6]. The grain size obtained for the material extruded from the powders after 5 and 10 rolling pre-treatments decreased from 9.2 µm (only extruded Mg) to 2.9 µm and 2.1 µm, respectively. The grain refinement resulted in an ultimate tensile strength increase from 242 MPa for extruded pure magnesium to a value of 270.6 MPa for material extruded after sintering of 10 times pre-rolled powder. Yield stress was improved from 170 MPa to 206.4 MPa by the pre-rolling of powder.
The aim of this study is to evaluate the microstructural, mechanical and electrochemical properties of pure magnesium-based PM processed materials when varying the processing parameters. Materials were prepared via hot pressing at temperatures of 300 °C, 400 °C and 500 °C applying pressures of 100 MPa, 200 MPa, 300 MPa, 400 MPa and 500 MPa. For comparison, cold compacted materials prepared under the same compaction pressures were analyzed in the same manner. The mechanical properties of compacts were analyzed in terms of the three-point bending test containing fractographic analysis and with microhardness testing. Electrochemical corrosion characteristics of the processed materials in HBSS and enriched HBSS were analyzed using electrochemical impedance spectroscopy, and the data were extended by metallographic analysis of the corrosion attack within the material volume.
2. Materials and Methods
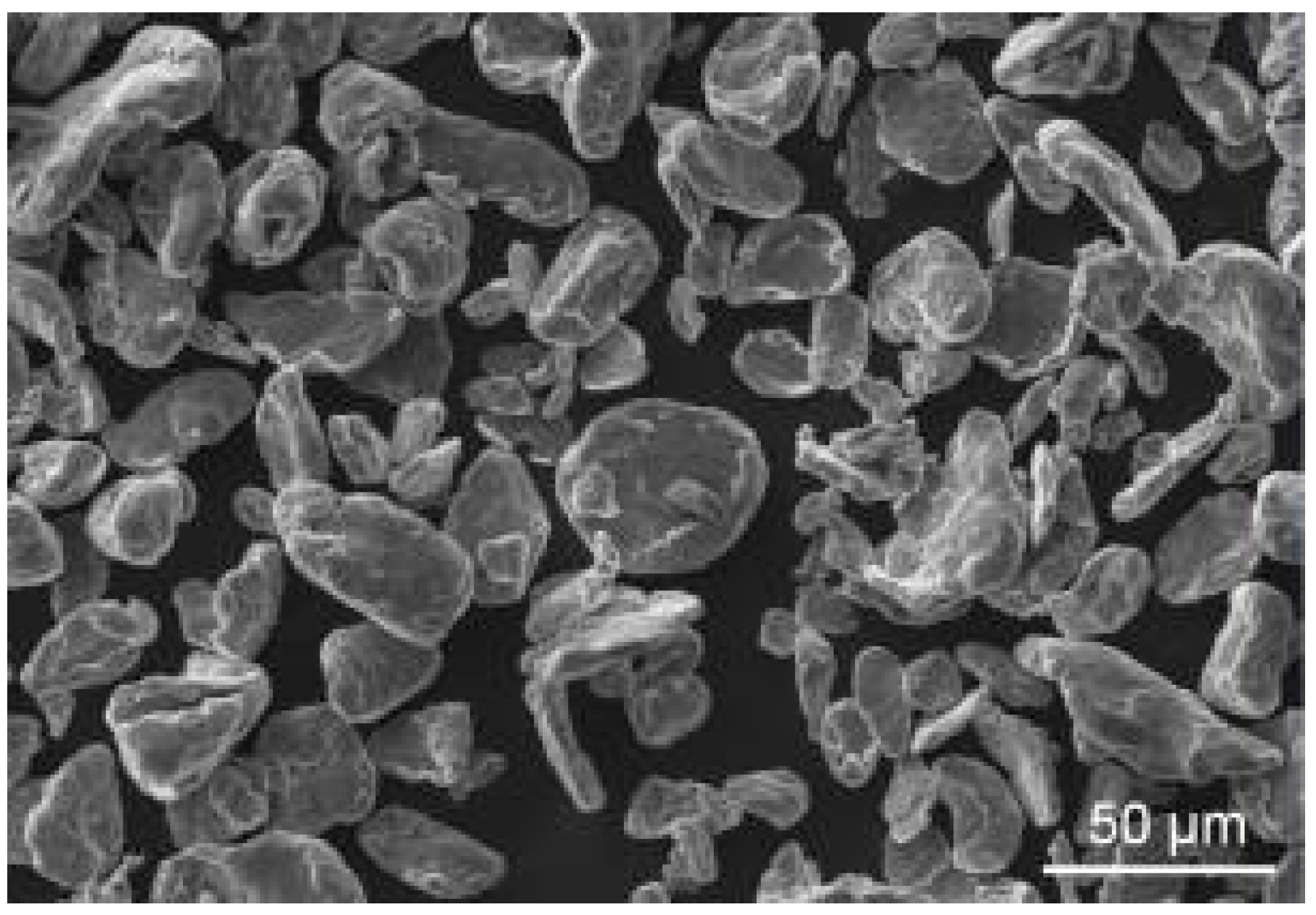
Magnesium powder used in this study (
Figure 1) was irregularly shaped with an average particle size of approximately 30 µm. The purity of the base material was 99.8% as declared by supplier Goodfellow (Huntingdon, UK); however, an oxide layer was found on the surface of powder particles using the scanning electron microscope (SEM, ZEISS EVO LS 10, Carl Zeiss Ltd., Cambridge, UK) using energy dispersive spectroscopy (EDS, Oxford Instruments plc, Abingdon, UK). This layer is to be expected on the surface of magnesium, and it was probably presented on the particles from the powder preparation.
For metallographic and electrochemical analysis, cylindrical compacts of 5 mm in height and 20 mm in diameter were prepared using a steel die. The preparation of the magnesium powder into the steel die for compaction was carried out under a nitrogen atmosphere to avoid further oxygen contamination. Magnesium powder (2.7 g) inserted into the steel die was compacted into compacts applying different uniaxial pressures; 100 MPa, 200 MPa, 300 MPa, 400 MPa and 500 MPa. Compaction was carried out using the Zwick Z250 Allround-Line machine (Zwick GmbH&Co. KG in Germany, Ulm, Germany) at room temperature and at elevated temperatures of 300 °C, 400 °C and 500 °C for one hour.
