Reduced Graphene Oxide Reinforced 7075 Al Matrix Composites: Powder Synthesis and Mechanical Properties
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Research Materials
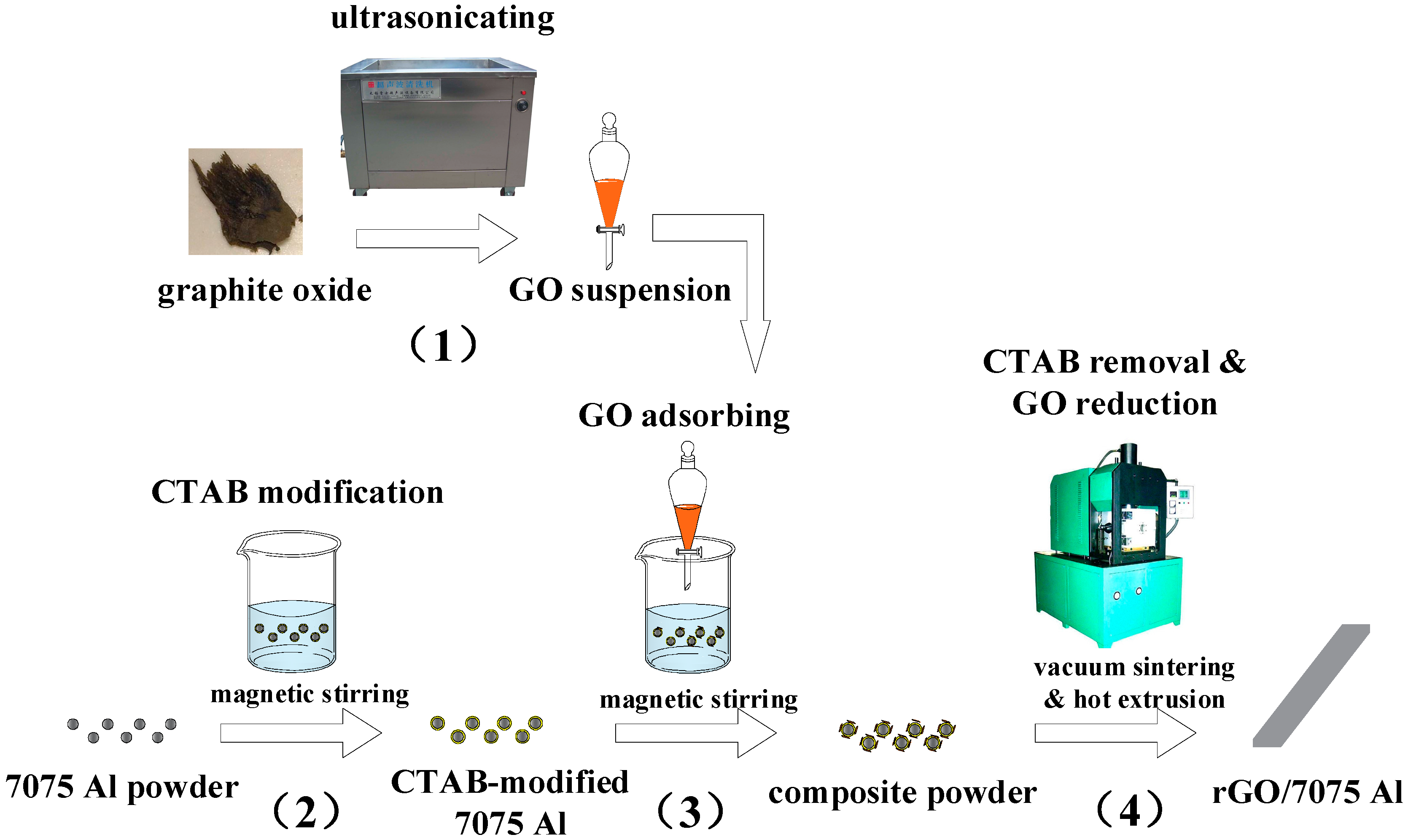
2.2. Composites Preparation
- (1)
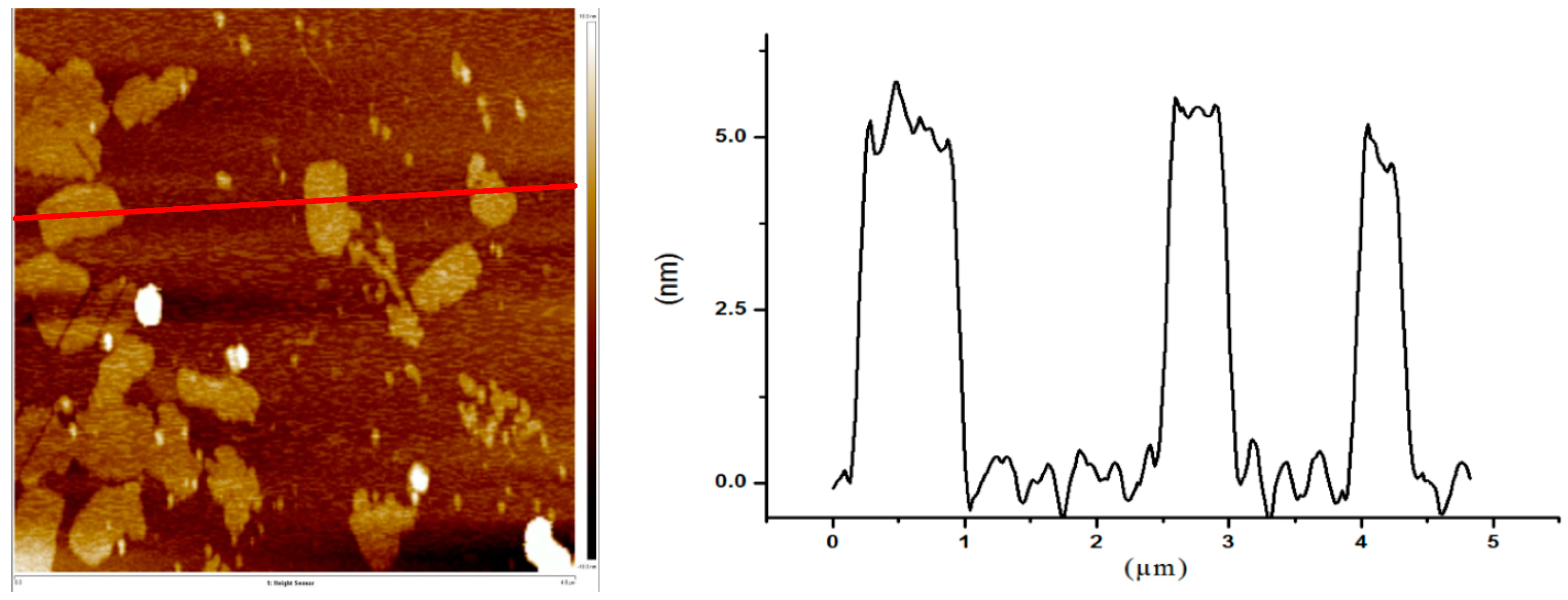
- Preparation of GO aqueous dispersion: To strip the as-received graphite oxides into GO nanaosheets with several-layers structure, the graphite oxide was added into deionized water and then ultrasonicated for 2 h to obtain a brown dispersion with no residual sediment. A 1 mg/mL GO aqueous dispersion was finally prepared. Figure 2 displays an AFM image of GO nanosheets obtained from the GO aqueous dispersion. As can be seen, the thickness of the GO nanosheets was ~5 nm. Considering the thickness of monolayer GO nanosheets was ~1 nm due to the attachment of oxygen functionalities [19], the GO nanosheets used in this investigation were no more than five layers.
