Effects of Matte Grade on the Distribution of Minor Elements (Pb, Zn, As, Sb, and Bi) in the Bottom Blown Copper Smelting Process
Abstract
:1. Introduction
2. Method
2.1. Research Methodology
2.2. Verification of SKSSIM
2.3. Calculation Process
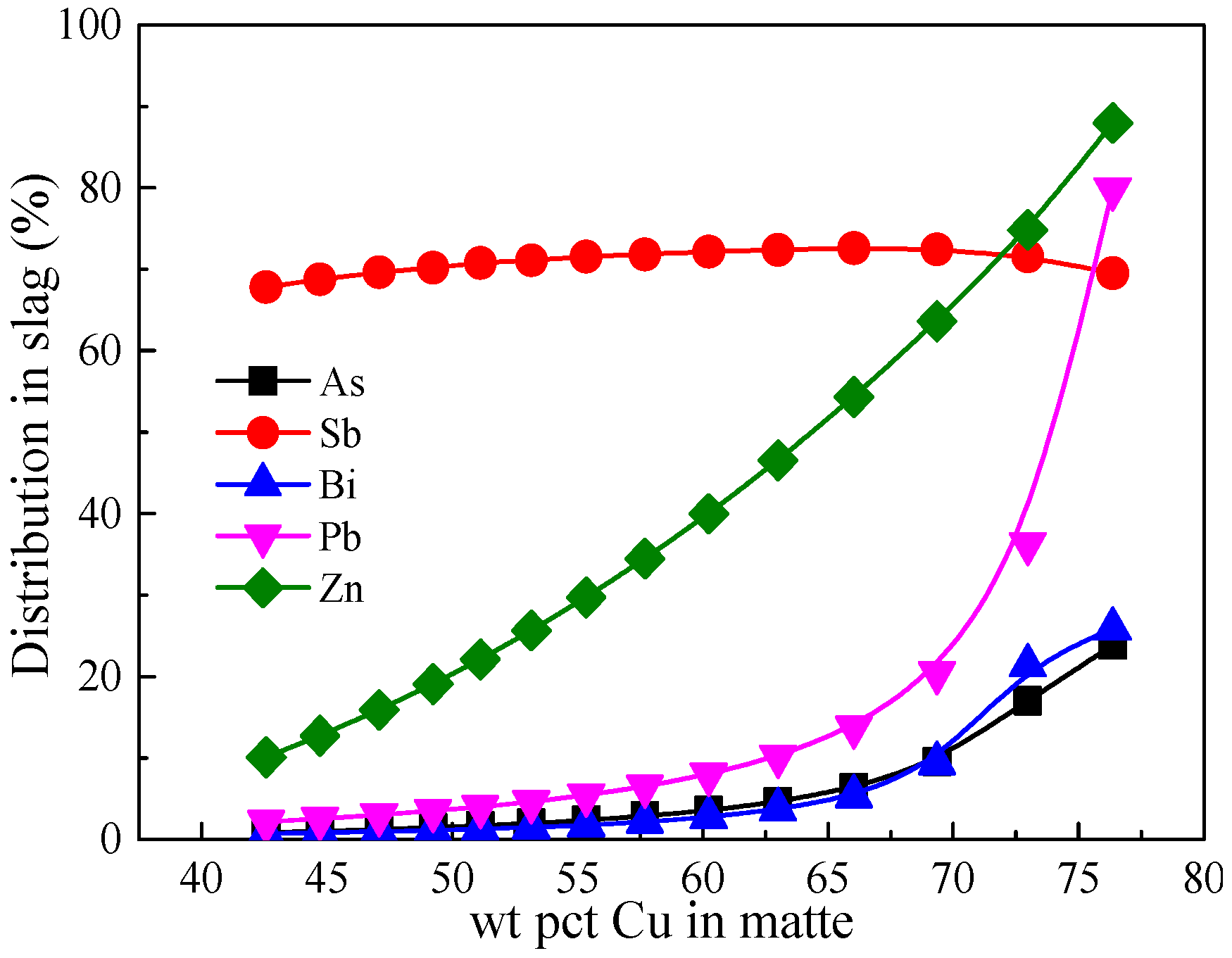
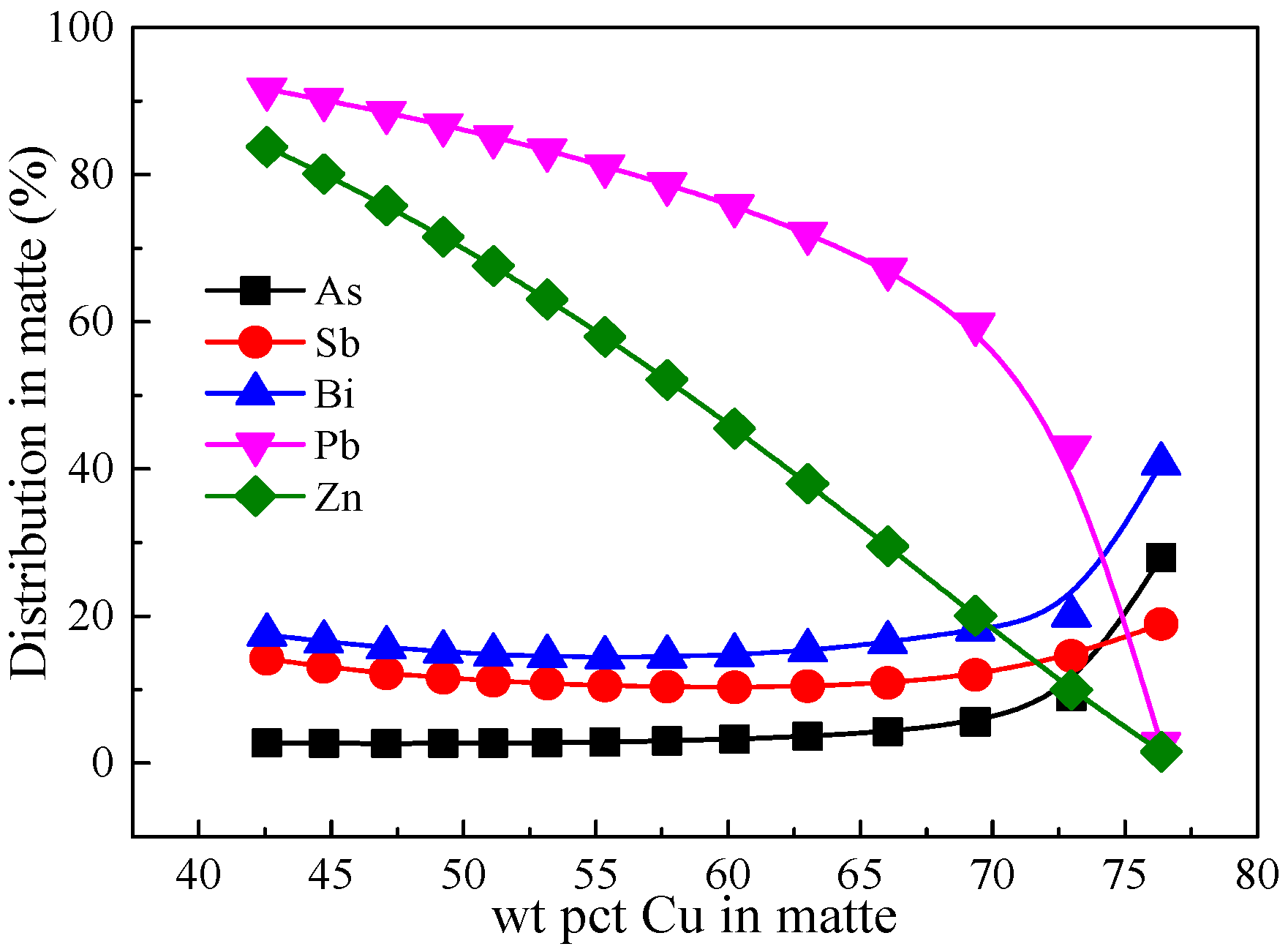
3. Results and Discussion
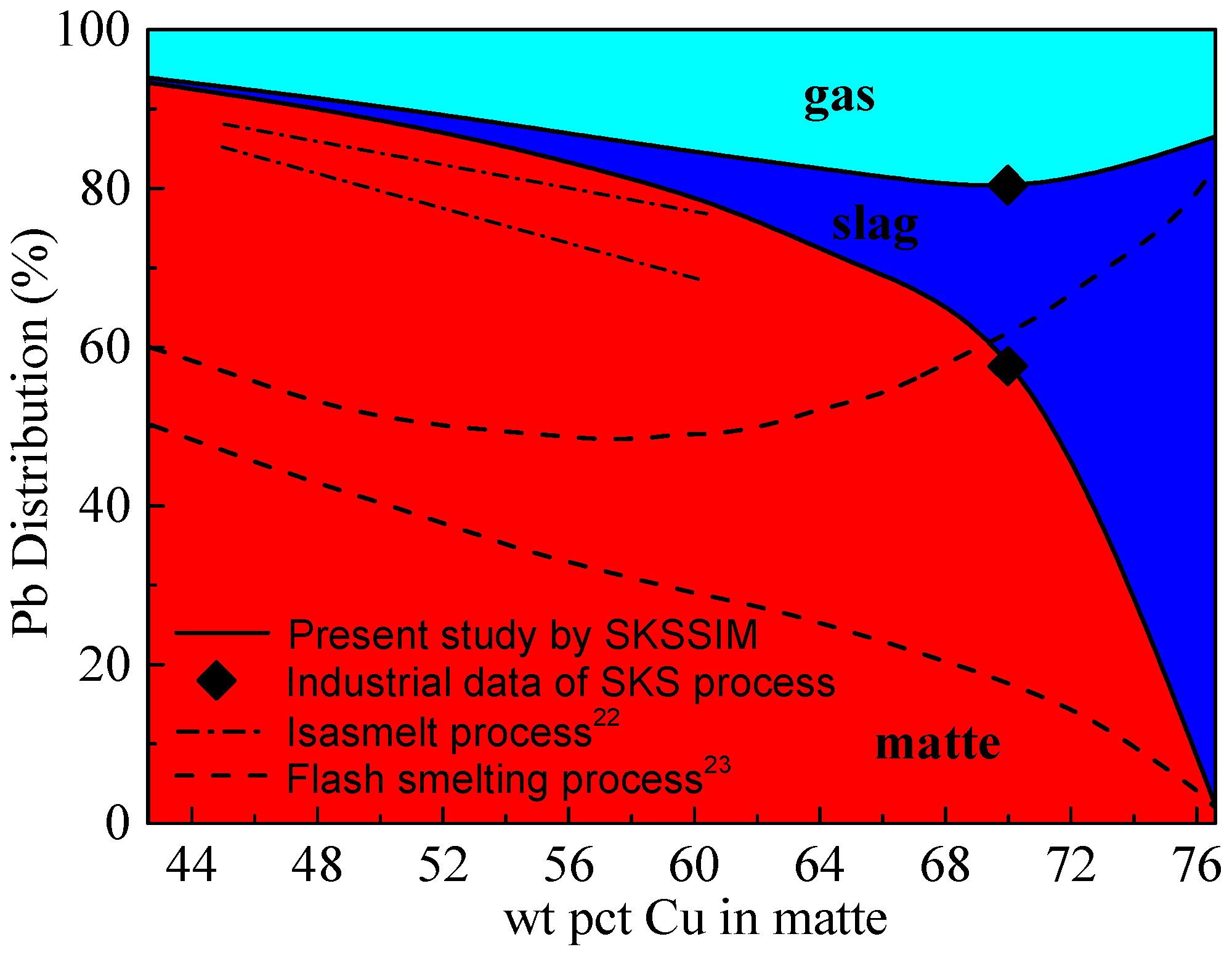
3.1. Distribution of Lead (Pb)
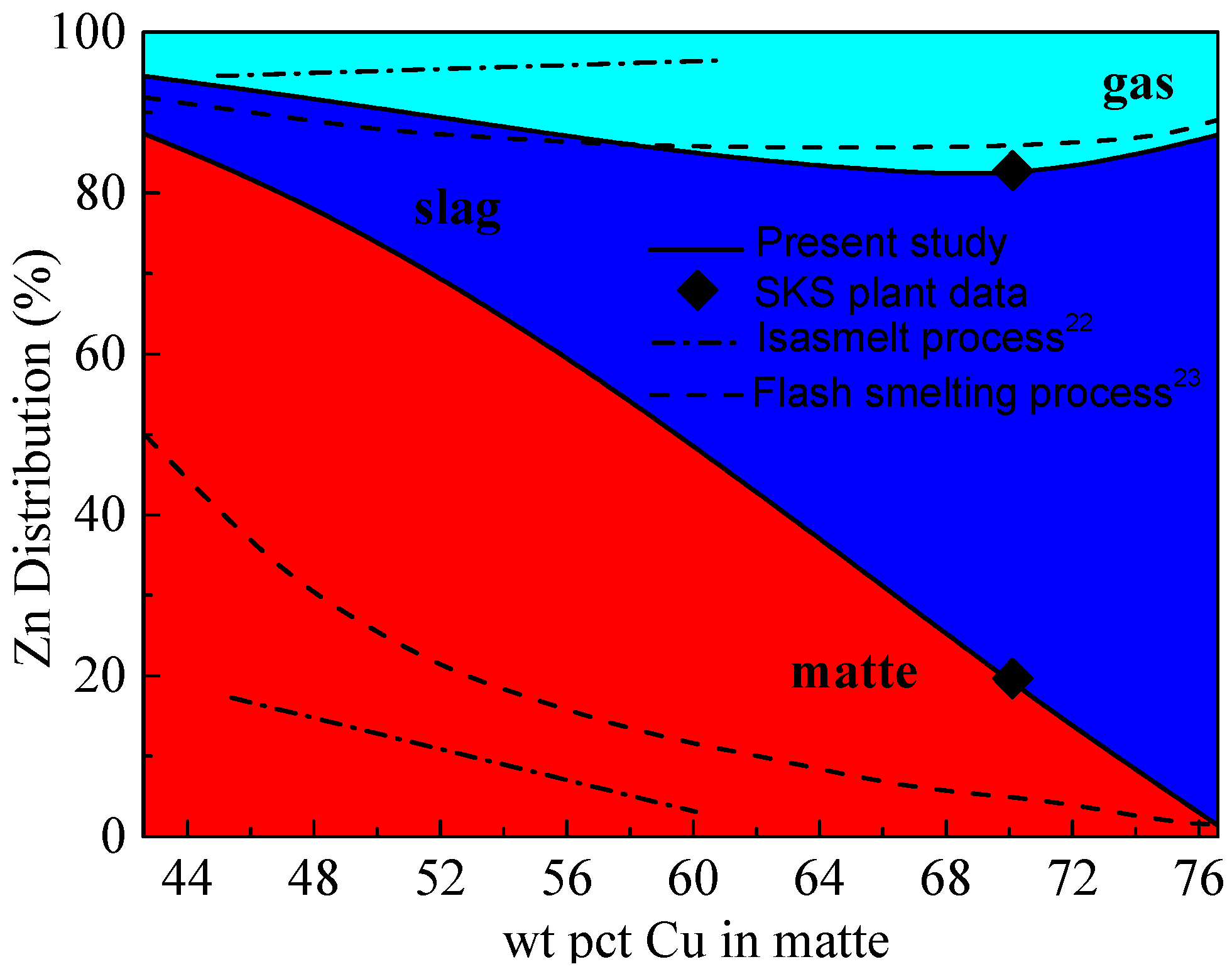
3.2. Distribution of Zinc (Zn)
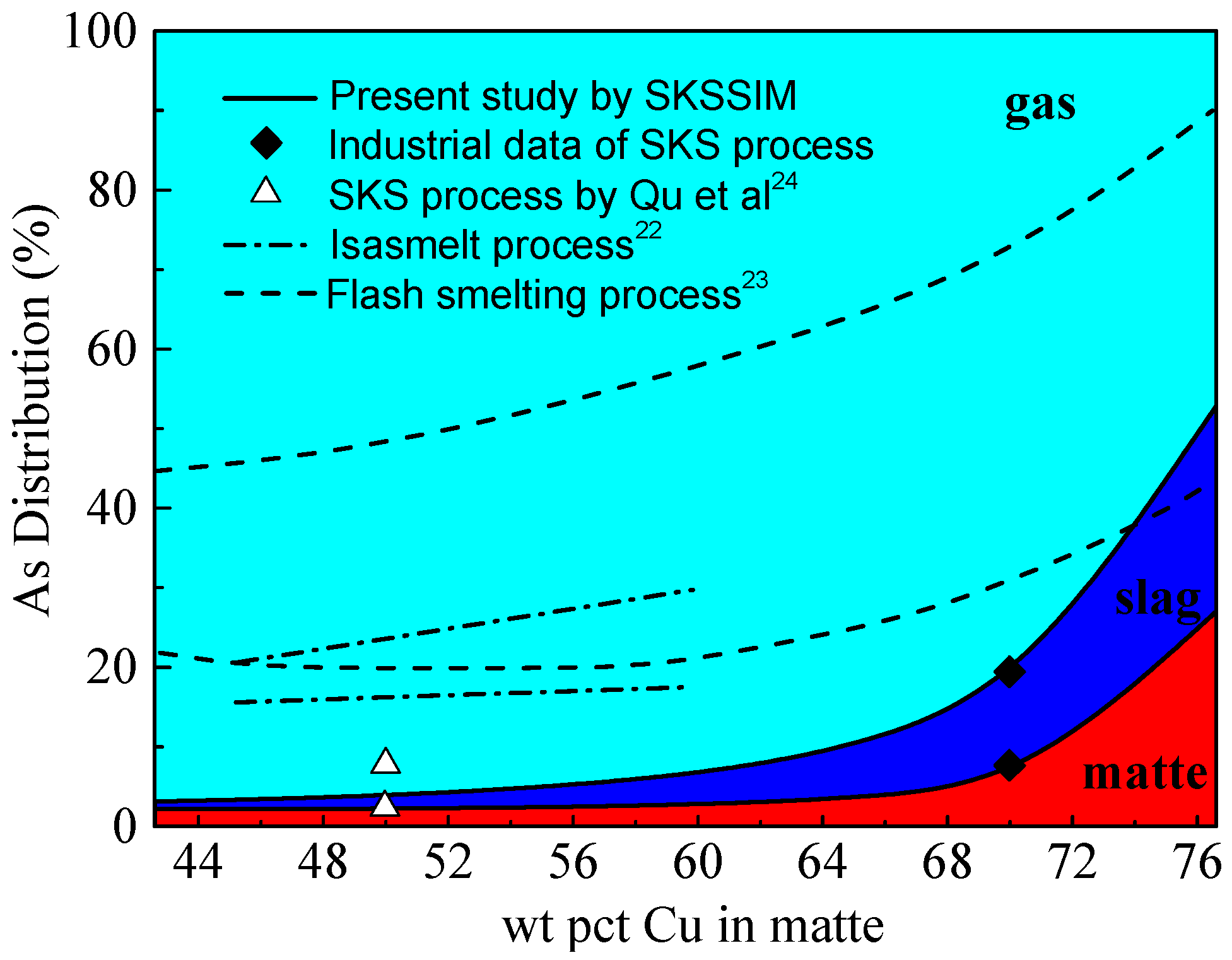
3.3. Distribution of Arsenic (As)
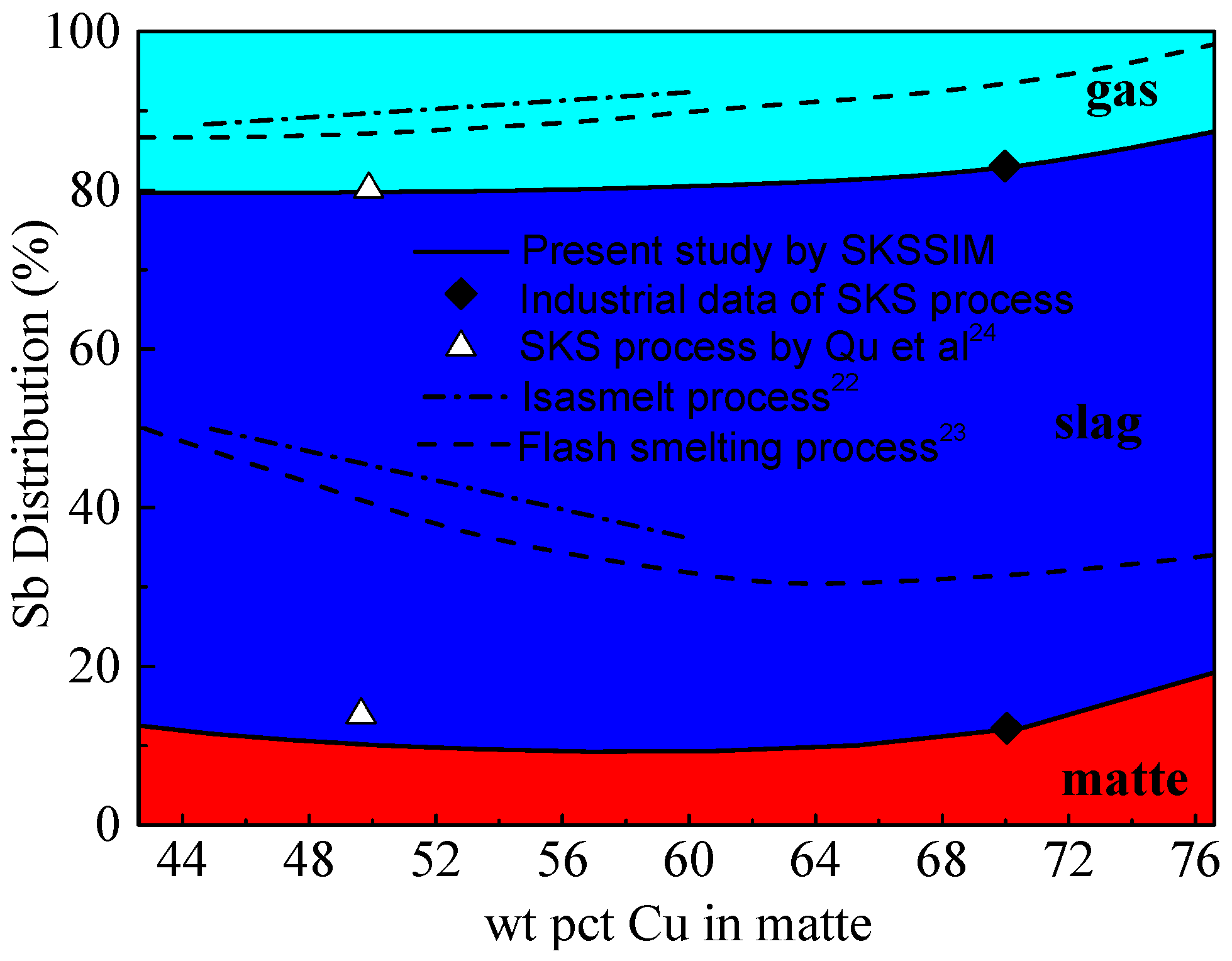
3.4. Distribution of Antimony (Sb)
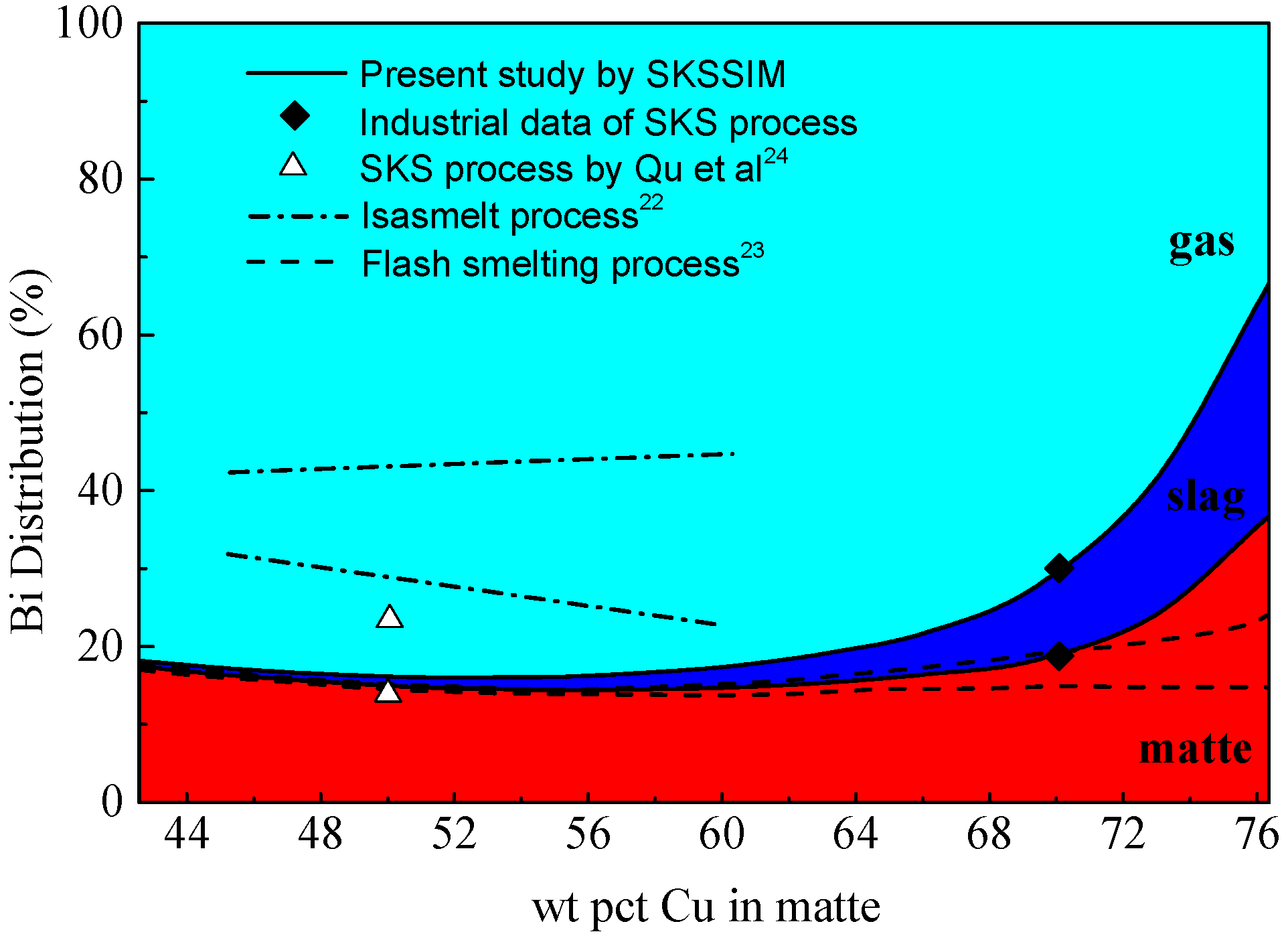
3.5. Distribution of Bismuth (Bi)
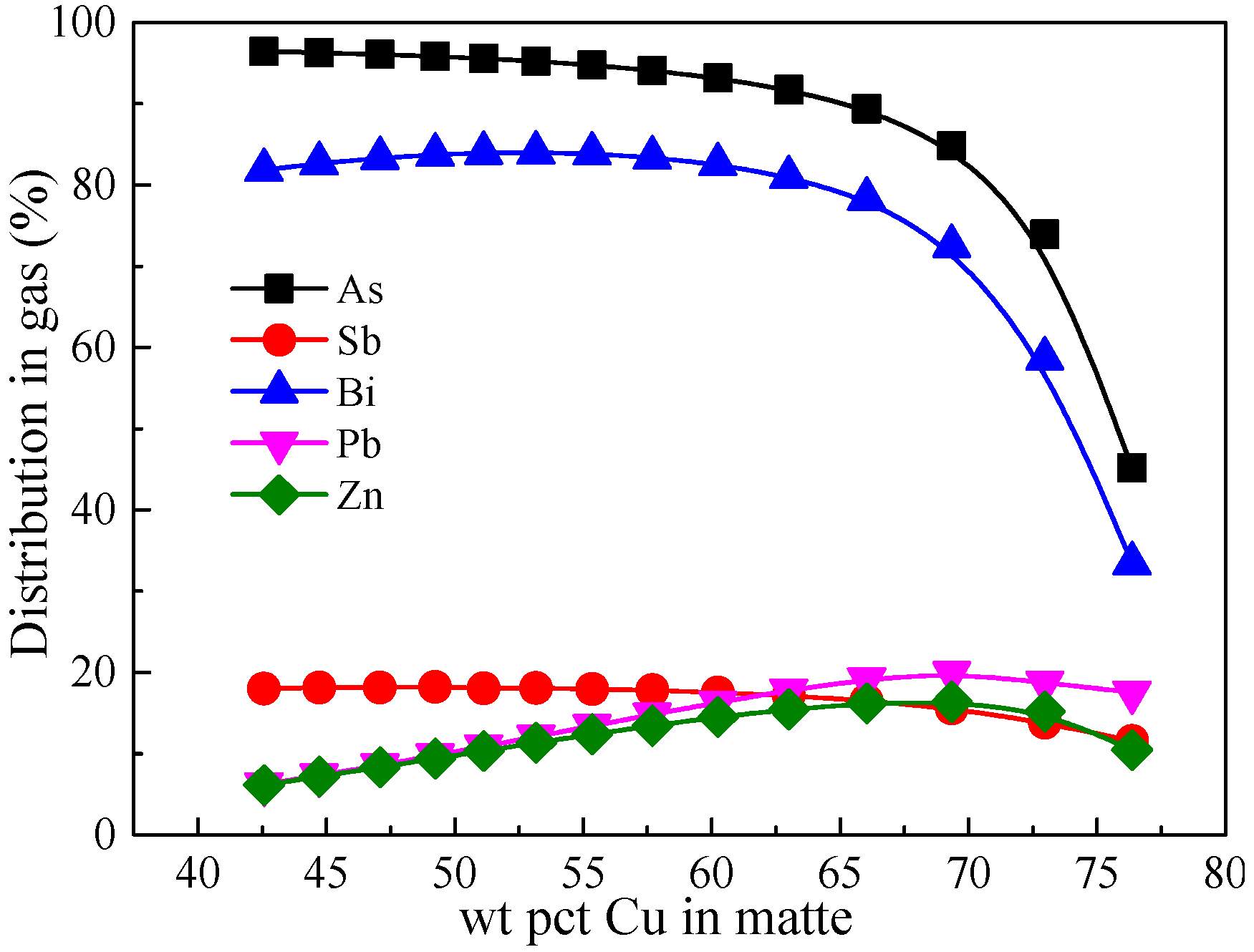
3.6. Comparison of Minor Elements Distribution
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, Q.M.; Guo, X.Y.; Tian, Q.H.; Chen, M.; Zhao, B.J. Reaction mechanism and distribution behavior of arsenic in the bottom blown copper smelting process. Metals 2017, 7, 302. [Google Scholar] [CrossRef]
