Inhibition of Brass (80/20) by 5-Mercaptopentyl-3-Amino-1,2,4-Triazole in Neutral Solutions
Abstract
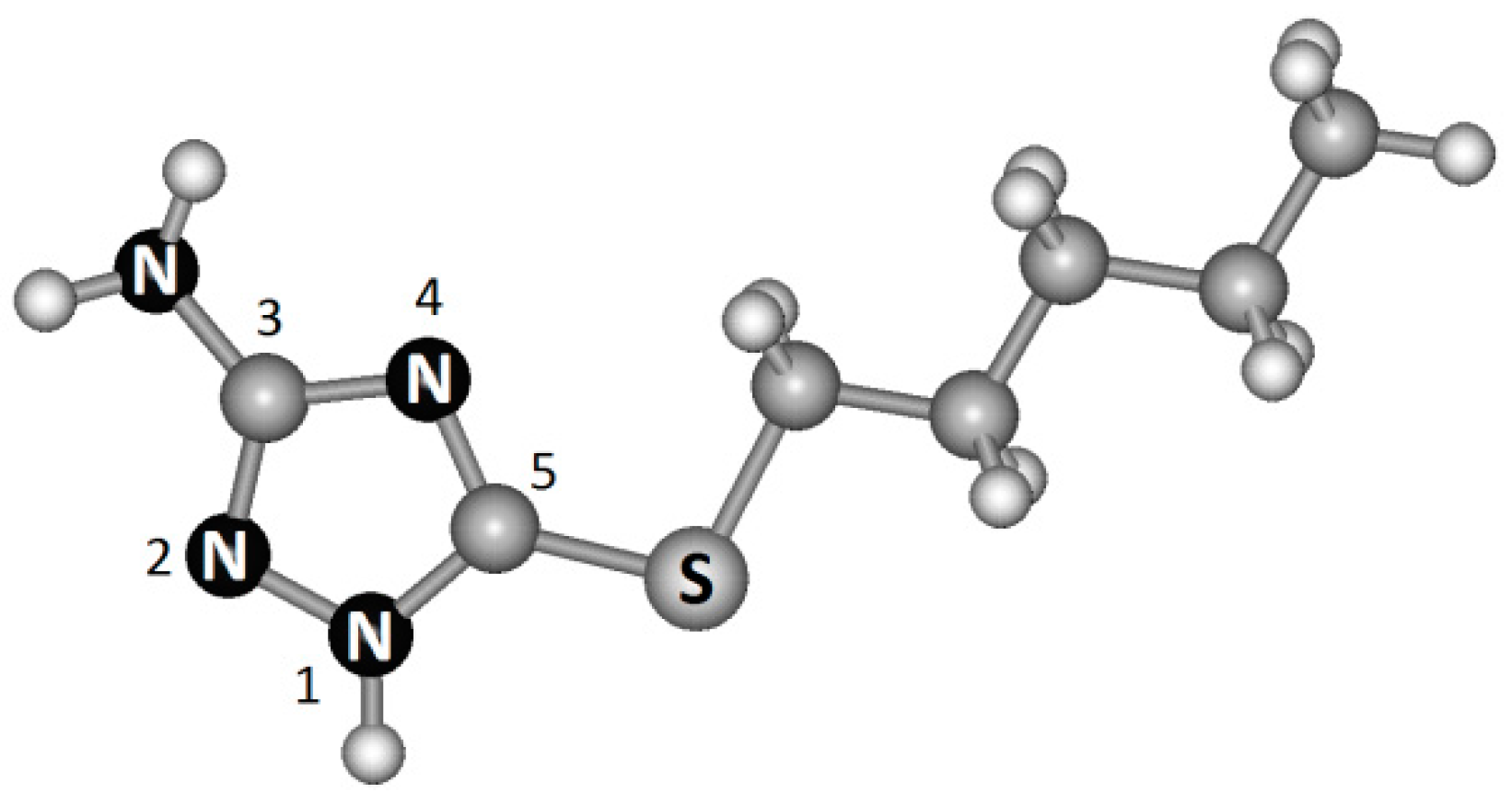
:1. Introduction
2. Experimental Section
3. Results and Discussion
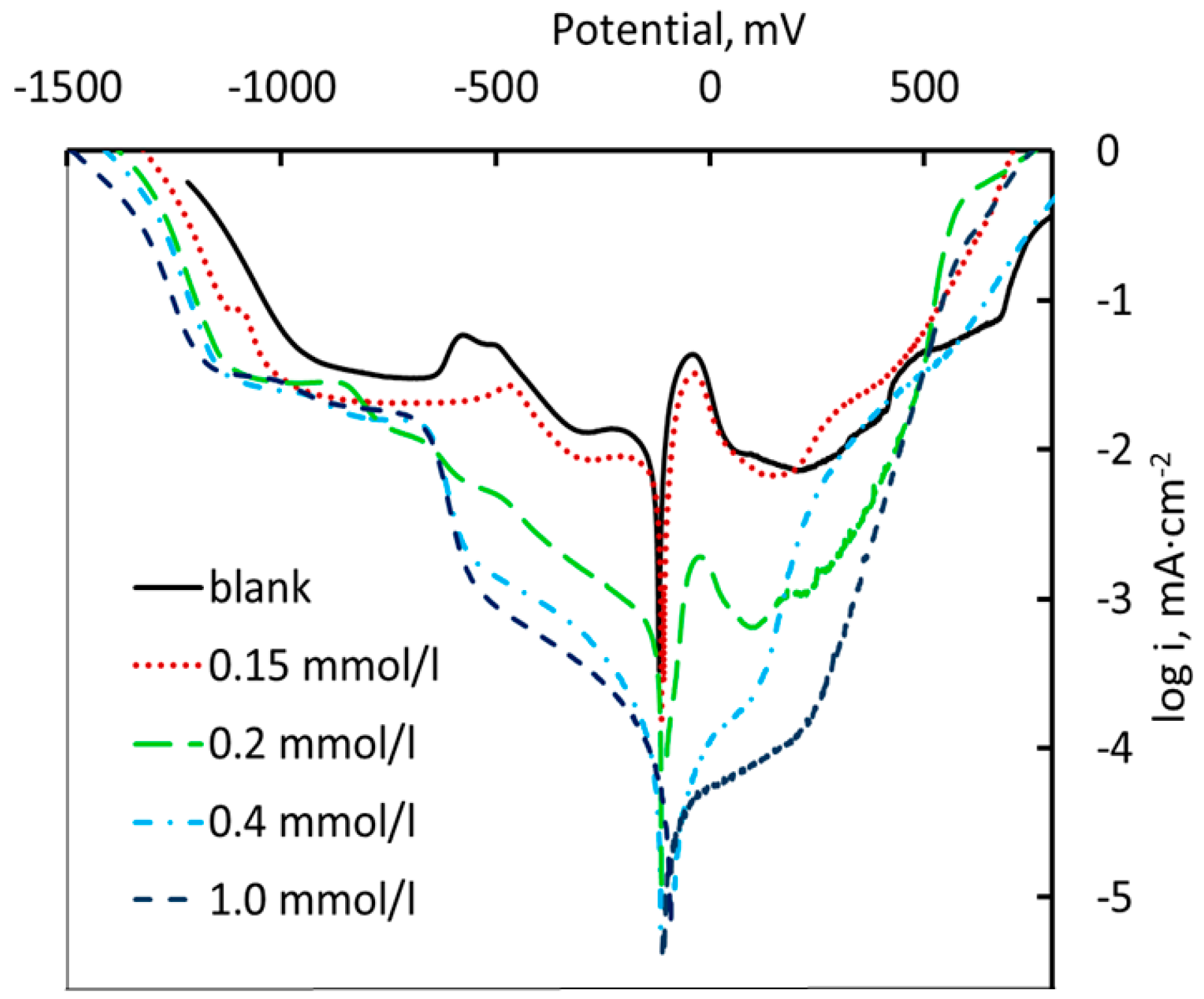
3.1. Electrochemical Research
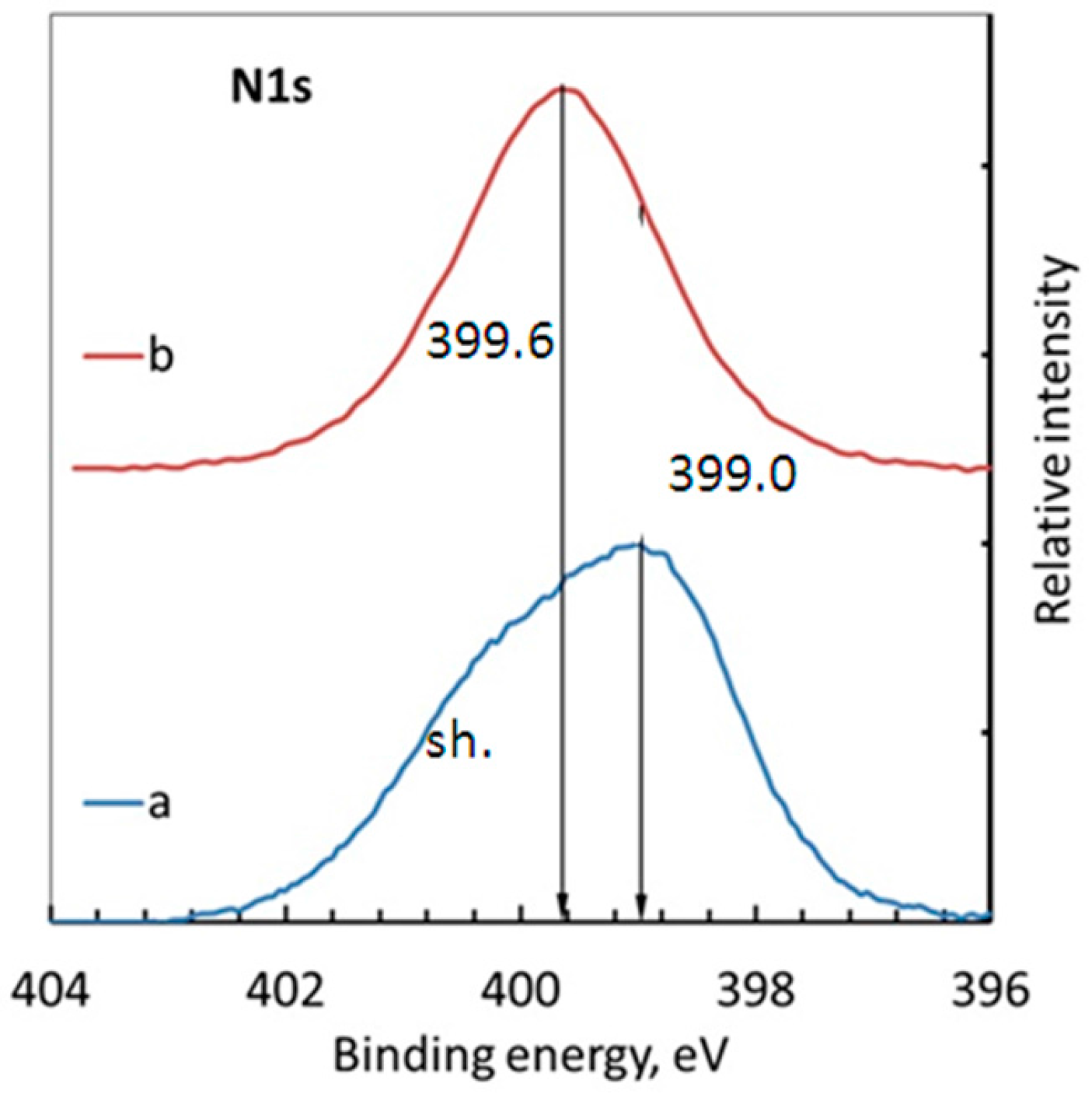