- (2)
- Modifying 7075 Al powders with CTAB: 50 g 7075 Al powders and 300 mL CTAB aqueous solution (0.8 wt %) were magnetically stirred for 2 h, filtered, and then rinsed with deionized water to obtain the CTAB-modified 7075 Al powders.
- (3)
- Adsorption of GO onto the 7075 Al powders: A powder slurry was prepared through adding CTAB-modified 7075 Al powders (~50 g) into deionized water. The GO aqueous dispersion was added drop by drop. The mixed slurry was magnetically stirred until the color changed to transparent, and was then filtered and rinsed to obtain the composite powders. The composite powders were finally vacuum dried at 70 °C for 8 h.
- (4)
- CTAB removal and rGO/7075 Al composites fabrication. No particular heating treatment was used to remove the CTAB and reduce the GO, because the sintering temperature (560 °C) was high enough to achieve the purpose. The green billets (30 mm in diameter and 40 mm in height) were prepared through compacting the composite powders under 140 MPa at room temperature. Subsequently, the green billets were heated in a vacuum furnace at 560 °C for 2 h, followed by hot pressing under 70 MPa for 10 min to ensure the density. After that, slabs with cross-sections 12 mm in width and 4 mm in thickness were obtained by hot extrusion at 450 °C. The extrusion ratio and ram speed used in present study were 14.7 and 1 mm/s, respectively. Subsequently, the slabs were solution-treated in a resistance furnace at 470 °C for 2 h, followed by water quenched, and then aged at 120 °C for 24 h. The contents of rGO in the composites were 0.15, 0.30, and 0.50 wt %, respectively. For comparison, a 7075 Al sample was also prepared using the same method without adding GO.