The microstructure of prepared samples was studied on cross-sectional cuts using SEM (ZEISS EVO LS 10) and a light optical microscope (LM, Zeiss Axio Observer Z1m, Carl Zeiss AG, Oberkochen, Germany). The internal structure of the compacts and the created grain size were analyzed with the electron backscattered diffraction (EBSD) technique (SEM, Tescan LYRA 3 XMU FEG/SEMxFIB, TESCAN Brno, s.r.o., Brno, Czech Republic). The step size of the method was 0.3 µm. Samples’ cross-sections intended for metallographic analysis were mold into a polymeric resin at room temperature, ground and polished according to the standard procedures of metallographic samples’ preparation. To reveal the microstructure, a 5% Nital etchant (5% nitric acid in ethanol) was used for several seconds. Compacts’ porosity was estimated from calculated density (calculated from sample dimensions and weight) considering a density of cast pure magnesium as the 0 porosity standard.
Microhardness testing was conducted on cross-sections of prepared compacts using the LECO AMH LM 248 microhardness tester (LECO Corporation, Saint Joseph, MI, USA) according to the ISO 6507-1 standard. For all the measurement, 25 g of load and a 10-s dwell time were used. The three-point bending test was carried out on samples with dimensions of 4 mm in height, 4 mm in width and 18 mm in length cut from the compacts, according to the ISO 7438 standard. The test machine used for the three-point bending test was the Zwick Z020 (Zwick GmbH&Co. KG in Germany, Ulm, Germany); the radius of the supports and the point was 2.5 mm; the distance between supports was 16 mm; and the velocity of applied loading was 1 mm/min. The three-point bending test was performed on one sample for each material preparation method.
Potentiostatic electrochemical impedance spectroscopy (EIS) was used as a method for the characterization of the prepared magnesium samples’ electrochemical corrosion characteristics. The three electrode cell system was used for the measurement with the Pt electrode as the counter electrode and the calomel electrode as the reference electrode, and the sample (1 cm
2 exposed area) served as the working electrode. With the aim to remove a layer of corrosion products created on the compacts’ surface, each sample was ground using 4000 SiC paper and rinsed with isopropanol just before the measurement. Measurements were carried out in HBSS and enriched HBSS (containing Mg
2+ and Ca
2+ ions). The composition of the used solutions is given in
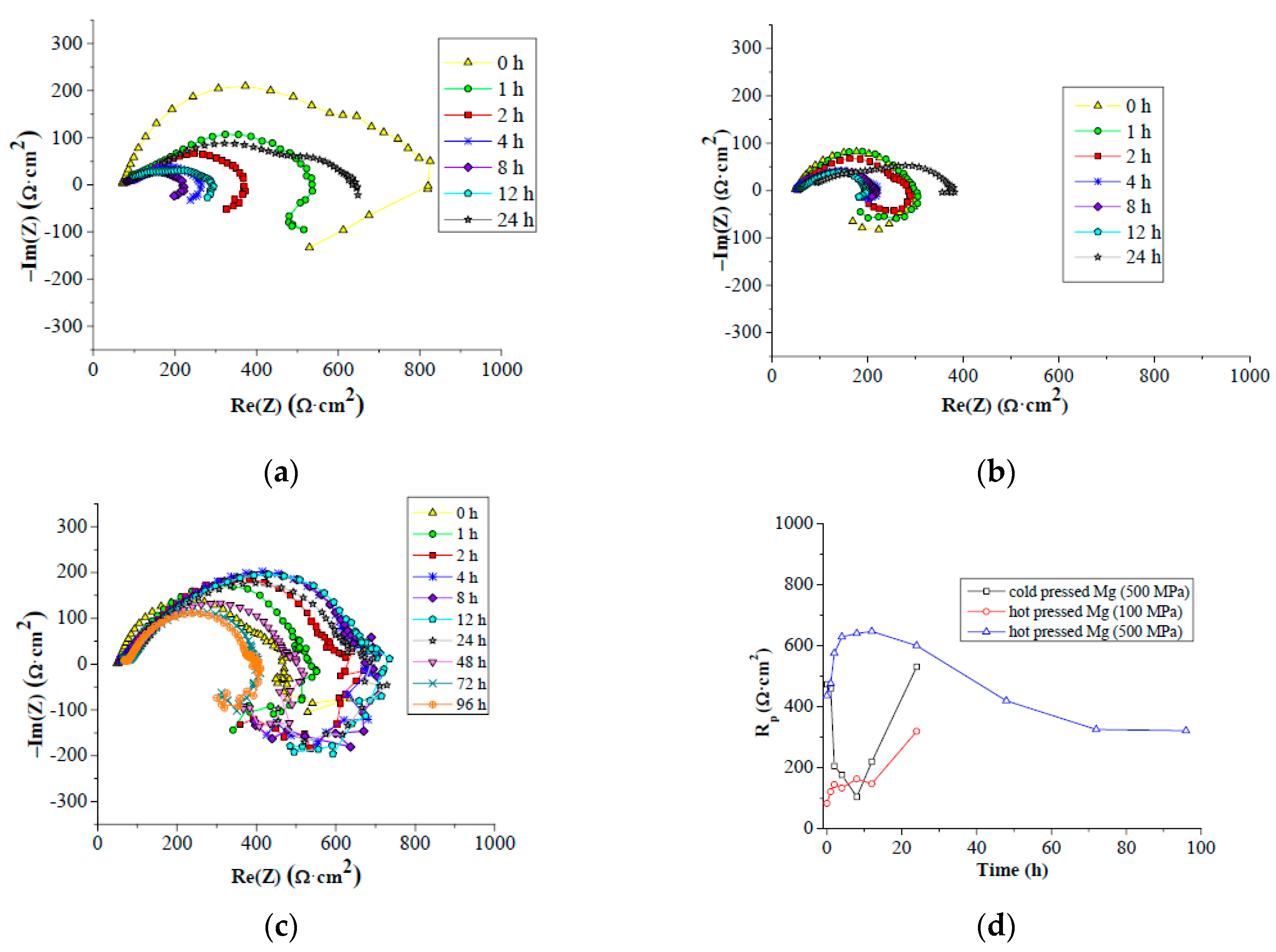
Table 1. The frequency used for the measurements was in the range from 100 kHz to 10 MHz, with the signal amplitude of 10 mV. All the measurements were carried out at laboratory temperature. EIS data were obtained after 5 min, 1 h, 2 h, 4 h, 8 h, 12 h, 24 h, 48 h, 72 h and 96 h of immersion. Each measurement time was 25.8 min. Due to the open porosity of the cold pressed samples, only cold pressed samples prepared under 500 MPa compacting pressure were used for the electrochemical corrosion characterization. In the case of hot pressing, samples prepared under 100 MPa and 500 MPa prepared at 400 °C were used for EIS measurements with the aim to reveal differences in material electrochemical corrosion behavior according to the processing conditions. Each material type was characterized by measurements performed on three samples.