- Coursol, P.; Mackey, P.J.; Kapusta, J.P.T.; Valencia, N.C. Energy consumption in copper smelting: A new Asian horse in the race. JOM 2015, 67, 1066–1074. [Google Scholar] [CrossRef]
- Li, W.F.; Zhan, J.; Fan, Y.Q.; Wei, C.; Zhang, C.F.; Hwang, J.Y. Research and industrial application of a process for direct reduction of molten high-lead smelting slag. JOM 2017, 69, 784–789. [Google Scholar] [CrossRef]
- Chen, L.; Hao, Z.D.; Yang, T.Z.; Liu, W.F.; Zhang, D.C.; Zhang, L.; Bin, S.; Bin, W.D. A comparison study of the oxygen-rich side blow furnace and the oxygen-rich bottom blow furnace for liquid high lead slag reduction. JOM 2015, 67, 1123–1129. [Google Scholar] [CrossRef]
- Liu, W.F.; Yang, T.Z.; Zhang, D.C.; Chen, L.; Liu, Y.F. A new pyrometallurgical process for producing antimony white from by-product of lead smelting. JOM 2014, 66, 1694–1700. [Google Scholar] [CrossRef]
- Yan, J. Recent operation of the oxygen bottom-blowing copper smelting and continuous copper converting technologies. In Proceedings of the 9th International Copper Conference (Copper 2016), Kobe, Japan, 13–16 November 2016; pp. 1015–1030. [Google Scholar]