2.3. Mechanical Properties and Density Measurements
2.4. Microstructure Characterizations
3. Results
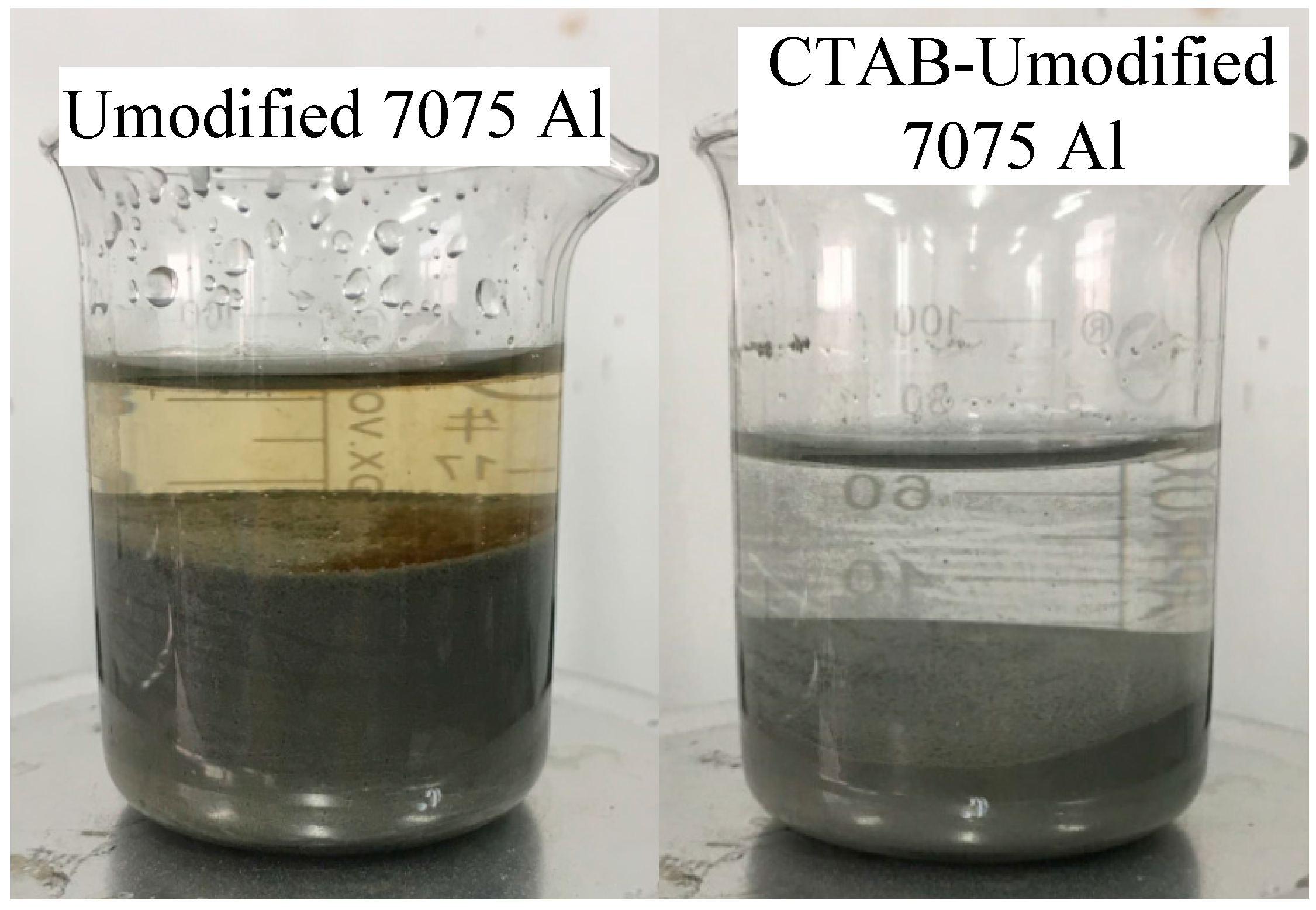
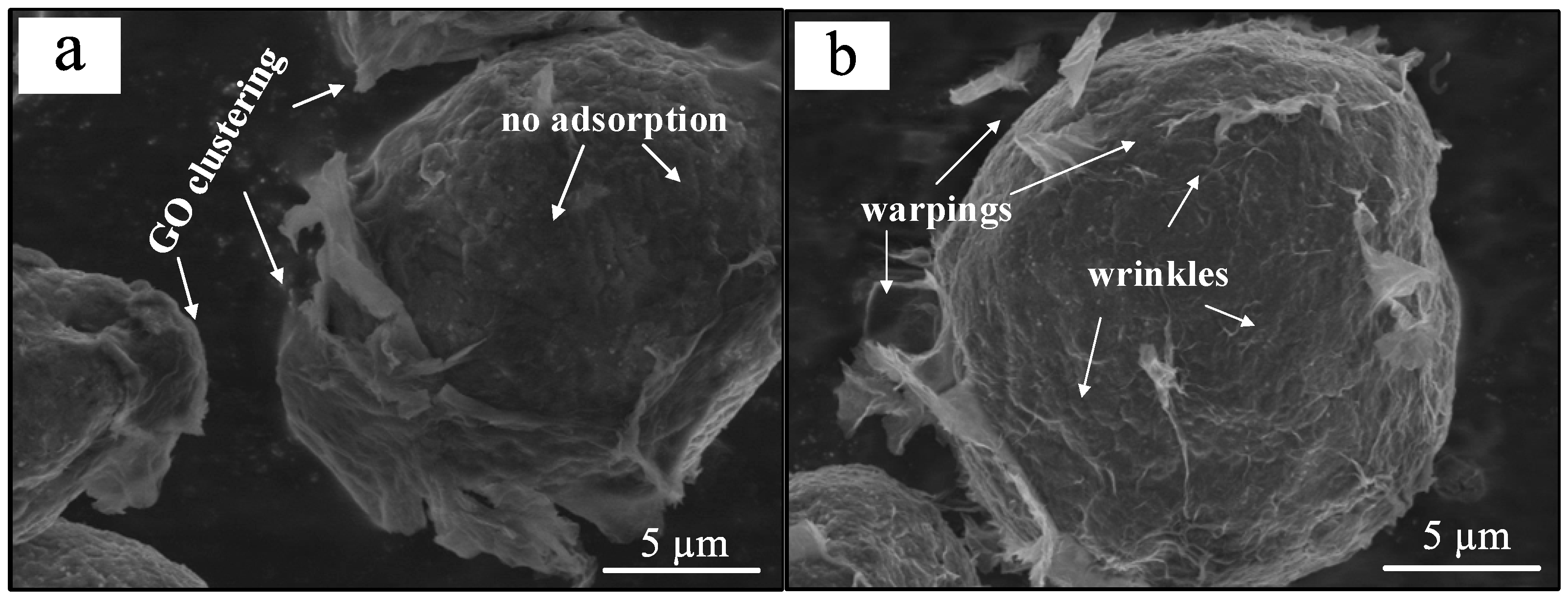
3.1. Improvement in Adsorption Uniformity of GOs by CTAB Modification
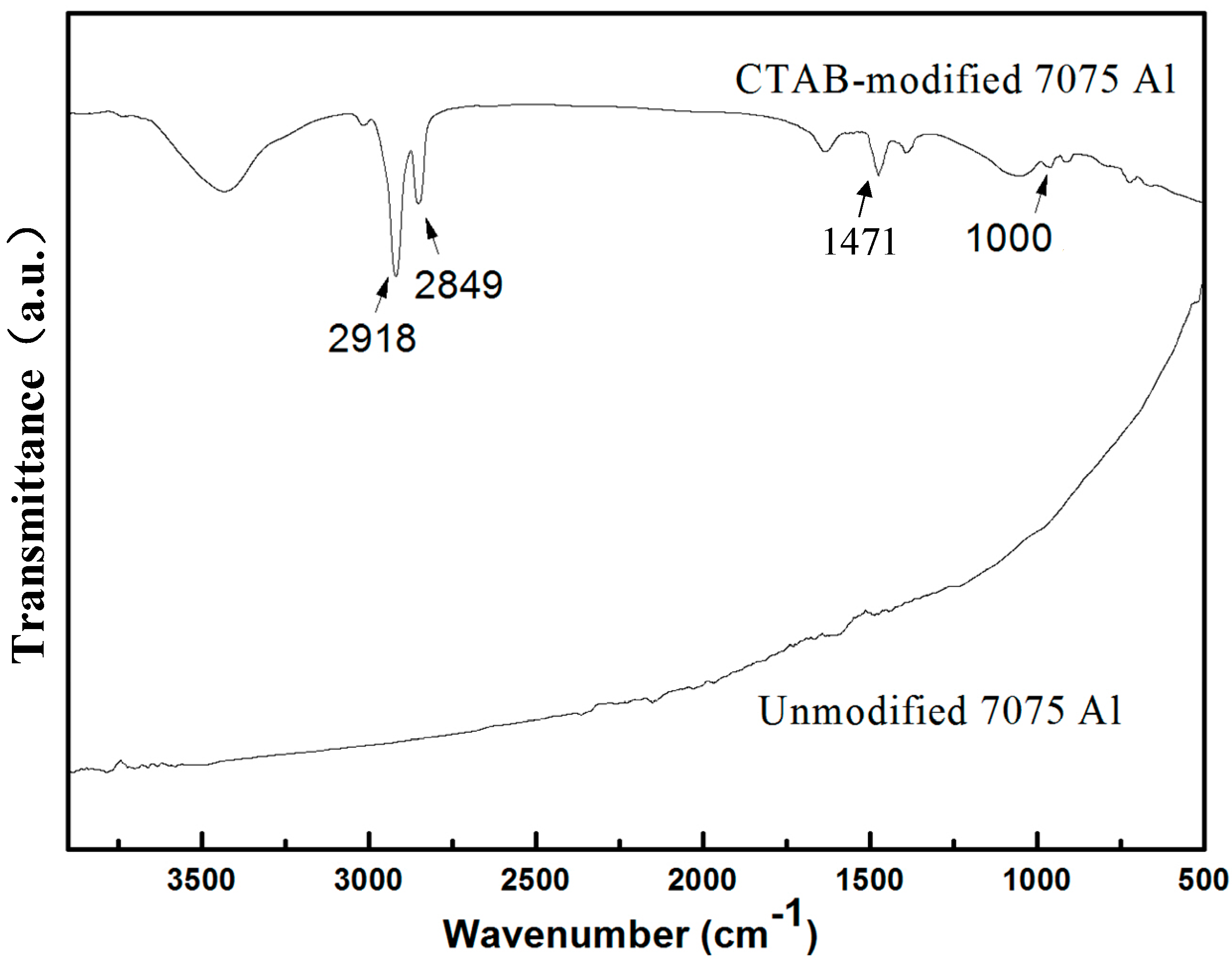
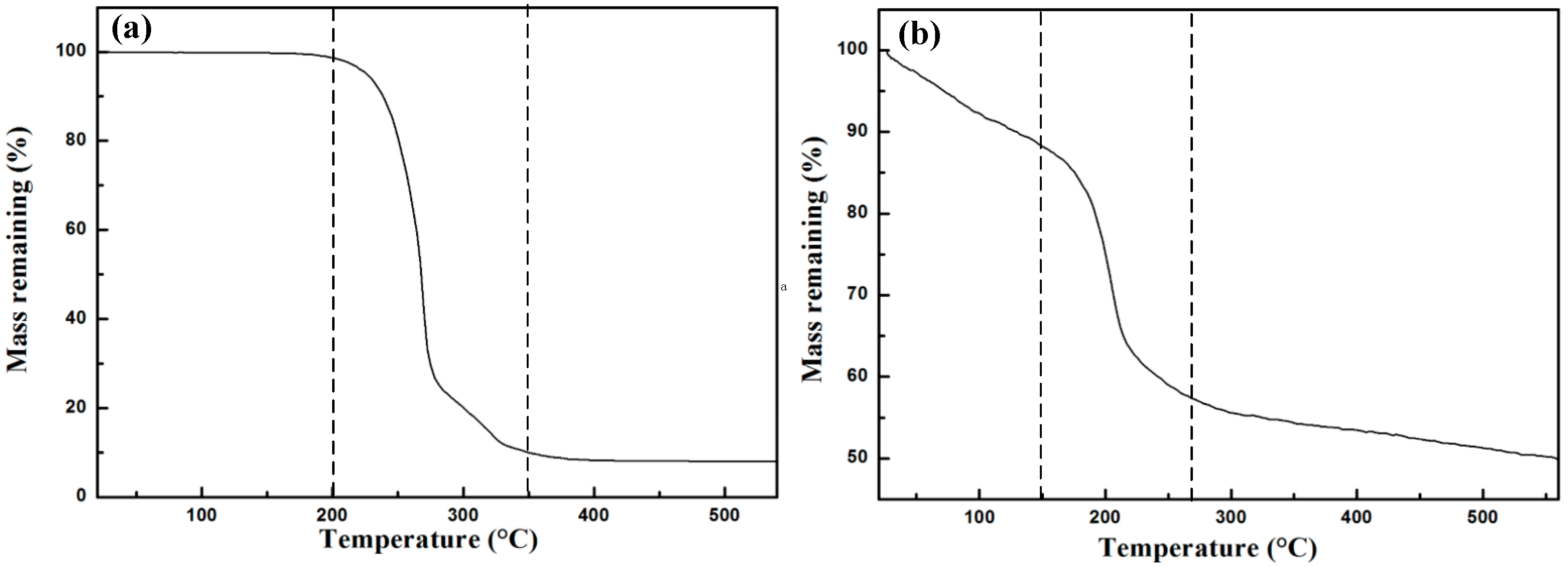
3.2. Removal of CTAB and Thermal Reduction of GO during Sintering Process
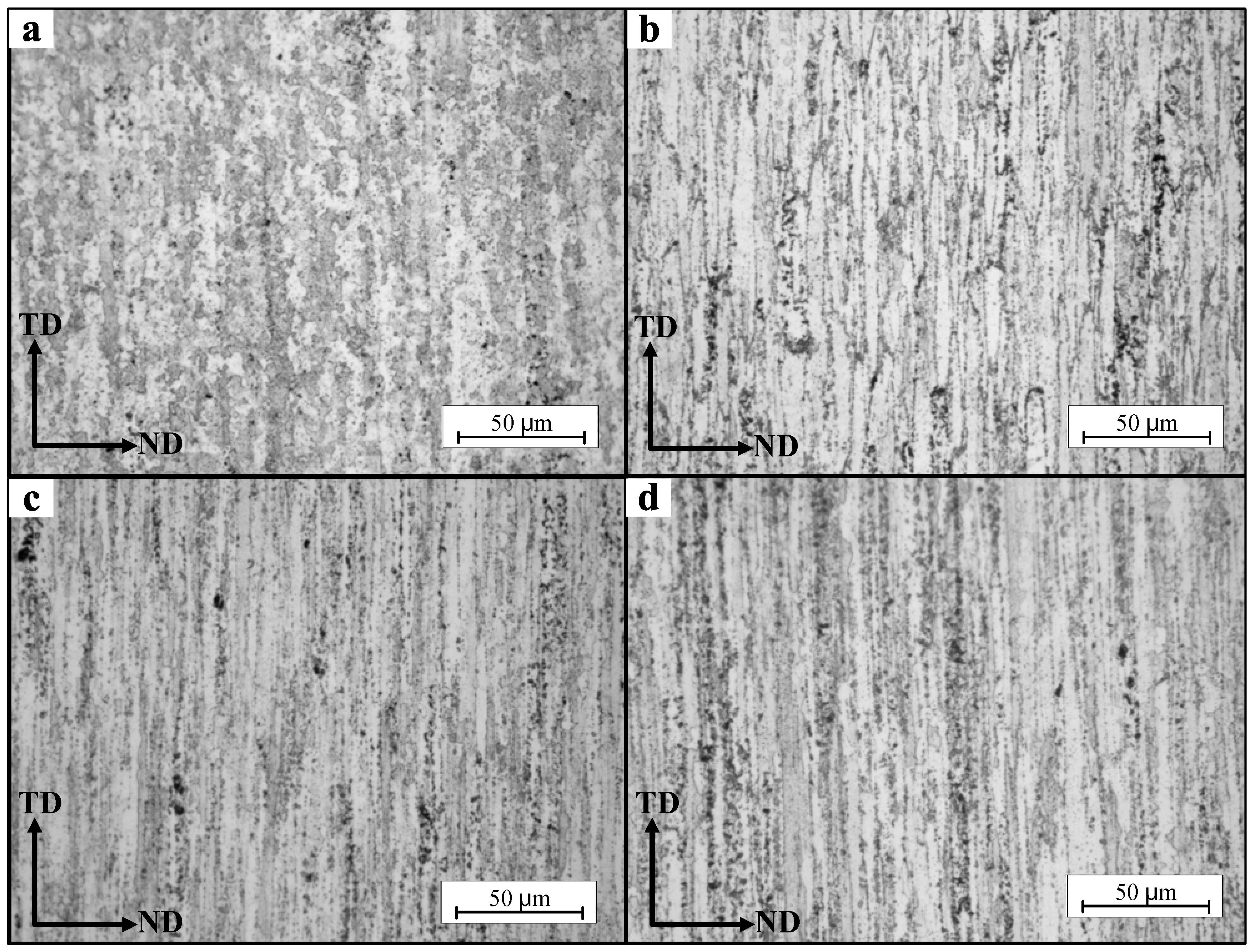
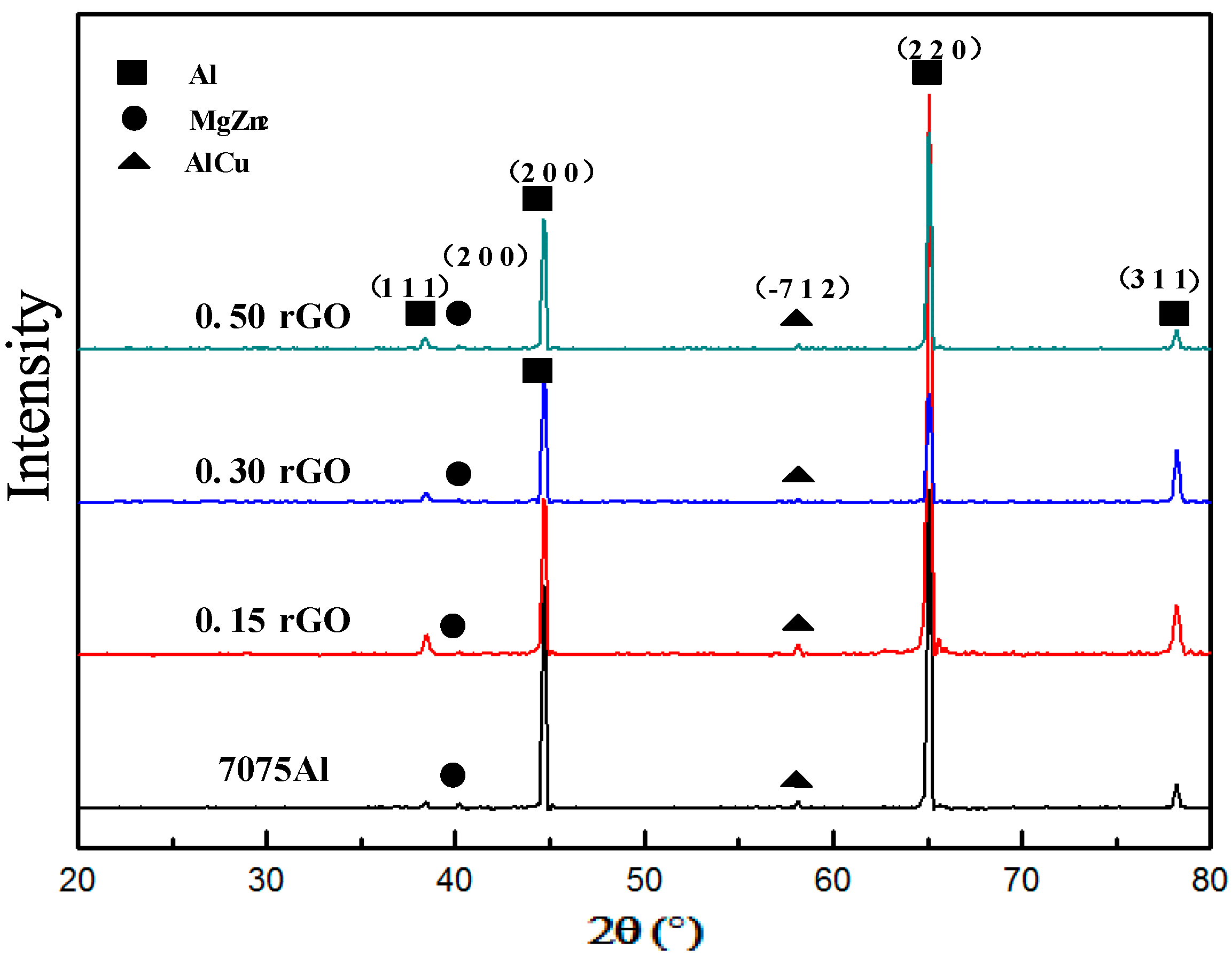
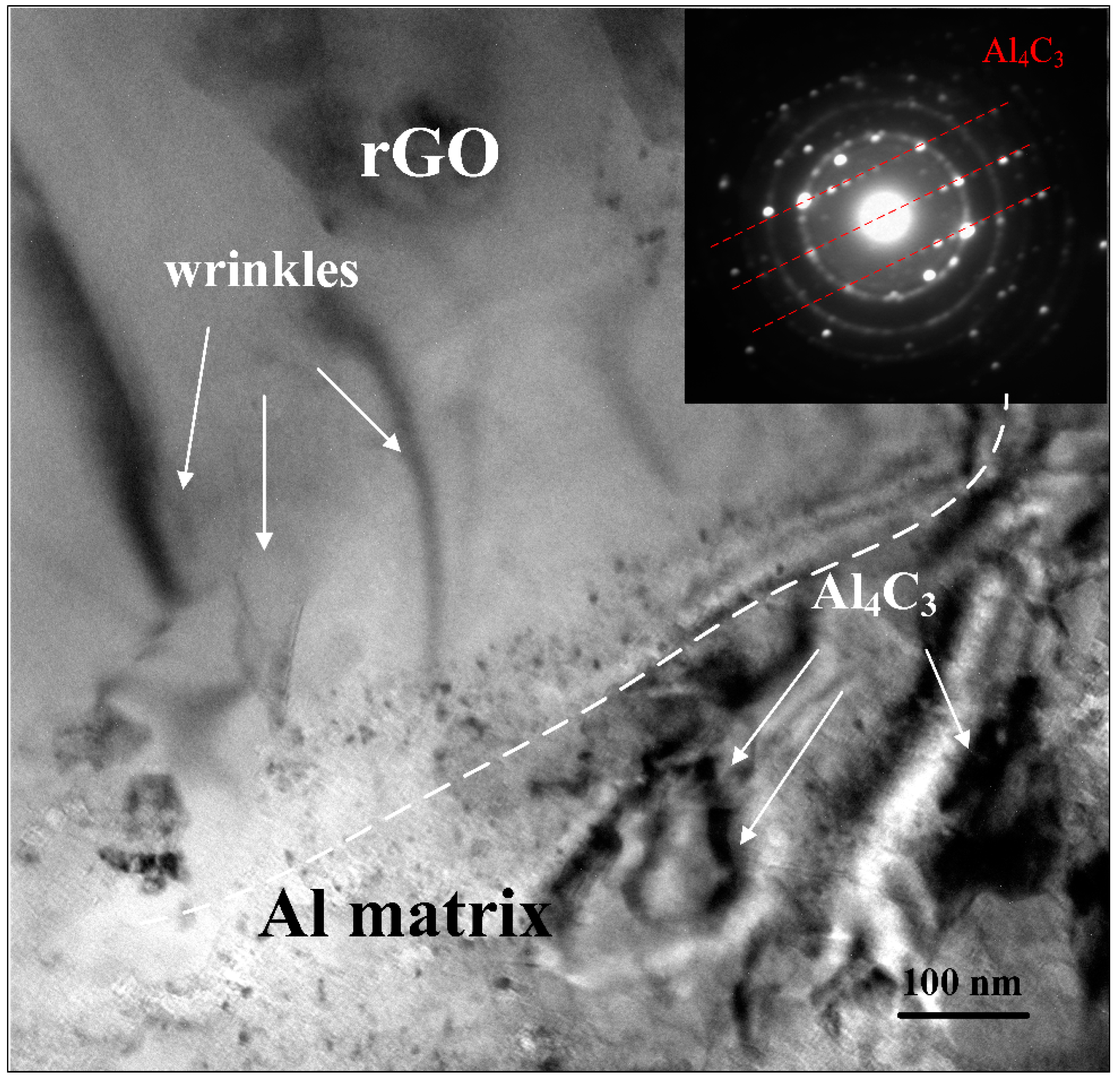
3.3. Microstructure
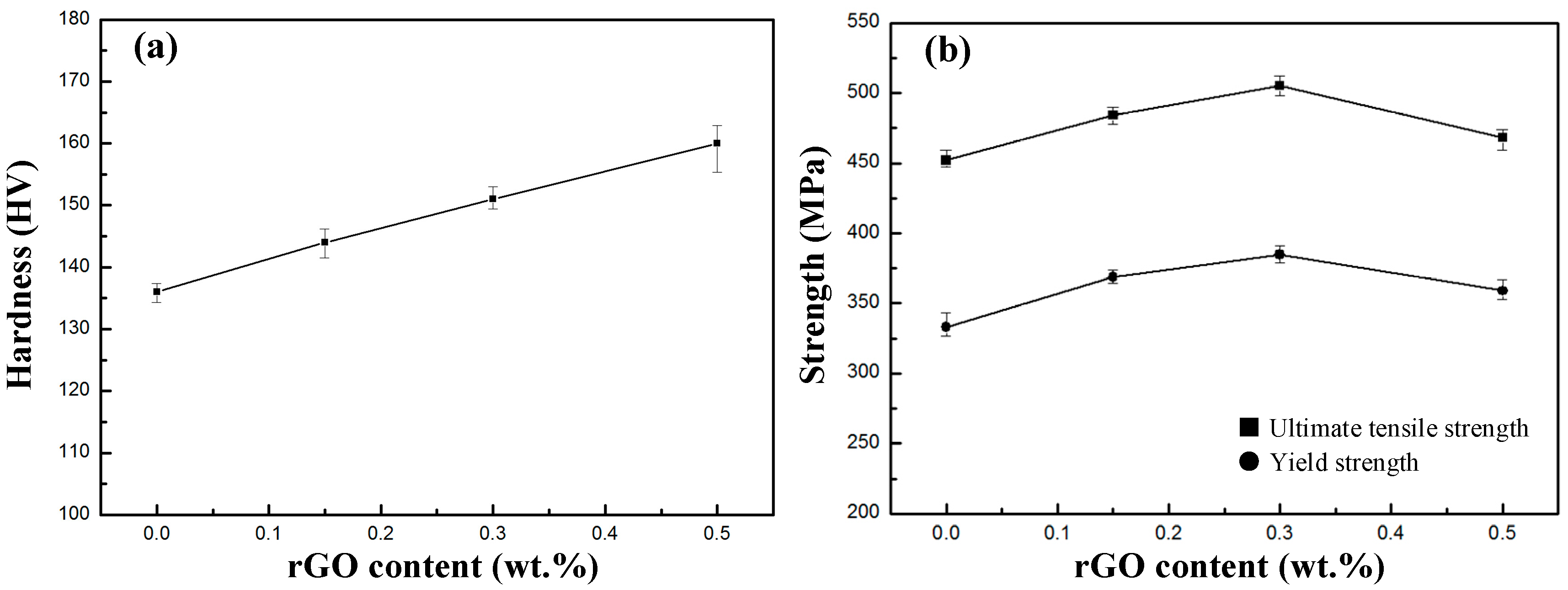
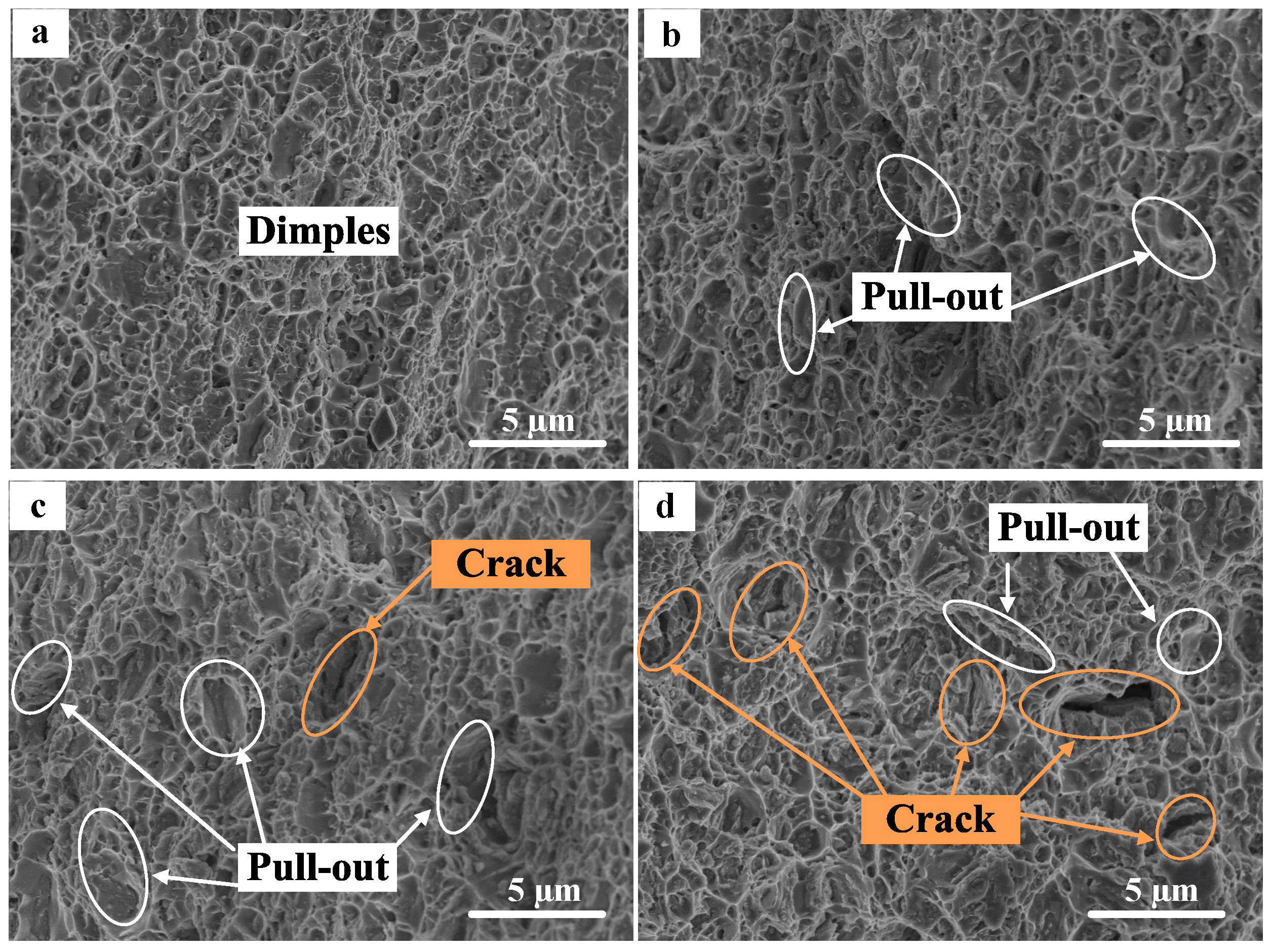
3.4. Mechanical Properties
4. Discussion
5. Conclusions
- (1)
- The significant improvement in the adsorption uniformity of GO is attributed to the formation of the cationic CTAB membrane on 7075 Al powders. Due to the negatively-charged nature, GO tends to absorb onto the cationic CTAB membrane through electrostatic attraction and, thus, a uniform distribution of GO in the CTAB-modified 7075 Al powders is effectively achieved.
- (2)
- During the sintering process, CTAB is effectively removed before 350 °C so that it would have little negative effect on the mechanical properties of composites. On the other hand, the thermal reduction of GO is demonstrated by the results of TGA and FTIR.
- (3)
- The Vickers hardness of rGO/7075 Al composites increases with the increase in rGO content. rGO has a significant effect on the tensile properties of 7075 Al alloys. Compared with 7075 Al, the composites all show higher tensile properties. The yield strength and the ultimate tensile