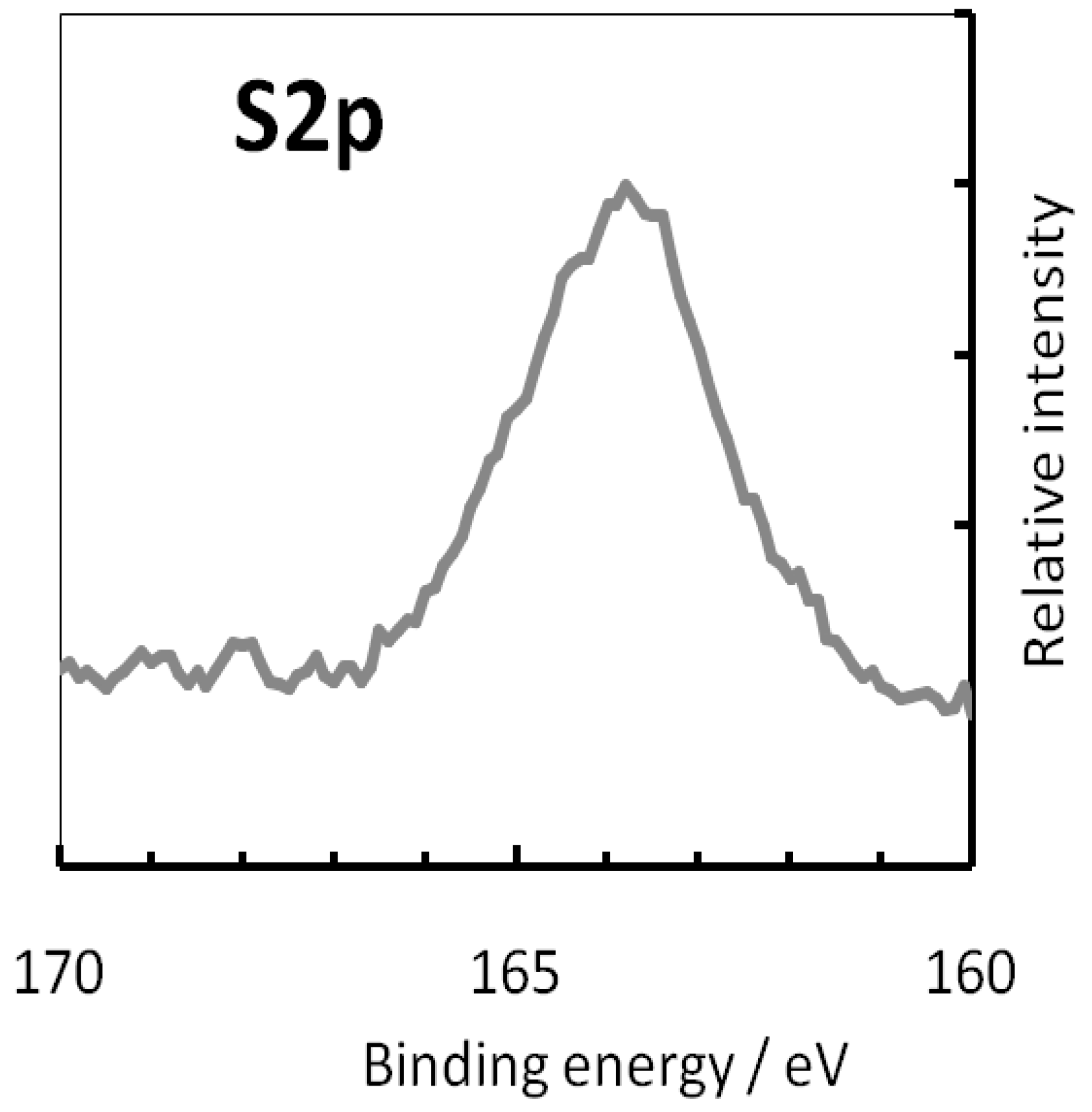
3.2. X-ray Photoelectron Spectroscopy (XPS) Measurements
3.3. Discussion
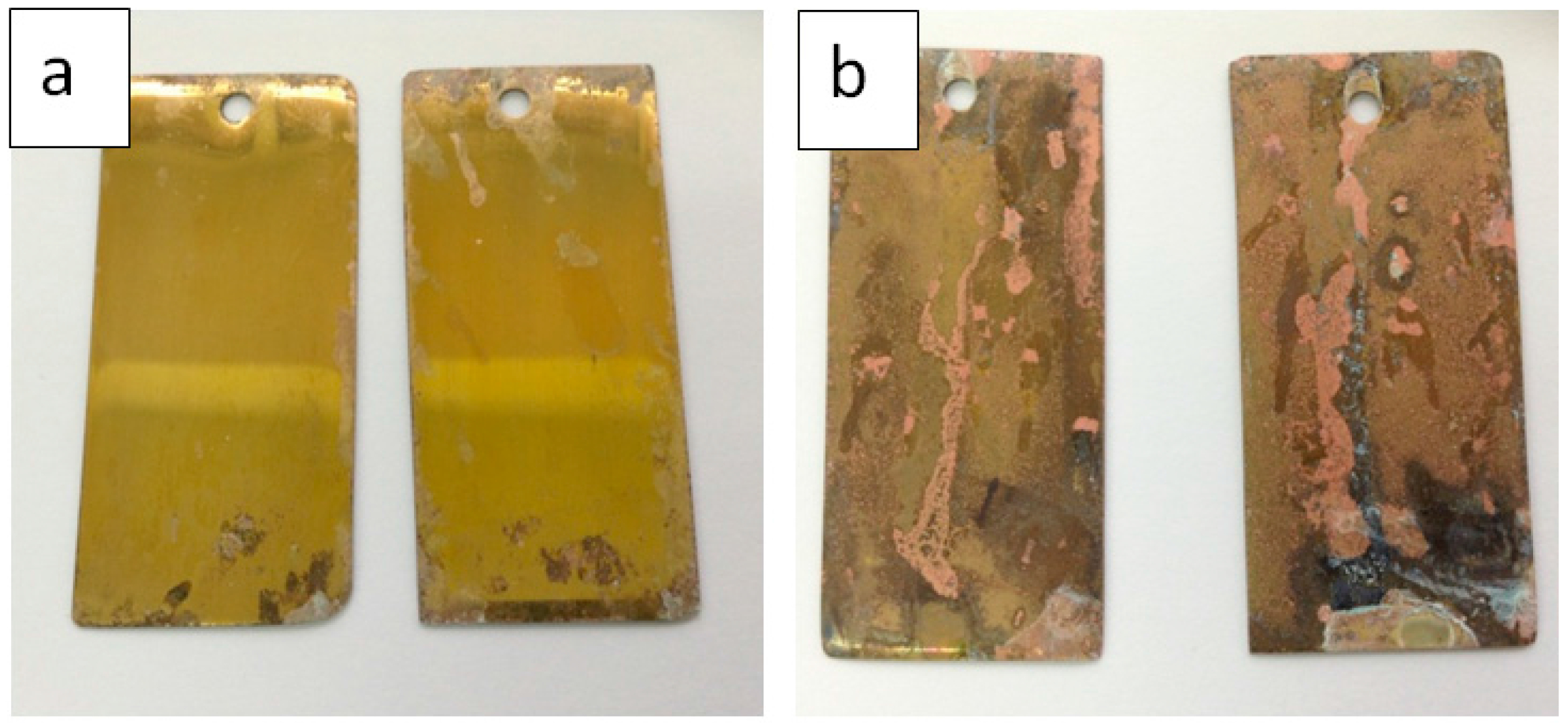
3.4. Corrosion Tests
4. Conclusions
- Electrochemical measurements have shown that addition of 1 mM MPATA results in complete suppression of anodic dissolution of copper: the main component of the brass alloy.
- The analysis of X-ray photoelectron spectra had shown that treatment of brass in MPATA solution results in formation a protective film consisted of the complexes [Cu1−xZnxMPATA] (where x < 0.2) whose thickness does not exceed 3–3.5 nm.