4. Discussion
Microstructural, mechanical and electrochemical corrosion characteristics of pure magnesium materials prepared by powder metallurgy were analyzed with the aim to identify the influence of processing parameters. Sets of cold compacted and hot pressed materials (300 °C, 400 °C and 500 °C) were prepared from magnesium powder under 100 MPa, 200 MPa, 300 MPa, 400 MPa and 500 MPa compacting pressures. While the material has potential in biomedical applications, the electrochemical corrosion characteristics of the processed materials were analyzed in HBSS and enriched HBSS using electrochemical impedance spectroscopy, and the data were extended with the analysis of the corrosion attack within the material volume.
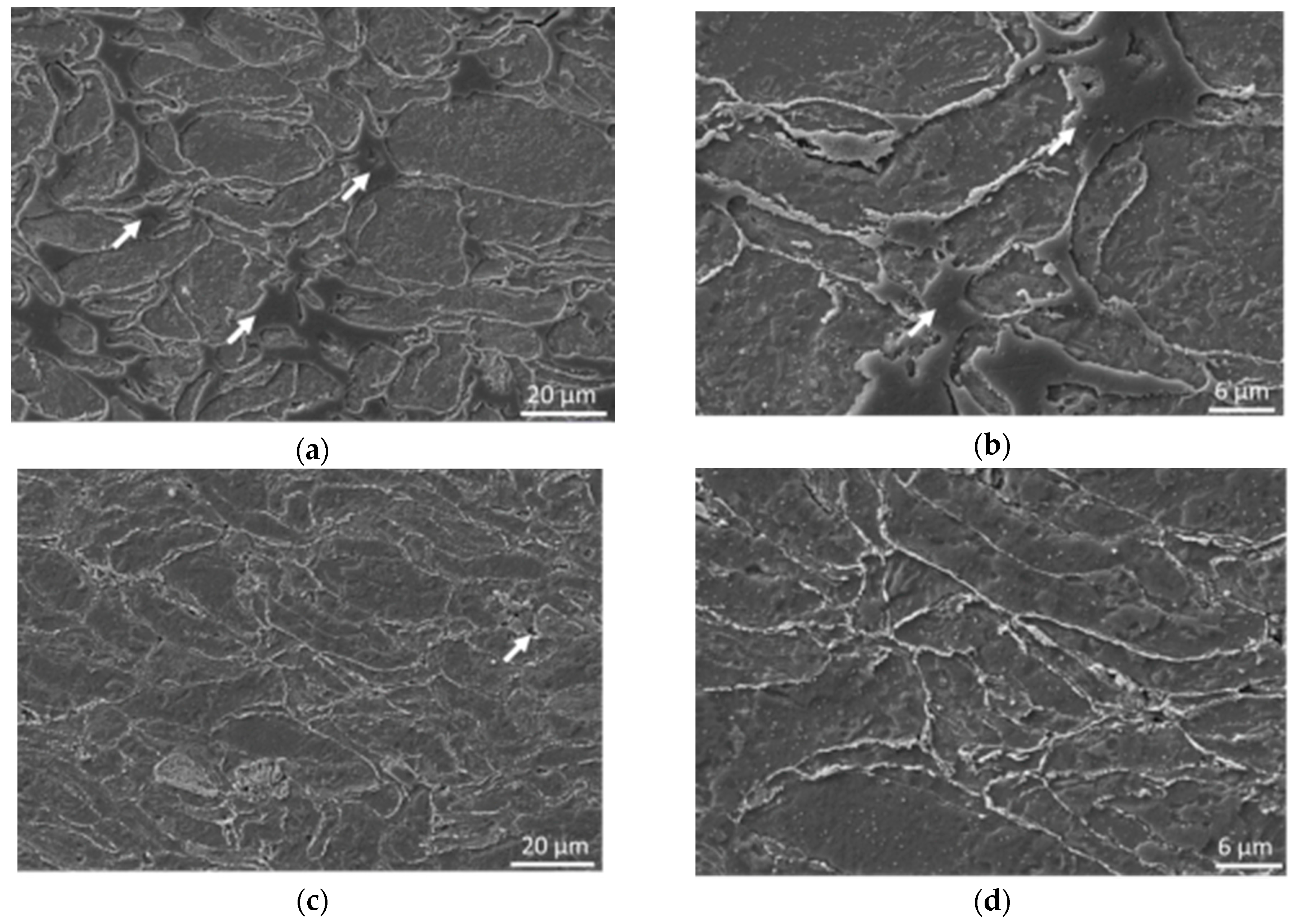
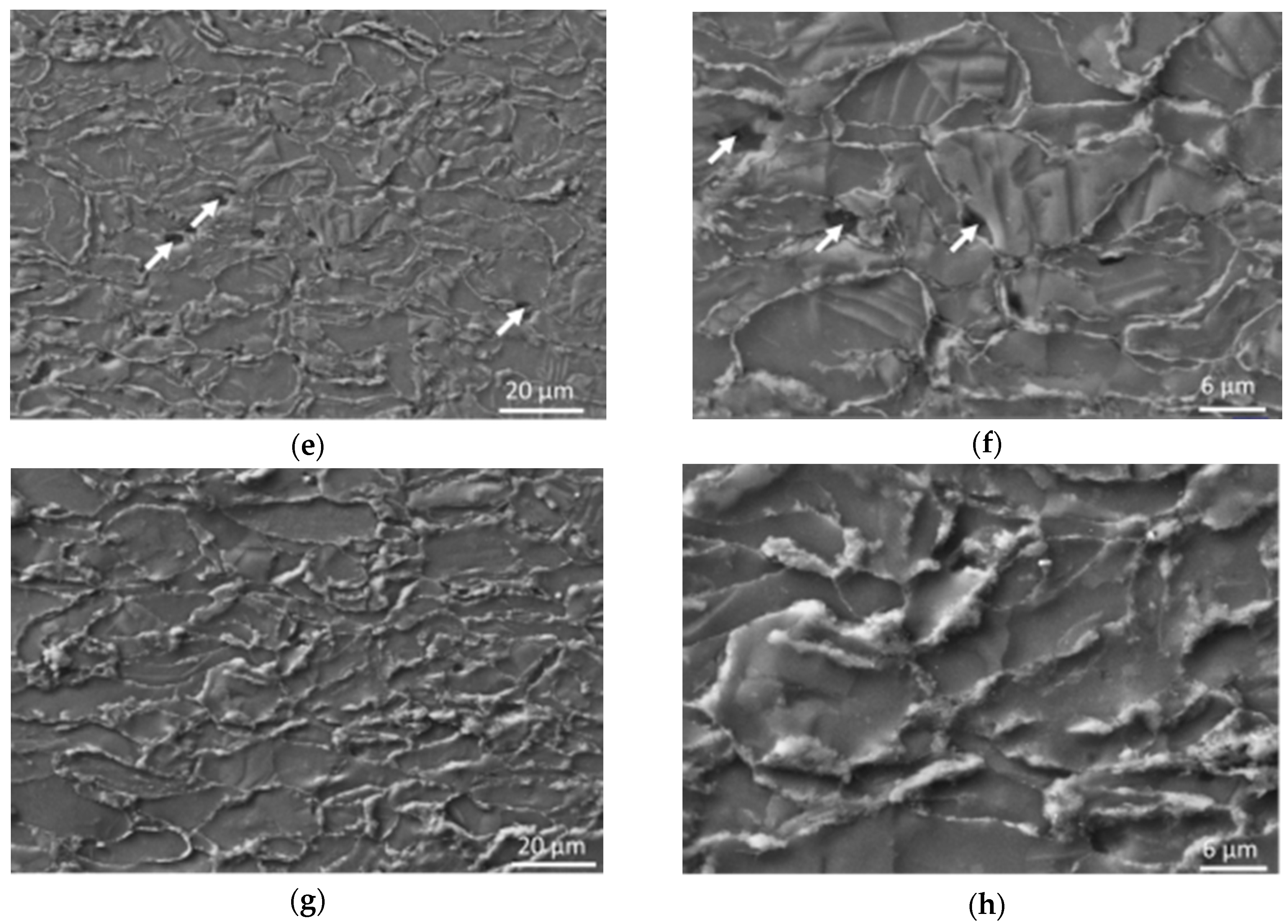
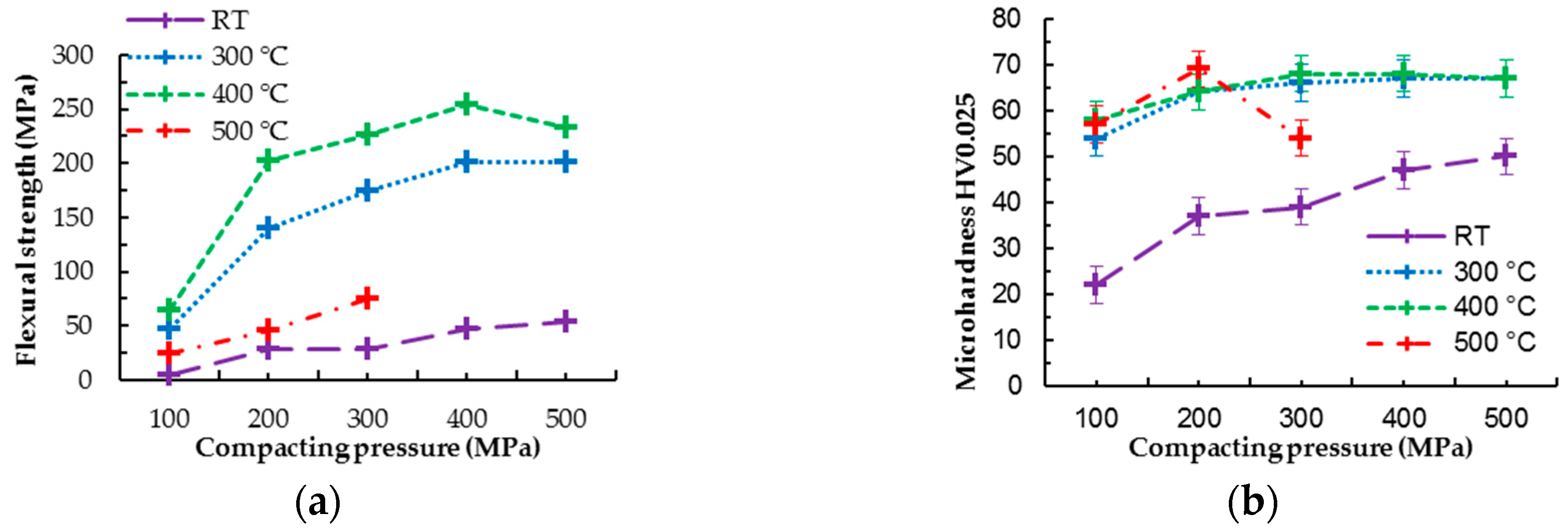
Low compacting pressures led only to minimal plastic deformation of magnesium powder particles during cold compaction or hot pressing (
Figure 2). Increasing the compaction pressure (above 300 MPa) at RT had an influence on the deformation of the powder particles while increased deformability of particles corresponded with increasing compaction pressure. The influence was also sufficient for changing the character of the porosity from open porosity to closed porosity and decreased the number of pores on the samples’ cross-sections, which is in agreement with [
11,
12,
13]. Hot pressing led to a significant decrease of the porosity of the compacts and also to a change in the character of the porosity (
Figure 2) [
4,
32]. The open porosity characteristic for cold pressed samples was replaced with closed localized pores observed in hot pressed samples’ microstructure. This change can be attributed to better plastic deformation of magnesium at a higher temperature, resulting in higher plastic deformation of powder particles and closing the open porosity present on particles’ boundaries due to the shape factor (spherical particles cannot fill the space 100%). The change of the magnesium deformability results from the hexagonal close-packed (HCP) crystallographic lattice structure of the material. While only one slip system is active at RT, the increase in temperature led to activation of more slip systems. Furthermore, the twinning mechanism is dominant for HCP lattice structures instead of the slip mechanism below 200 °C [
34,
35].