strength both reach the peak values for rGO/7075 Al composite with 0.30 wt % rGO addition. The yield strength and ultimate tensile strength of 0.30 rGO/7075 Al are increased by 15.6% and 11.7% compared with 7075 Al. The improvement in strength of rGO/7075 Al composites is attributed to stress transfer and dislocation strengthening. With rGO content reaching 0.50 wt %, however, the excessive addition of rGO gives rise to a weakening in the enhancement of the tensile properties compared with 0.30 rGO/Al composite, due to the increasing amounts of brittle Al4C3 and cracks.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miracle, D.B. Metal matrix composites-from science to technological significance. Compos. Sci. Technol. 2005, 65, 2526–2540. [Google Scholar]
- Surappa, M.K. Aluminum matrix composites: Challenges and opportunities. Sadhana 2003, 28, 319–334. [Google Scholar]
- Williams, J.C.; Strake, E.A., Jr. Process in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar]
- Li, B.; Pan, Q.L.; Chen, C.P.; Wu, H.H.; Yin, Z.M. Effects of solution treatment on microstructural and mechanical properties of Al-Zn-Mg alloy by microalloying with Sc and Zr. J. Alloys Compd. 2016, 664, 553–564. [Google Scholar]
- Davydov, V.G.; Rostova, T.D.; Zakharov, V.V.; Filatov, Y.A.; Yelagin, V.I. Scientific principles of making an alloying addition of scandium to aluminum alloys. Mater. Sci. Eng. A 2000, 280, 30–36. [Google Scholar]
- Jones, M.J.; Humphreys, F.J. Interaction of recrystallization and precipitation: The effect of Al3Sc on the recrystallization behavior of deformed aluminum. Acta Mater. 2003, 51, 2149–2159. [Google Scholar]