- During corrosion tests, it was found that even after 5 days in a salt fog chamber with hourly spraying, brass samples protected with an inhibitor retain their gloss and have damage less than 1% of the surface.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, R.C.; Burstein, G.T. The anodic behavior of freshly generated α-brass surfaces. Corros. Sci. 1981, 21, 119–128. [Google Scholar] [CrossRef]
- Polunin, A.V.; Pschelnikov, A.P.; Losev, V.V.; Marshakov, I.K. Electrochemical studies of the kinetics and mechanism of brass dezincification. Electrochim. Acta 1982, 27, 467–475. [Google Scholar] [CrossRef]
- Dinnappa, R.K.; Mayanna, S.M. The dezincification of brass and its inhibition in acidic chloride and sulphate solutions. Corros. Sci. 1987, 27, 349–361. [Google Scholar] [CrossRef]
- Trethewey, R.; Pinwill, I. The dezincification of free-machining brasses in sea water. Surf. Coat. Technol. 1987, 30, 289–307. [Google Scholar] [CrossRef]
- Zou, J.; Wang, D.; Qiu, W. Solid-state diffusion during the selective dissolution of brass: Chronoamperometry and positron annihilation study. Electrochim. Acta 1997, 42, 1733–1737. [Google Scholar] [CrossRef]
- Morales, J.; Esparza, P.; Gonzales, S.; Vasquez, L.; Salvarezza, R.C.; Arvia, A.J. Kinetics and mechanism of α-brass dealloying in aqueous 0.5 M sodium chloride solution derived from combined scanning tunneling microscopy and electrochemical data. Langmuir 1996, 12, 500–507. [Google Scholar] [CrossRef]
- Kumar, S.; Sankara Narayanan, T.S.N.; Manimaran, A.; Suresh Kumar, M. Effect of lead content on the dezincification behavior of leaded brass in neutral and acidified 3.5% NaCl solution. Mater. Chem. Phys. 2007, 106, 134–141. [Google Scholar] [CrossRef]
- Kabasakaloglu, M.; Kıyak, T.; Sendil, O.; Asan, A. Electrochemical behavior of brass in 0.1 M NaCl. Appl. Surf. Sci. 2002, 193, 167–174. [Google Scholar] [CrossRef]
- Karpagavalli, R.; Balasubramaniam, R. Development of novel brasses to resist dezincification. Corros. Sci. 2007, 49, 963–979. [Google Scholar] [CrossRef]
- Cocco, F.; Elsener, B.; Fantauzzi, M.; Atzei, D.; Rossi, A. Nanosized surface films on brass alloys by XPS and XAES. RSC Adv. 2016, 6, 31277. [Google Scholar] [CrossRef]
- Hollander, O.; May, R.C. The Chemistry of azole copper corrosion inhibitors in cooling waters. Corrosion 1985, 41, 39–45. [Google Scholar] [CrossRef]
- De Costa, S.L.F.A.; Agostinho, S.M.L.; Nobe, K. Rotating ring-disk electrode studies of Cu-Zn alloy electrodissolution in 1M HCl effect of benzotriazole. J. Electrochem. Soc. 1993, 140, 3483–3488. [Google Scholar] [CrossRef]
- Shih, H.C.; Tzou, R.J. Effect of benzotriazole on the stress corrosion cracking and the electrochemical polarization of 70/30 brass in fluoride solutions. J. Electrochem. Soc. 1991, 138, 958–961. [Google Scholar] [CrossRef]