- Cui, Z.X.; Wang, Z.; Wang, H.B.; Wei, C.B. Two-step copper smelting process at Dongying Fangyuan. In Proceedings of the 9th International Copper Conference (Copper 2016), Kobe, Japan, 13–16 November 2016; pp. 1043–1052. [Google Scholar]
- Chen, M.; Cui, Z.X.; Zhao, B.J. Slag chemistry of bottom blown copper smelting furnace at Dongying Fangyuan. In Proceedings of the 6th International Symposium on High-Temperature Metallurgical Processing, Orlando, FL, USA, 15–19 March 2015; pp. 257–264. [Google Scholar]
- Shui, L.; Cui, Z.X.; Ma, X.D.; Rhamdhani, M.A.; Nguyen, A.V.; Zhao, B.J. Mixing phenomena in a bottom blown copper smelter: A water model study. Metall. Mater. Trans. B 2015, 46, 1218–1225. [Google Scholar] [CrossRef]
- Shui, L.; Cui, Z.X.; Ma, X.D.; Rhamdhani, M.A.; Nguyen, A.V.; Zhao, B.J. Understanding of bath surface wave in bottom blown copper smelting furnace. Metall. Mater. Trans. B 2016, 47, 135–144. [Google Scholar] [CrossRef]
- Liu, H.Q.; Cui, Z.X.; Chen, M.; Zhao, B.J. Phase equilibrium study of ZnO-“FeO”-SiO2 system at fixed Po2 10−8 atm. Metall. Mater. Trans. B 2016, 47, 164–177. [Google Scholar] [CrossRef]
- Liu, H.Q.; Cui, Z.X.; Chen, M.; Zhao, B.J. Phase equilibria study of the ZnO-“FeO”-SiO2-Al2O3 system at Po2 10−8 atm. Metall. Mater. Trans. B 2016, 47, 1113–1123. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Chen, Z.; Yan, H.J.; Liu, F.K.; Liu, L.; Cui, Z.X.; Shen, D.B. Numerical simulation of gas-liquid multi-phase flows in oxygen enriched bottom-blown furnace. Chin. J. Nonferr. Met. 2012, 22, 1826–1834. (In Chinese) [Google Scholar]
- Yan, H.J.; Liu, F.K.; Zhang, Z.Y.; Gao, Q.; Liu, L.; Cui, Z.X.; Shen, D.B. Influence of lance arrangement on bottom-blowing bath smelting process. Chin. J. Nonferr. Met. 2012, 22, 2393–2400. (In Chinese) [Google Scholar]
- Guo, X.Y.; Wang, Q.M.; Tian, Q.H.; Zhang, Y.Z. Mechanism and multiphase interface behavior of copper sulfide concentrate smelting in oxygen-enriched bottom blowing furnace. Nonferr. Met. Sci. Eng. 2014, 5, 28–34. (In Chinese) [Google Scholar]