- Shen, Q.; Wu, C.D.; Luo, G.Q.; Fang, P.; Li, C.Z.; Wang, Y.Y.; Zhang, L.M. Microstructure and mechanical properties of Al-7075/B4C composites fabricated by plasma activated sintering. J. Alloys Compd. 2014, 588, 265–270. [Google Scholar]
- Panigrahi, S.K.; Jayaganthan, R. Effect of ageing on microstructure and mechanical properties of bulk, cryorolled, and room temperature rolled Al 7075 alloy. J. Alloys Compd. 2011, 509, 9609–9616. [Google Scholar]
- Taleghani, M.A.J.; Ruiz Navas, E.M.; Torralba, J.M. Microstructural and mechanical characterization of 7075 aluminium alloy consolidated from a premixed powder by cold compaction and hot extrusion. Mater. Des. 2014, 55, 674–682. [Google Scholar]
- Seiner, H.; Ramirez, C.; Koller, M.; Sedlak, P.; Landa, M.; Miranzo, P.; Belmonte, M.; Osendi, M.I. Elastic properties of silicon nitride ceramics reinforced with graphene nanofillers. Mater. Des. 2015, 87, 675–680. [Google Scholar]
- Wang, H.D.; Kurata, K.; Fukunaga, T.; Zhang, X.; Takamatsu, H. Width dependent intrinsic thermal conductivity of suspended monolayer graphene. Int. J. Heat Mass Transf. 2017, 105, 76–80. [Google Scholar]
- Bolotin, K.I.; Sikes, K.J.; Jiang, Z.; Klima, M.; Fudenberg, G.; Hone, J.; Kim, P.; Stormer, H.L. Ultrahigh electron mobility in suspended graphene. Solid State Commun. 2008, 146, 351–355. [Google Scholar]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [PubMed]
- Begum, K.R.; Sankeshwar, N.S. Electronic thermal conduction in suspended graphene. Phys. E Low-Dimens. Syst. Nanostruct. 2015, 73, 27–34. [Google Scholar]
- Rashad, M.; Pan, F.S.; Tang, A.T.; Asif, M. Effect of Graphene Nanoplatelets addition on mechanical properties of pure aluminum using a semi-powder method. Prog. Nat. Sci. Mater. Int. 2014, 24, 101–108. [Google Scholar]