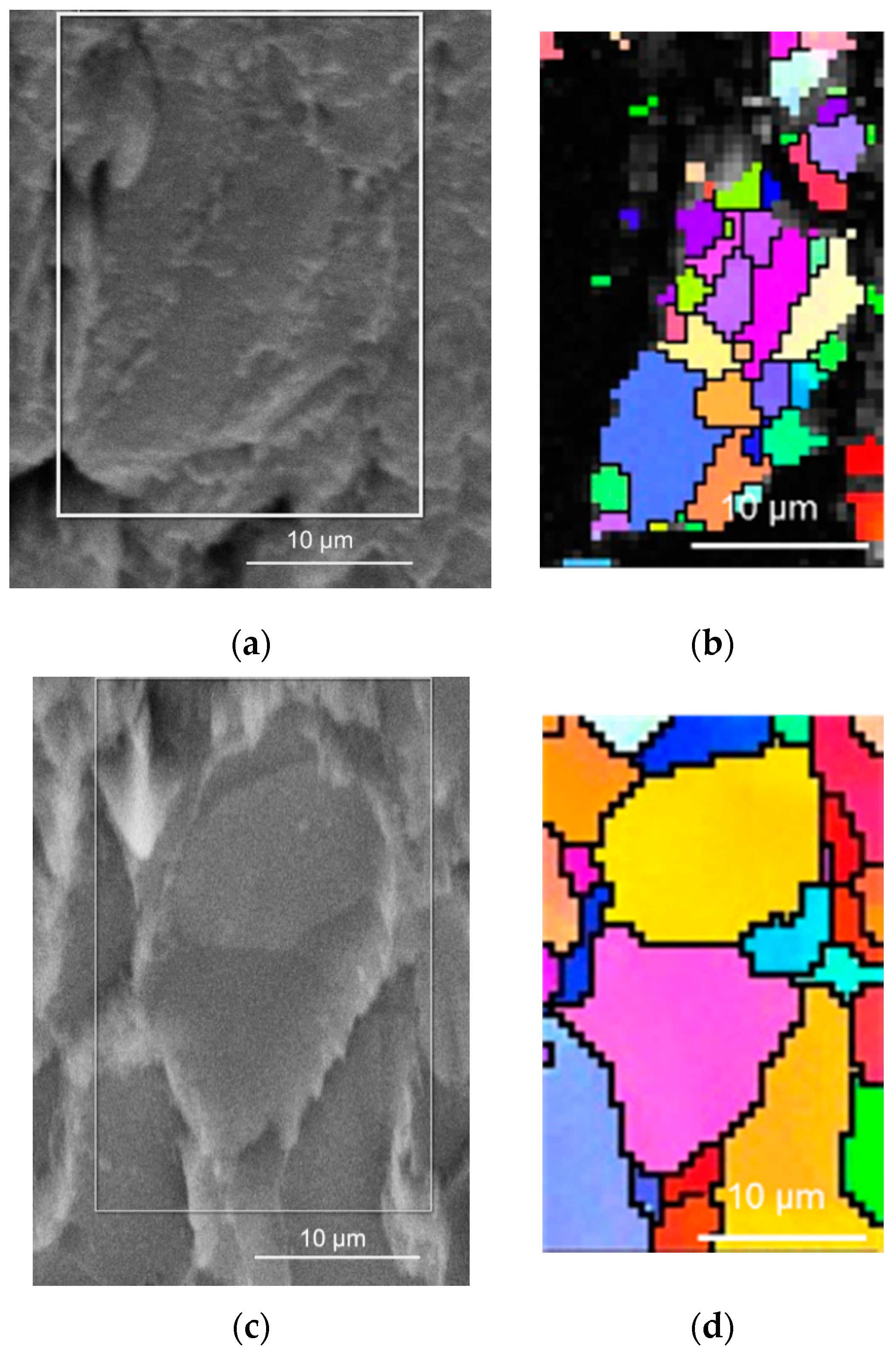
Besides the improvement of magnesium deformability, the elevated temperature also influenced the evolution of grains in the compacted powder particles, which was revealed with EBSD analysis shown in
Figure 3. The cold compacted sample revealed a very fine microstructure within the powder particle, but wide gaps between powder particles were observed. EBSD analysis of the hot pressed sample revealed coarser metallic grains in powder particles; however, the gaps between powder particles were not visible as diffusion bonding at higher temperatures took place between powder particles.
A coarser metallic microstructure generally leads to a decrease in hardness and tensile strength compared to the fine-grained material [
4,
5]. However, the observed fine microstructure corresponds only to the powder particles, while the particles’ bonding was observed to be weak (also, an open porosity was observed). Therefore, this fine microstructure obtained by cold compacting of magnesium did not affect the mechanical properties of the samples in a positive way due to the low compactness of particle boundaries.
Improvement of mechanical properties by hot pressing of pure magnesium is visible in
Figure 3 and
Figure 4. Hot pressed samples prepared at 300 °C revealed the same trend in increasing flexural strength with increasing compaction pressure as samples prepared at 400 °C; however, the values of flexural strength are slightly higher for the series of samples prepared at 400 °C. This fact can be attributed to a higher diffusion rate of magnesium at 400 °C, which leads to better diffusion bonding between powder particles and possibly more pronounced grain coarsening at higher temperature [
11]. Samples prepared at 500 °C reached much lower values of flexural strength compared to other hot pressed samples (comparing samples prepared under the same pressures) and only slightly higher than cold compacted samples (
Figure 4a). At the temperature of 500 °C, the diffusion of magnesium is even higher than at 400 °C, which should lead to better powder particle diffusion bonding; however, the higher temperature (500 °C) can lead to intensive coarsening of metallic grains created in the powder particles. As a result, the grain coarsening could be a reason for the flexural strength decrease (
Figure 4a), considering good material compaction based on EBSD analysis.