- Guo, X.Y.; Wang, Q.M.; Tian, Q.H.; Zhao, B.J. Performance analysis and optimization of oxygen bottom blowing copper smelting process. Chin. J. Nonferr. Met. 2016, 26, 689–698. (In Chinese) [Google Scholar]
- Liao, L.L.; Wang, Q.M.; Tian, Q.H.; Guo, X.Y. Multiphase equilibrium modeling study on the oxygen bottom blowing copper smelting (SKS) process. In Proceedings of the 9th International Copper Conference (Copper 2016), Kobe, Japan, 13–16 November 2016; pp. 1252–1264. [Google Scholar]
- Wu, W. Mathematical Model Research and System Development of Multiphase Equilibrium in Copper Flash Smelting. Master’s Thesis, Jiangxi University of Science and Technology, Ganzhou, China, June 2007. (In Chinese). [Google Scholar]
- Shimpo, R.; Goto, S.; Ogawa, O. An improved computer program for equilibrium calculations. Metall. Trans. A 1993, 24, 1882–1889. [Google Scholar] [CrossRef]
- Tan, P.; Zhang, C. Computer model of copper smelting process and distribution behaviors of accessory elements. J. Cent. South Univ. Technol. 1997, 4, 36–41. [Google Scholar] [CrossRef]