- Otieno-Alego, V.; Schweinsberg, D.P.; Hope, G.A.; Notoya, T. An electrochemical and SERS study of the effect of 1-[N,N-bis-(hydroxyethyl)aminomethyl]-benzotriazole on the acid corrosion and dezincification of 60/40 brass. Corros. Sci. 1996, 38, 213–223. [Google Scholar] [CrossRef]
- Fenelon, M.; Breslin, C.B. An electrochemical study of the formation of benzotriazole surface films on copper, zinc and a copper-zinc alloy. J. Appl. Electrochem. 2001, 31, 509–516. [Google Scholar] [CrossRef]
- Nagiub, A.; Mansfeld, F. Evaluation of corrosion inhibition of brass in chloride media using EIS and ENA. Corros. Sci. 2001, 43, 2147–2171. [Google Scholar] [CrossRef]
- Kosec, T.; Merl, D.K.; Milošev, I. Impedance and XPS study of benzotriazole films formed on copper, copper-zinc alloys and zinc in chloride solution. Corros. Sci. 2008, 50, 1987–1997. [Google Scholar] [CrossRef]
- Mamas, S.; Kiyak, T.; Kabasakaloglu, M.; Koc, A. The effect of benzotriazole on brass corrosion. Mater. Chem. Phys. 2005, 93, 41–47. [Google Scholar] [CrossRef]
- Kosec, T.; Milošev, I.; Pihlar, B. Benzotriazole as an inhibitor of brass corrosion in chloride solution. Appl. Surf. Sci. 2007, 253, 8863–8873. [Google Scholar] [CrossRef]
- Milošev, I.; Kosec, T. Electrochemical and spectroscopic study of benzotriazole films formed on copper, copper-zinc alloys and zinc in chloride solution. Chem. Biochem. Eng. Q. 2009, 23, 53–60. [Google Scholar]
- Gerengi, H.; Darowicki, K.; Bereket, G.; Slepski, P. Evaluation of corrosion inhibition of brass-118 in artificial seawater by benzotriazole using dynamic EIS. Corros. Sci. 2009, 51, 2573–2579. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Bogdanovic, G.D.; Radovanovic, M.B.; Petrovic, M.B.; Stamenkovic, A.T. Influence of pH and chloride ions on electrochemical behavior of brass in alkaline solution. Int. J. Electrochem. Sci. 2009, 4, 654–661. [Google Scholar]
- Zerjav, G.; Milošev, I. Protection of copper against corrosion in simulated urban rain by the combined action of benzotriazole, 2-mercaptobenzimidazole and stearic acid. Corros. Sci. 2015, 98, 180–191. [Google Scholar] [CrossRef]
- Ravichandran, R.; Rajendran, N. Influence of benzotriazole derivatives on the dezincification of 65–35 brass in sodium chloride. Appl. Surf. Sci. 2005, 239, 182–192. [Google Scholar] [CrossRef]
- Cohen, S.L.; Brusic, V.A.; Kaufman, F.B.; Frankel, G.S.; Motakef, S.; Rush, B. X-ray photoelectron spectroscopy and ellipsometry studies of the electrochemically controlled adsorption of benzotriazole on copper surfaces. J. Vac. Sci. Technol. 1990, A8, 2417–2424. [Google Scholar] [CrossRef]
- Yanardag, T.; Özbay, S.; Dinçer, S.; Aksüt, A.A. Corrosion inhibition efficiency of benzimidazole and benzimidazole derivatives for zinc, copper and brass. Asian J. Chem. 2012, 24, 47–52. [Google Scholar]