- Wang, J.Y.; Li, Z.Q.; Fan, G.L.; Pan, H.H.; Chen, Z.X.; Zhang, D. Reinforcement with graphene nanosheets in aluminum matrix composites. Scr. Mater. 2012, 66, 594–597. [Google Scholar]
- Bartolucci, S.F.; Paras, J.; Rafiee, M.A.; Rafiee, J.; Lee, S.; Kapoor, D.; Koratkar, N. Graphene-aluminum nanocomposites. Mater. Sci. Eng. A 2011, 528, 7933–7937. [Google Scholar]
- Gao, X.; Yue, H.Y.; Guo, E.J.; Zhang, H.; Lin, X.Y.; Yao, L.H.; Wang, B. Preparation and tensile properties of homogeneously dispersed graphene reinforced aluminum matrix composites. Mater. Des. 2016, 94, 54–60. [Google Scholar]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar]
- ASTM Standard E8/E8M, 2009. Standard Test Methods for Tension Testing of Metallic Materials; ASTM International: Montgomery County, PA, USA, 2009. [CrossRef]
- Zhu, J.X.; He, H.P.; Zhu, L.Z.; Wen, X.Y.; Deng, F. Characterization of organic phases in the interlayer of montmorillonite using FTIR and 13C NMR. J. Colloid Interface Sci. 2005, 286, 239–244. [Google Scholar] [PubMed]
- Yu, W.H.; Ren, Q.Q.; Tong, D.S.; Zhou, C.H.; Wang, H. Clean production of CATB-montmorillonite: Formation mechanism and swelling behavior in xylene. Appl. Clay Sci. 2014, 97–98, 222–234. [Google Scholar]
- Han, N.M.; Zhang, X.M.; Liu, S.D.; He, D.G.; Zhang, R. Effect of solution treatment on the strength and fracture toughness of aluminum alloy 7050. J. Alloys Compd. 2011, 509, 4138–4145. [Google Scholar]
- Meng, Q.N.; Wen, M.; Mao, F.; Nedfors, N.; Jansson, U.; Zheng, W.T. Deposition and characterization of reactive magnetron sputtered zirconium carbide films. Surf. Technol. 2013, 232, 876–883. [Google Scholar]