- Chaubal, P.C.; Sohn, H.Y.; George, D.B.; Bailey, L.K. Mathematical modeling of minor-element behavior in flash smelting of copper concentrates and flash converting of copper mattes. Metall. Trans. B 1989, 20, 39–51. [Google Scholar] [CrossRef]
- Nagamori, M.; Errington, W.J.; Mackey, P.J.; Poggi, D. Thermodynamic simulation model of the isasmelt process for copper matte. Metall. Mater. Trans. B 1994, 25, 839–859. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.F. Study on choosing the best grade matte copper smelting process. World Nonferrous Met. 2016, 9, 21–22. (In Chinese) [Google Scholar]
- Qu, S.L.; Dong, Z.Q.; Chen, T. Distribution of minor elements in complex copper concentrates in oxygen-enriched bottom blown smelting process. China Nonferrous Metall. 2016, 3, 22–24. (In Chinese) [Google Scholar]
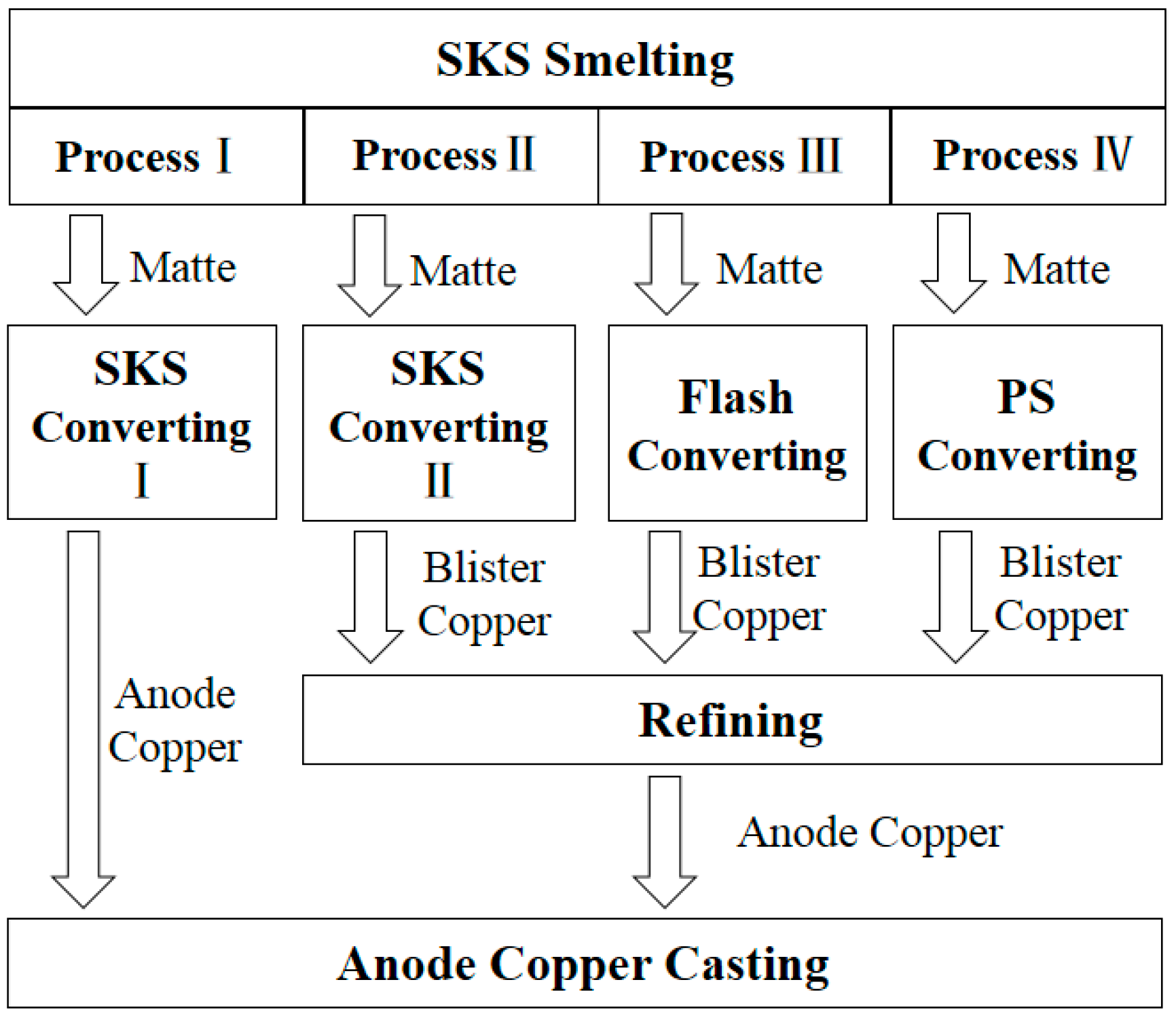
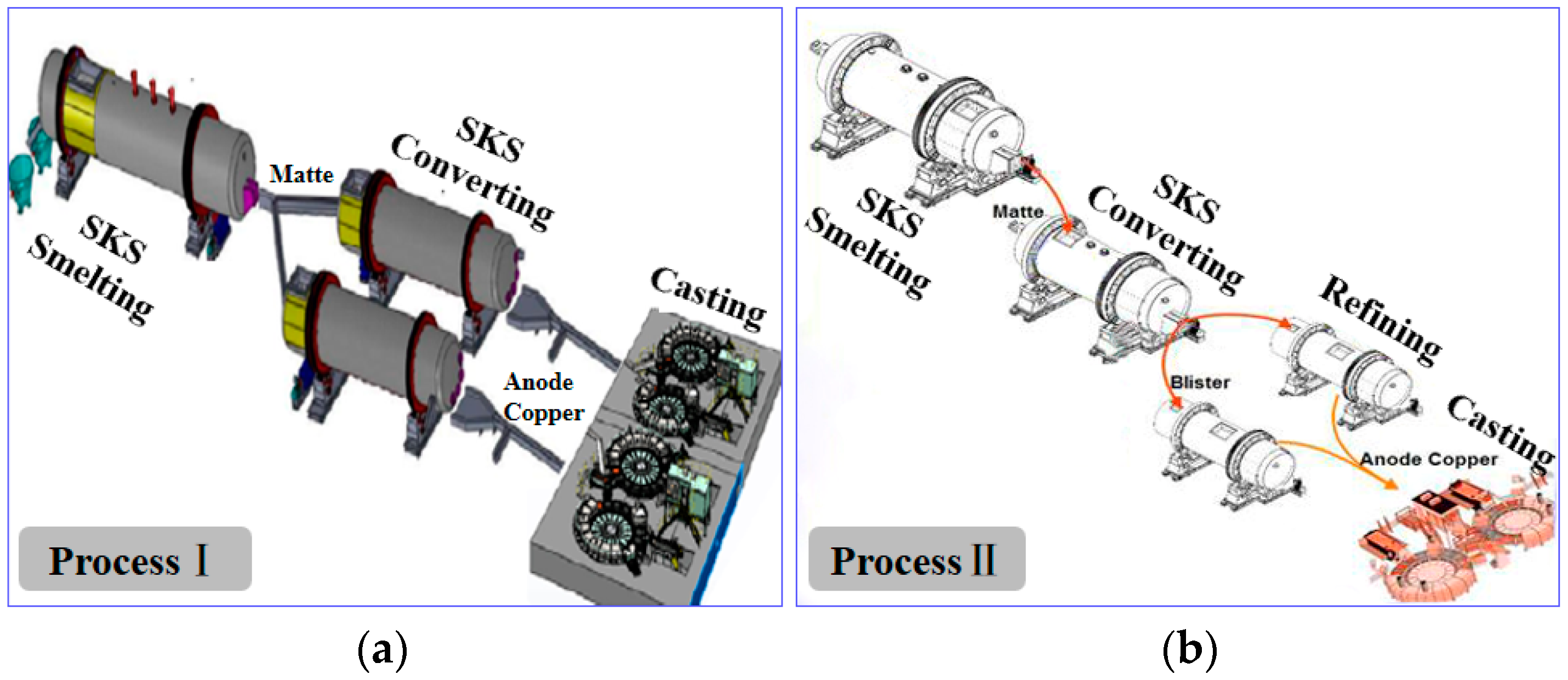
| Phases | Chemical Components |
|---|---|
| Gas | SO2, SO3, S2, O2, N2, H2O, PbO, PbS, Zn, ZnS, As2, AsO, AsS, SbO, SbS, BiS |
| Slag | FeO, Cu2S, Cu2O, Fe3O4, FeS, PbO, ZnO, As2O3, Sb2O3, Bi2O3, SiO2, CaO, MgO, Al2O3 |