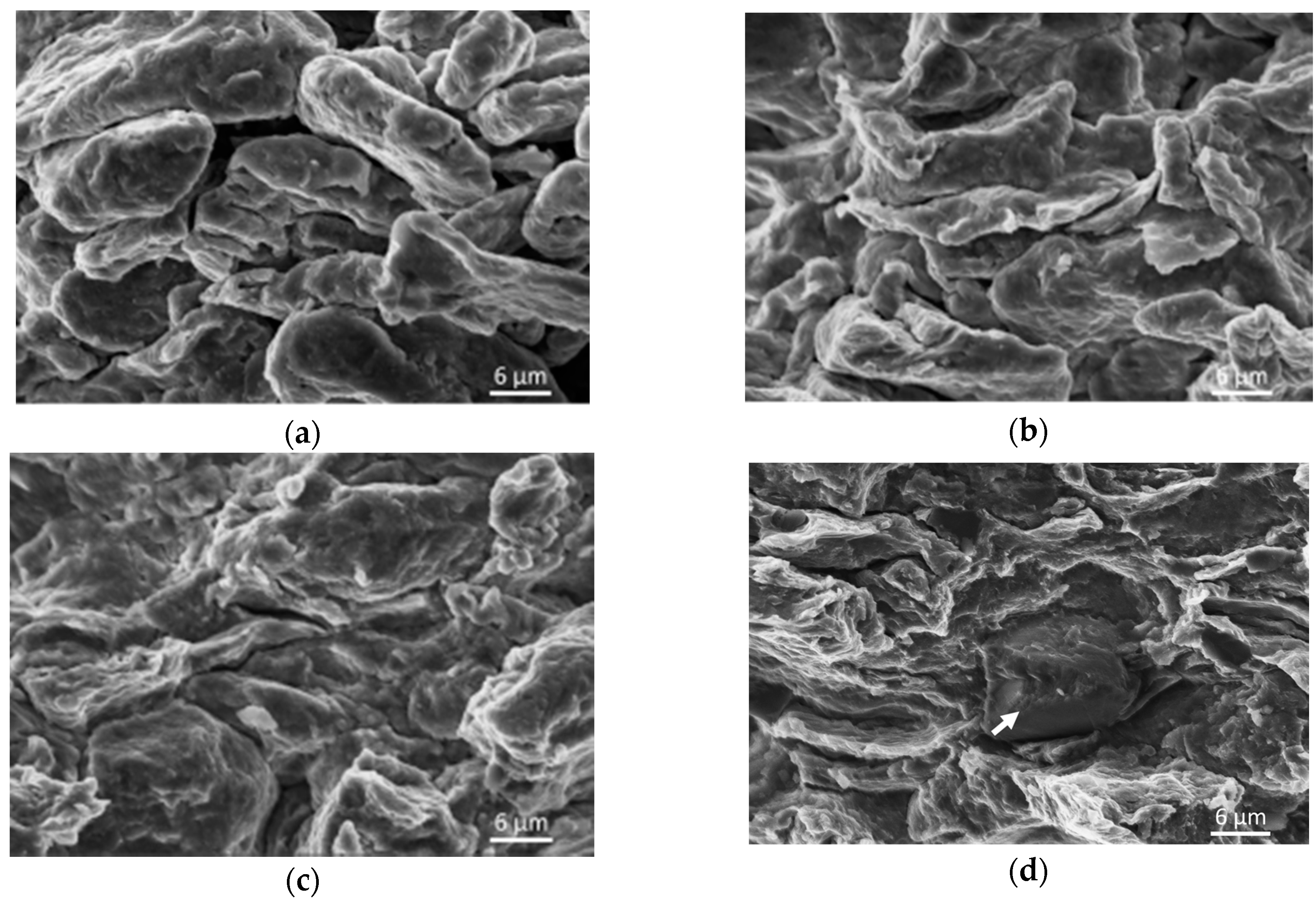
Bonding of powder particles into the coherent sample is also apparent from the microhardness test results. Cold compacted samples follow the same trend of increasing microhardness with increasing compaction pressure as samples prepared via hot pressing; however, the obtained values are significantly lower. This can be attributed to the good cohesion of samples prepared via hot pressing. Cold compacted samples hold together only due to the powder particles’ interlocking as a result of plastic deformation, while the hot pressed samples are interlocked and diffusion bonded. The good cohesion of powder particles in the case of hot pressed samples is also visible from the fractographic analysis provided in
Figure 5, as inter-granular failure was visible only on fracture surfaces of samples prepared by hot pressing. Fractographic analysis from cold compacted samples revealed only the trans-granular failure of samples, and no inter-granular failure was observed. While the character of fracture surfaces differs, only brittle fracture was reported in all the measured samples. The character of fracture surfaces furthermore confirms the assumption that the samples prepared by cold pressing are not diffusion bonded.
Differences between cold compacted and hot pressed samples were also observed during EIS measurement. Low compactness of cold compacted samples compared to the hot pressed samples resulted in lower corrosion resistance of cold compacted samples. This was proven by the corrosion rate and the depth to which corrosion progressed. It is clearly evident that by dissolving of magnesium under H
2 evolution, the corrosion environment is alkalized (increase pH value), and the corrosion rate of magnesium slows down [
30]. Corrosion products created on magnesium are usually based on MgO and Mg(OH)
2. The layer of corrosion products, especially the Mg(OH)
2 compound, is easily disturbed and dissolved in environments containing Cl
−. The layer offers only very low protection to magnesium against corrosion especially in environments containing aggressive ions [
36]. HBSS is a more complex environment containing phosphates and hydrogen phosphates. More compounds in the corrosion environment enable more chemical reactions between the metallic surface, corrosion environment and corrosion products [
30]. Products of these reactions can create a layer of corrosion products (outer layer) over the created MgO and Mg(OH)
2 inner primary layer created on the sample surface. Both layers develop during the corrosion process and are porous by their nature or the damage of the compact areas can occur. All the types of corrosion products are created during the material exposure to the environment and grow on the material surface at the same time. These layers cannot be exactly distinguished.