- Gao, G.; Liang, C.H. 1,3-Bis-diethylamino-propan-2-ol as volatile corrosion inhibitor for brass. Corros. Sci. 2007, 49, 3479–3493. [Google Scholar] [CrossRef]
- Tuck, C.D.S.; Powell, C.A.; Nuttall, J. Corrosion of Copper and Its Alloys; Elsevier: Houston, TX, USA, 2010; pp. 1938–1968. [Google Scholar]
- Milic, S.M.; Antonijevic, M.M. Some aspects of copper corrosion in presence of benzotriazole and chloride ions. Corros. Sci. 2009, 51, 28–34. [Google Scholar] [CrossRef]
- Karpagavalli, R.; Cairns Darran, R.; Rajeswari, S. Synergistic inhibition effect of 2-mercaptobenzothiazole and Tween-80 on the corrosion of brass in NaCl solution. Appl. Surf. Sci. 2008, 254, 4483–4493. [Google Scholar]
- Ebrahimzadeha, M.; Gholami, M.; Momeni, M.; Kosari, A.; Moayeda, M.H.; Davoodi, A. Theoretical and experimental investigations on corrosion controlof 65Cu-35Zn brass in nitric acid by two thiophenolderivatives. Appl. Surf. Sci. 2015, 332, 384–392. [Google Scholar] [CrossRef]
- Qiu, P.; Leygraf, C. Initial oxidation of brass induced by humidified air. Appl. Surf. Sci. 2011, 258, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lan, Y.; He, Y.; Li, Y.; Luo, J. 1,2,4-Triazole as a corrosion inhibitor in copper chemical mechanical polishing. Thin Solid Films 2014, 556, 395–404. [Google Scholar] [CrossRef]
- Mountassir, Z.; Srhiri, A. Electrochemical behaviour of Cu-40Zn in 3% NaCl solution polluted by sulphides: Effect of aminotriazole. Corros. Sci. 2007, 49, 1350–1361. [Google Scholar] [CrossRef]
- Rajkumar, G.; Sethuraman, M.G. Corrosion protection ability of self-assembled monolayer of 3-amino-5-mercapto-1,2,4-triazole on copper electrode. Thin Solid Films 2014, 562, 32–36. [Google Scholar] [CrossRef]
- Bag, S.K.; Chakraborty, S.B.; Roy, A.; Chaudhuri, S.R. 2-Aminobenzimidazole as corrosion inhibitor for 70–30 brass in ammonia. Br. Corros. J. 1996, 31, 207–212. [Google Scholar] [CrossRef]
- Morales, J.; Esparza, P.; Fernandez, G.T.; Gonzalez, S.; Garcia, J.E.; Caceres, J.; Salvarezza, R.C.; Arvia, A.J. A comparative study on the passivation and localized corrosion of α- and β-brass in borate buffer solutions containing sodium chloride-II. X-ray photoelectron and Auger electron spectroscopy data. Corros. Sci. 1995, 37, 231–239. [Google Scholar] [CrossRef]
- Al-kharafi, F.M.; El-Shamy, A.M.; Ateya, B.G. Comparative effects of tolytriazole and benzotriazole against sulfide attack on copper. Int. J. Electrochem. Sci. 2009, 4, 1351–1364. [Google Scholar]
- Viswanathan, S.S. A review on recent patents in corrosion Inhibitors. Recent Pat. Corros. Sci. 2010, 2, 6–12. [Google Scholar]
- Kuznetsov, Y.I.; Agafonkina, M.O.; Shikhaliev, K.S.; Andreeva, N.P.; Potapov, A.Y. Adsorption and passivation of copper by triazoles in neutral aqueous solution. Int. J. Corros. Scale Inhib. 2014, 3, 137–148. [Google Scholar] [CrossRef]