| Matte | Cu2S, Cu, FeS, FeO, Fe3O4, Pb, PbS, ZnS, As, Sb, Bi |
| Components | Phase | Activity Coefficient |
|---|---|---|
| Cu2S | Matte | 1 |
| FeS | Matte | |
| Cu | Matte | 14 |
| FeO | Matte | |
| Fe3O4 | Matte | |
| Pb | Matte | 23 |
| PbS | Matte | |
| ZnS | Matte | |
| As | Matte | |
| Sb | Matte | |
| Bi | Matte | |
| FeO | Slag | |
| SiO2 | Slag | 2.1 |
| Fe3O4 | Slag | |
| Cu2O | Slag | |
| FeS | Slag | 70 |
| Cu2S | Slag | |
| PbO | Slag | |
| ZnO | Slag | |
| As2O3 | Slag | |
| Sb2O3 | Slag | |
| Bi2O3 | Slag |
| Components | State | Aij | Bij |
|---|---|---|---|
| Cu2S | liquid | −145,349 | 43.06 |
| FeS | liquid | −135,556 | 43.06 |
| PbS | liquid | −151,881 | 79.67 |
| ZnS | solid | −391,434 | 203.08 |
| Cu2O | liquid | −137,139 | 54.25 |
| FeO | liquid | −259,244 | 62.38 |
| Fe3O4 | solid | −1,097,693.74 | 305.93 |
| As2O3 | liquid | −1,215,325.18 | 457.37 |
| Sb2O3 | liquid | −687,438 | 237.86 |
| Bi2O3 | liquid | −563,470 | 257.66 |
| PbO | liquid | −196,818 | 79.15 |
| ZnO | solid | −475,260 | 208.63 |