- Li, G.; Xiong, B.W. Effect of graphene content on microstructures and tensile property of graphene-nanosheets/aluminum composites. J. Alloys Compd. 2017, 697, 31–36. [Google Scholar]
- Jiang, L.; Fan, G.L.; Li, Z.Q.; Kai, X.Z.; Zhang, D.; Chen, Z.X.; Humphries, S.; Heness, G.; Yeung, W.Y. An approach to the uniform dispersion of a high volume fraction of carbon nanotubes in aluminum powder. Carbon 2011, 49, 1968–1971. [Google Scholar]
- Yang, Y.J.; Li, W.K. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306. [Google Scholar] [PubMed]
- Tegou, E.; Pseiropoulos, G.; Filippidou, M.K.; Chatzandroulis, S. Low-temperature thermal reduction of graphene oxide films in ambient aptmosphere: Infra-red spectroscopic studies and gas sensing applications. Microelectron. Eng. 2016, 159, 146–150. [Google Scholar]
- Dixit, S.; Mahata, A.; Mahapatra, D.R.; Kailas, S.V.; Chattopadhyay, K. Multi-layer graphene reinforced aluminum—Manufacturing of high strength composite by friction stir alloying. Compos. Part B Eng. 2018, 136, 63–71. [Google Scholar]
- Bisht, A.; Srivastava, M.; Kumar, R.M.; Lahiri, I.; Lahiri, D. Strengthening mechanism in graphene nanoplatelets reinforced aluminum composite fabricated through spark plasma sintering. Mater. Sci. Eng. A 2017, 695, 20–28. [Google Scholar]
- Arsenault, R.J.; Shi, N. Dislocation generation due to differences between the coefficients of thermal expansion. Mater. Sci. Eng. 1986, 81, 175–187. [Google Scholar]