- Agafonkina, M.O.; Kuznetsov, Y.I.; Andreeva, N.P.; Shikhaliev, K.S.; Potapov, A.Y. Adsorption and passivation of copper by S-containing heterocyclic compounds in neutral aqueous solution. Corros. Mater. Prot. 2016, 3, 29–38. [Google Scholar]
- Makarychev, Y.B.; Arkhipushkin, I.A.; Karpukhina, T.A.; Shikhaliev, H.S.; Kazansky, L.P. Formation of protective layers by some azoles on zinc in aqueous solutions. Corros. Mater. Prot. 2016, No 2, 20–27. [Google Scholar]
- Arkhipushkin, I.A.; Shikhaliev, K.S.; Vagramyan, T.A.; Kazansky, L.P. Adsorption of 5-mercaptopentyl-3-amino-1,2,4-triazole on copper in neutral solutions. Corros. Mater. Prot. 2016, 7, 17–23. [Google Scholar]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4713. [Google Scholar] [CrossRef]
- Scofield, H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectrosc. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
- Mohai, M. MultiQuant: Multimodel XPS quantification software. Surf. Interface Anal. 2004, 36, 828–832. [Google Scholar] [CrossRef]
- Cumpson, P.J.; Seah, M.P. Elastic scattering corrections in AES and XPS. II. Estimating attenuation lengths and conditions required for their valid use in overlayer/substrate experiments. Surf. Interface Anal. 1997, 25, 430–446. [Google Scholar] [CrossRef]
- Speckmann, H.D.; Haupt, S.; Strehblow, H.H. A quantitative surface analytical study of electrochemically-formed copper oxides by XPS and X-ray-induced Auger spectroscopy. Surf. Interface Anal. 1988, 11, 148–155. [Google Scholar] [CrossRef]
- Milošev, I.; Strehblow, H.-H. Electrochemical behavior of Cu-xZn alloys in borate buffer solution at pH 9.2. J. Electrochem. Soc. 2003, 150, B517–B524. [Google Scholar]
- Arjmand, F.; Adriaens, A. Influence of pH and chloride concentration on the corrosion of unalloyed copper in NaCl solution: A comparative study between the micro and macro scales. Materials 2012, 5, 2439–2464. [Google Scholar] [CrossRef] [Green Version]
- Milošev, I.; KosecMikic, T.; Gaberscek, M. The effect of Cu-rich sub-layer on the increased corrosion resistance of Cu-xZn alloys in chloride containing borate buffer. Electrochim. Acta 2006, 52, 415–426. [Google Scholar] [CrossRef]
- Kazansky, L.P.; Pronin, Y.E.; Arkhipushkin, I.A. XPS study of adsorption of 2-mercaptobenzothiazole on a brass surface. Corros. Sci. 2014, 89, 21–29. [Google Scholar] [CrossRef]
- Ismail, K.M.; El-Egami, S.S.; Abdelatah, M. Effect of Zn and Pb as alloying elements on the electrochemical behavior of brass in borate solution. J. Appl. Electrochem. 2001, 31, 663–670. [Google Scholar] [CrossRef]
- Kear, G.; Barker, B.D.; Walsh, F.C. Electrochemical corrosion of unalloyed copper in chloride media—A critical review. Corros. Sci. 2004, 46, 109–135. [Google Scholar] [CrossRef]
- Otmacic, H.; Telegdi, J.; Papp, K.; Stupnišek-Lisac, E. Protective properties of an inhibitor layer formed on copper in neutral chloride solution. J. Appl. Electrochem. 2004, 34, 545–550. [Google Scholar] [CrossRef]