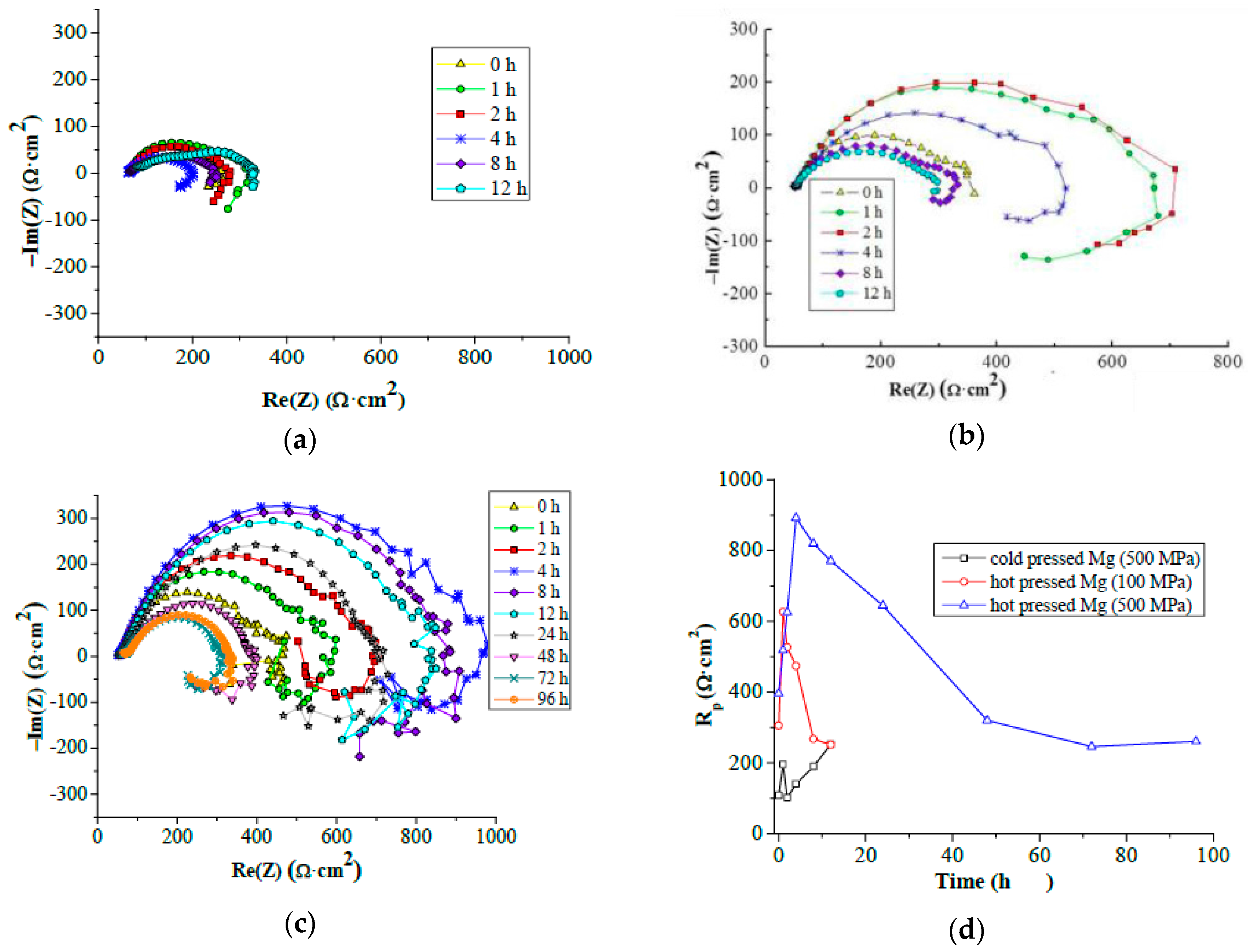
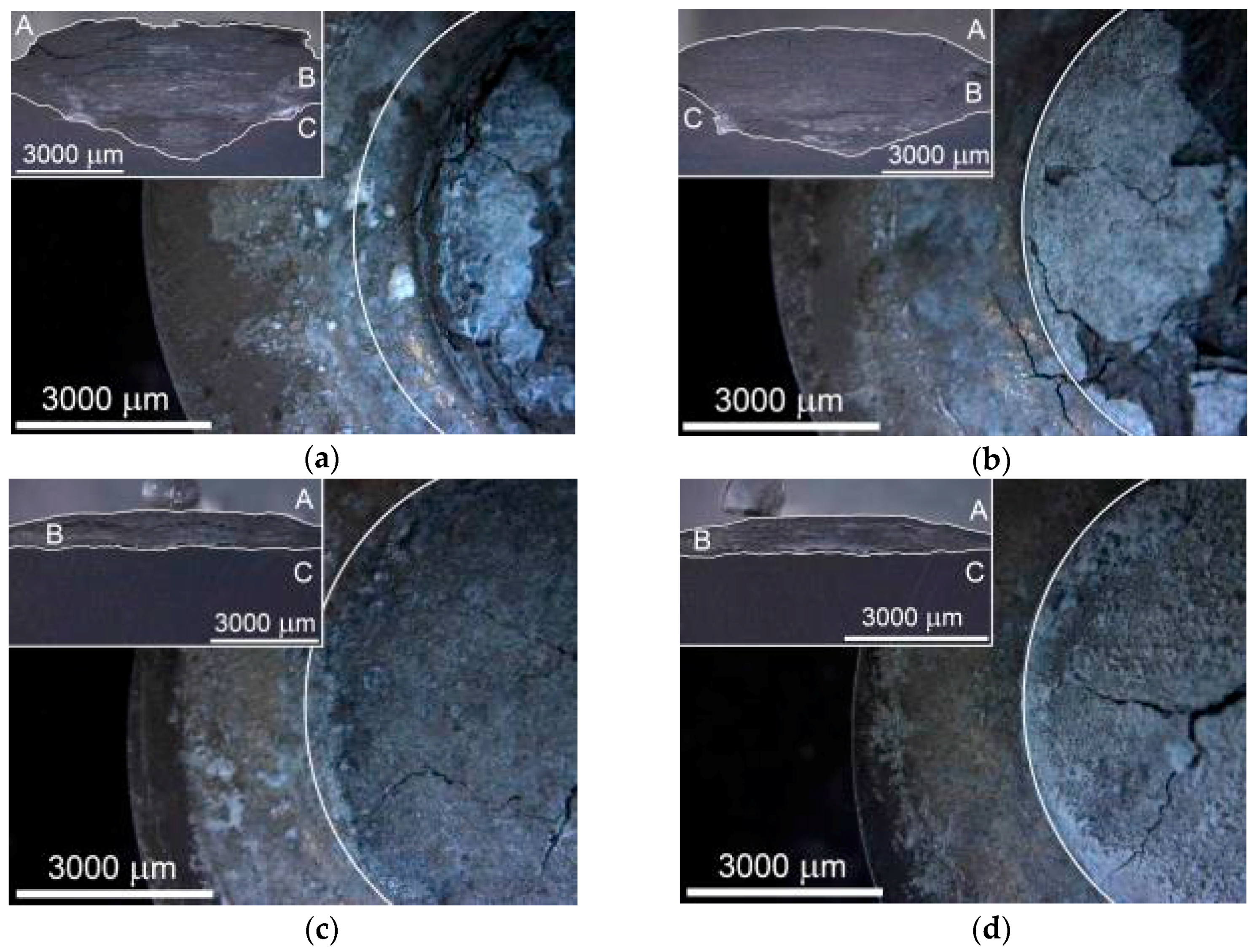
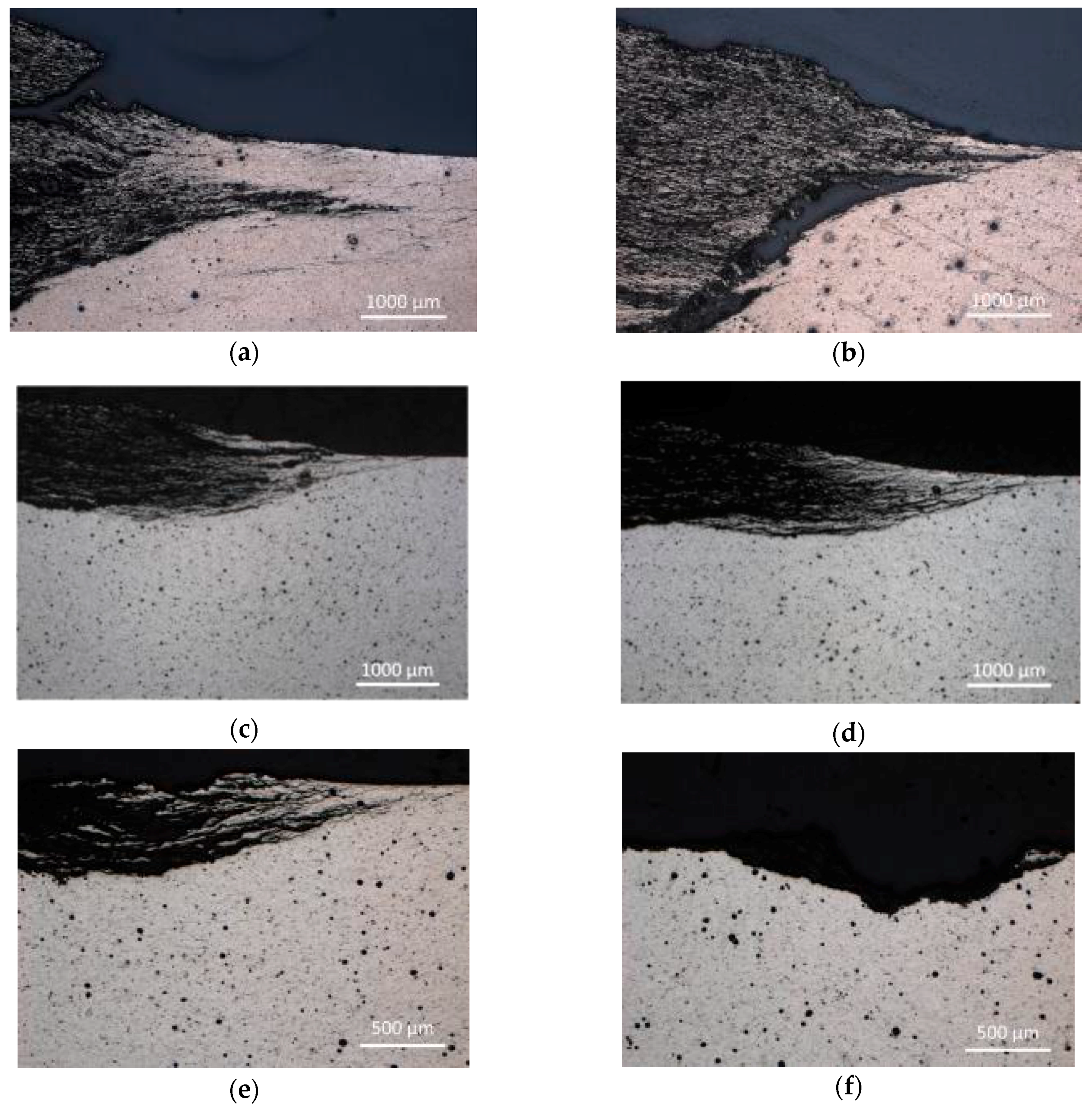
According to the Le Chatelier theory, HBSS should be more aggressive for magnesium than enriched HBSS. Comparing the corrosion process character of examined magnesium materials (
Figure 9 and
Figure 10), this theory was not clearly proven. The similar thickness of the layers of corrosion products (
Figure 9), even the progress of corrosion attack within the material volume (
Figure 10), was observed considering the same processing parameters. Corrosion attack progress was the only observed difference between the cold compacted and hot pressed samples; whereas the cold compacted samples that were only mechanically-bonded exhibited a more pronounced degradation process when compared to hot pressed samples. The higher compacting pressure used (500 MPa), resulting in even better material compaction, had a positive influence also on material corrosion resistance.
The highest corrosion resistance was reached for hot pressed magnesium materials prepared under 500 MPa in both corrosion mediums. This was proven by higher polarization resistance and duration of the corrosion experiment compared to other material states. The time of experiments indicates more pronounced corrosion attack of samples in HBSS compared to enriched HBSS. This difference was not observed for hot pressed samples prepared under 500 MPa. However, the values of Rp determined for the materials tested in both solution types did not exhibit significant differences, and the higher aggressiveness of HBSS compared to enriched HBSS was not proven with the results. Slightly higher values of Rp reached for different material states in enriched HBSS when compared to HBSS did not show a significant influence of corrosion environment on material properties. However, similar values of Rp were reached at the end of EIS test for all the materials in each corrosion environment. Different material types exhibit similar corrosion behavior regardless of the corrosion environment used.