- Yin, C.M.; Wang, J.F. Effects of activation temperature on the deoxygenation specific surface area and supercapacitor performance of graphene. Carbon 2016, 109, 558–565. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Consideration of Orowan strengthening effect in particulate-reinforced metal matrix nanocomposites: A model for predicting their yield strength. Scr. Mater. 2006, 54, 1321–1326. [Google Scholar] [CrossRef]


| Elements | Zn | Mg | Cu | Fe | Si | Mn | Others | Al |
|---|---|---|---|---|---|---|---|---|
| (wt %) | 5.720 | 2.310 | 1.560 | 0.092 | 0.087 | 0.080 | 0.050 | bal. |
| Materials | Density (g·cm−3) | Theoretical Density (g·cm−3) | Relative Density (%) |
|---|---|---|---|
| 7075 Al | 2.7863 | 2.8000 | 99.51 |
| 0.15 rGO/7075 Al | 2.7830 | 2.7989 | 99.43 |
| 0.30 rGO/7075 Al | 2.7823 | 2.7979 | 99.44 |
| 0.50 rGO/7075 Al | 2.7795 | 2.7966 | 99.39 |
| Materials | Yield Strength (MPa) | UTS (MPa) | Elongation |
|---|---|---|---|
| 7075 Al | |||
| 0.15 rGO/7075 Al | |||
| 0.30 rGO/7075 Al | |||
| 0.50 rGO/7075 Al |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, C.; Liu, B.; Meng, Q.; Ma, S.; Dai, W. Reduced Graphene Oxide Reinforced 7075 Al Matrix Composites: Powder Synthesis and Mechanical Properties. Metals 2017, 7, 499. https://doi.org/10.3390/met7110499
Sun Y, Zhang C, Liu B, Meng Q, Ma S, Dai W. Reduced Graphene Oxide Reinforced 7075 Al Matrix Composites: Powder Synthesis and Mechanical Properties. Metals. 2017; 7(11):499. https://doi.org/10.3390/met7110499
Chicago/Turabian StyleSun, Youhong, Chi Zhang, Baochang Liu, Qingnan Meng, Shaoming Ma, and Wenhao Dai. 2017. "Reduced Graphene Oxide Reinforced 7075 Al Matrix Composites: Powder Synthesis and Mechanical Properties" Metals 7, no. 11: 499. https://doi.org/10.3390/met7110499