- Finšgar, M.; Peljhan, S.; Kokalj, A.; Kovac, J.; Milošev, I. Determination of the Cu2O thickness on btah-inhibited copper by reconstruction of auger electron spectra. J. Electrochem. Soc. 2010, 157, C295–C301. [Google Scholar] [CrossRef]
- Chadwick, D.; Hashemi, T. Electron spectroscopy of corrosion inhibitors: Surface films formed by 2-mercaptobenzothiazole and 2-mercaptobenzimidazole on copper. Surf. Sci. 1979, 89, 649–659. [Google Scholar] [CrossRef]
- Finšgar, M. 2-Mercaptobenzimidazole as a copper corrosion inhibitor: Part II. Surface analysis using X-ray photoelectron spectroscopy. Corros. Sci. 2013, 72, 90–98. [Google Scholar] [CrossRef]
- Hashemi, T.; Hogarth, C.A. X-ray induced Auger spectroscopy of the surface films formed by benzotriazole on zinc and zinc alloys. Spectrochim. Acta 1988, 43, 783–787. [Google Scholar] [CrossRef]
- Finšgar, M.; Milošev, I. Inhibition of copper corrosion by 1,2,3-benzotriazole: Areview. Corros. Sci. 2010, 52, 2737–2749. [Google Scholar] [CrossRef]
- Antonijevic, M.M.; Petrovic, M.B. Copper corrosion inhibitors. A review. Int. J. Electrochem. Sci. 2008, 3, 1–28. [Google Scholar]
- Kuznetsov, Y.I.; Kazansky, L.P. Physico-chemical aspects of metal protection by azoles as corrosion inhibitors. Russ. Chem. Rev. 2008, 77, 219–232. [Google Scholar] [CrossRef]
- Al-Kharafi, F.M.; Ateya, B.G.; Abd Allah, R.M. Selective dissolution of brass in saltwater. J. Appl. Electrochem. 2004, 34, 47–53. [Google Scholar] [CrossRef]
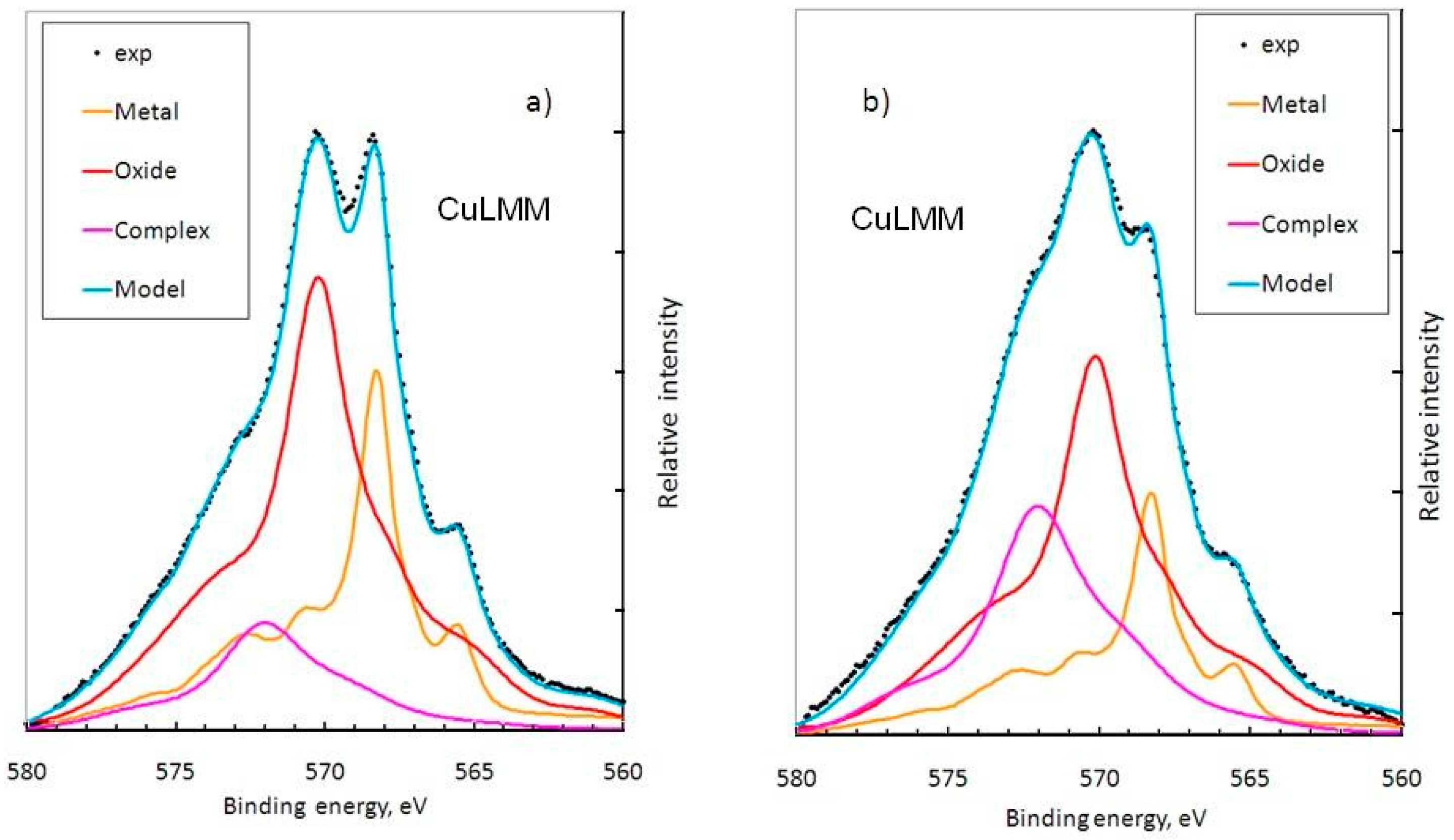
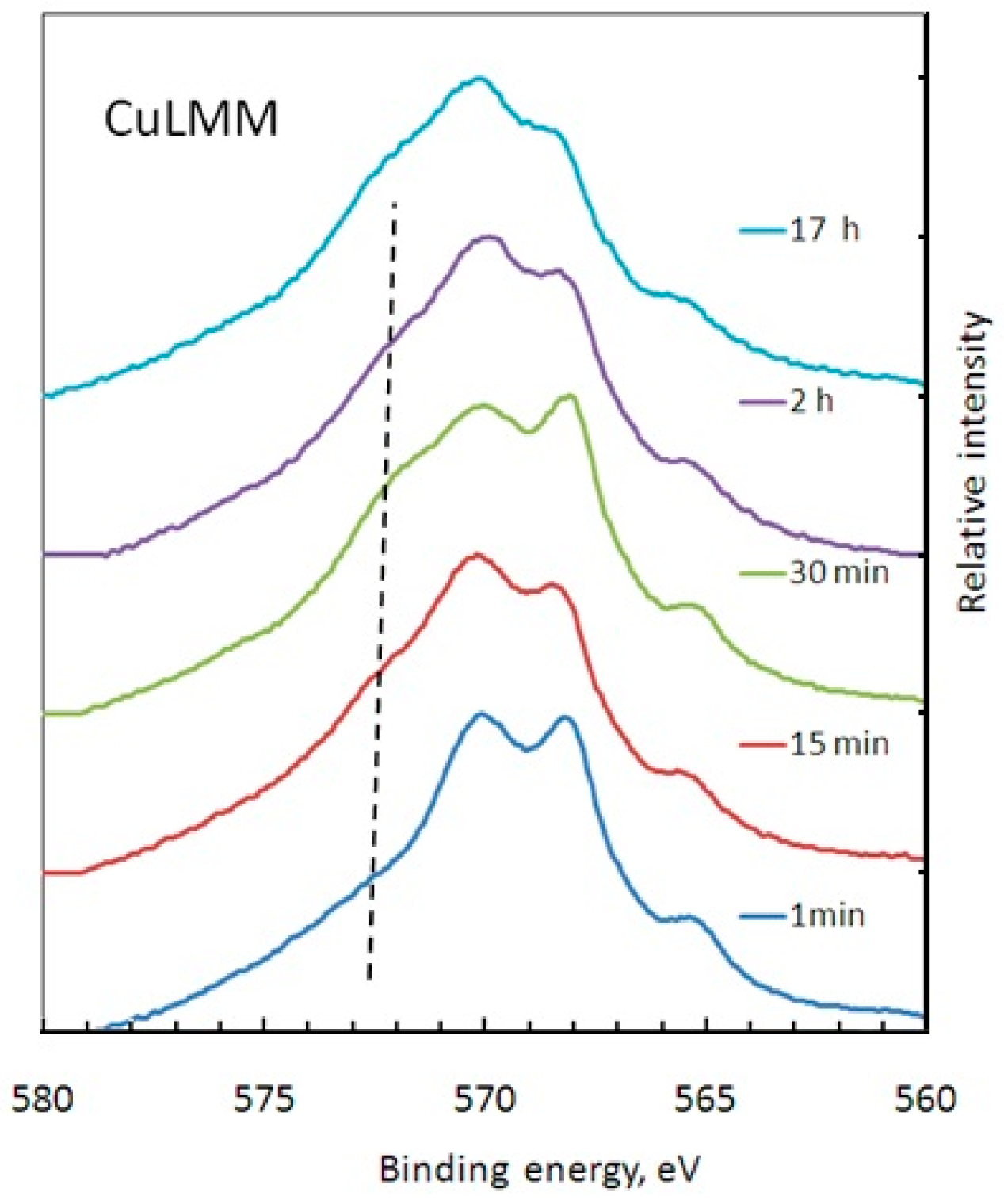

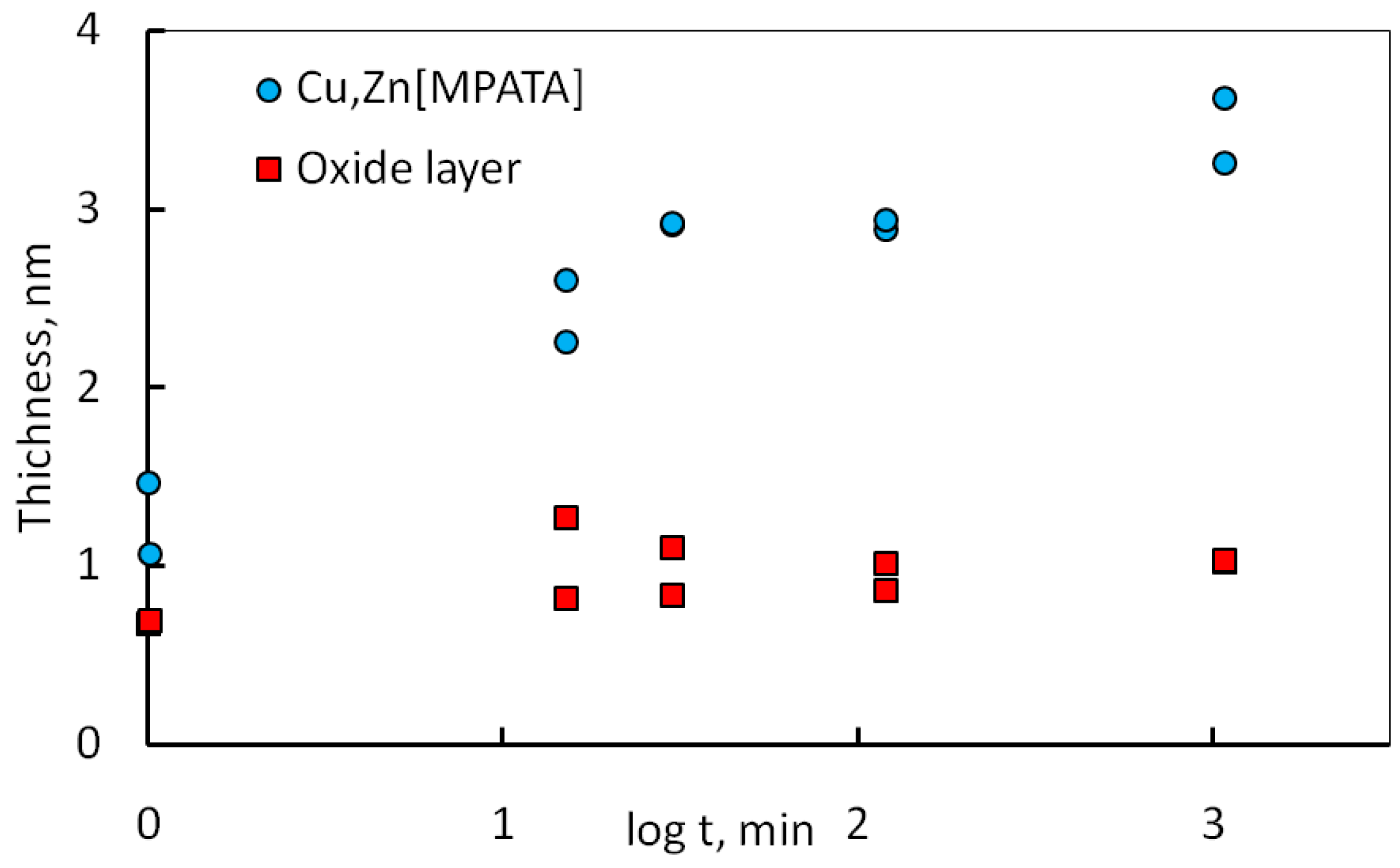
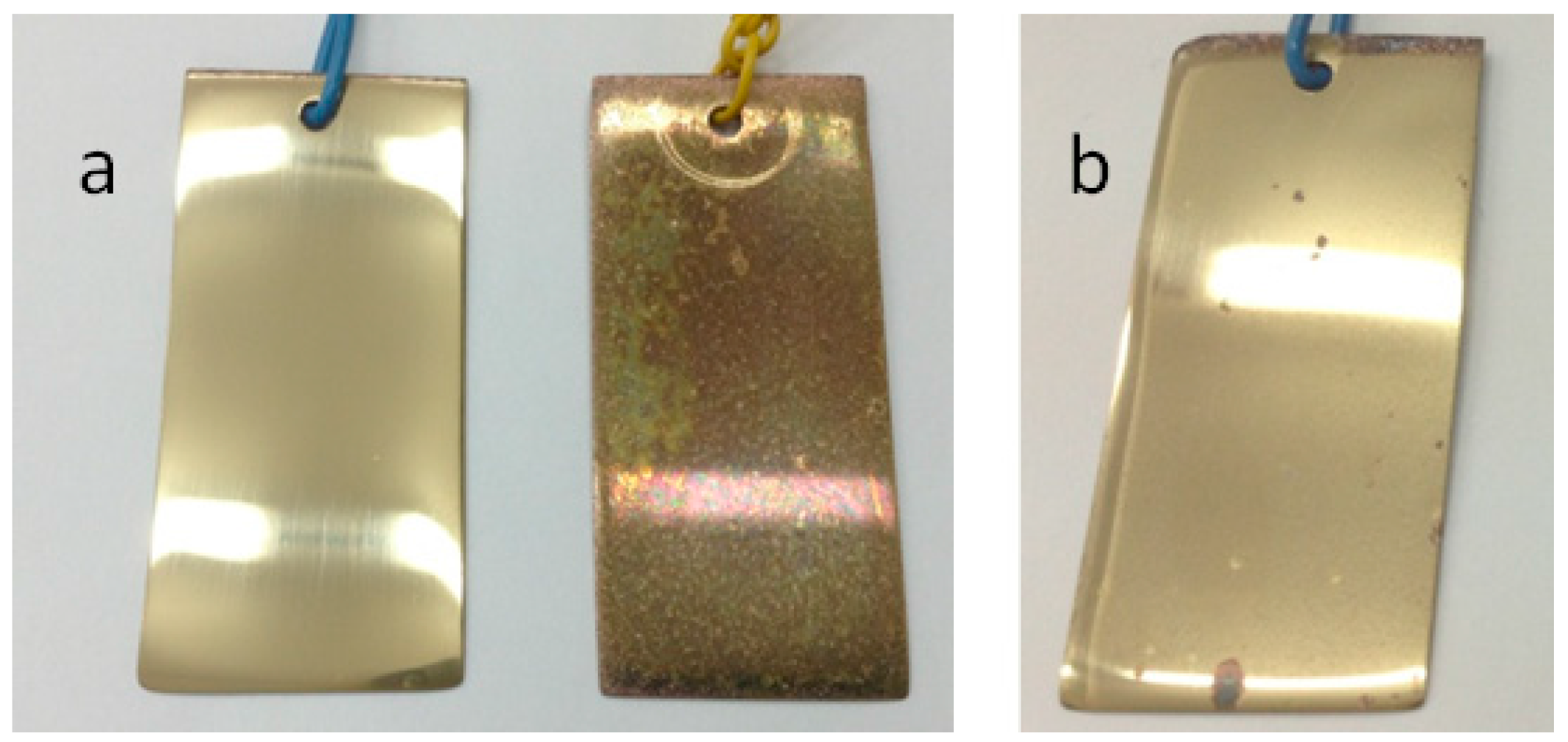


| Time | Zn(OH)2 | ZnO | Zn(m) | CuMPATA | Cu2O | Cu(m) |
|---|---|---|---|---|---|---|
| 1 min | 4.42 | 0.30 | 2.64 | 12.80 | 37.78 | 42.06 |
| 15 min | 1.80 | 0.40 | 2.40 | 30.26 | 37.07 | 28.06 |
| 30 min | 1.50 | 0.58 | 2.07 | 33.68 | 31.61 | 30.56 |
| 120 min | 2.94 | 0.65 | 1.59 | 36.02 | 30.84 | 27.95 |
| 1020 min | 4.26 | 1.93 | 1.05 | 45.41 | 27.54 | 19.81 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkhipushkin, I.А.; Shikhaliev, K.S.; Potapov, A.Y.; Sapronova, L.V.; Kazansky, L.P. Inhibition of Brass (80/20) by 5-Mercaptopentyl-3-Amino-1,2,4-Triazole in Neutral Solutions. Metals 2017, 7, 488. https://doi.org/10.3390/met7110488
Arkhipushkin IА, Shikhaliev KS, Potapov AY, Sapronova LV, Kazansky LP. Inhibition of Brass (80/20) by 5-Mercaptopentyl-3-Amino-1,2,4-Triazole in Neutral Solutions. Metals. 2017; 7(11):488. https://doi.org/10.3390/met7110488
Chicago/Turabian StyleArkhipushkin, Ivan А., Khidmet S. Shikhaliev, Andrei Y. Potapov, Lyudmila V. Sapronova, and Leonid P. Kazansky. 2017. "Inhibition of Brass (80/20) by 5-Mercaptopentyl-3-Amino-1,2,4-Triazole in Neutral Solutions" Metals 7, no. 11: 488. https://doi.org/10.3390/met7110488