| SiO2 | liquid | −912,677 | 180.92 |
| SO3 | gas | −459,543 | 165.15 |
| SO2 | gas | −361,500 | 72.49 |
| As2 | gas | −415,418 | 113.24 |
| AsS | gas | −184,465 | 45.88 |
| AsO | gas | −257,759 | 46.12 |
| SbO | gas | −126,601 | −60.35 |
| SbS | gas | 103,194 | −59.91 |
| BiS | gas | −0.057 | 96.74 |
| PbO | gas | 60,860 | −54.39 |
| PbS | gas | 57,812 | −53.83 |
| ZnS | gas | 13,200 | 32.15 |
| Component | Cu | Fe | S | Pb | Zn | As | Sb | Bi | SiO2 | MgO | CaO | Al2O3 | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Composition (wt %) | 24.4 | 26.8 | 28.6 | 0.96 | 1.9 | 0.37 | 0.10 | 0.10 | 6.4 | 1.9 | 2.4 | 2.3 | 3.9 |
| Operation Parameters | SKS Plant Data |
|---|---|
| Charging speed of dry mixed concentrates (t/h) | 66 |
| Water percent in the mixed concentrates (%) | 10.21 |
| Charging speed of flux (t/h) | 5.277 |
| Smelting temperature (K) | 1473 |
| Negative pressure in furnace (Pa) | 50–200 |
| Volume of pure oxygen (Nm3/h) | 10,885 |
| Volume of air (Nm3/h) | 5651 |
| Volume of O2 in oxygen-enriched air (%) | 73 |
| Matte grade (%) | 70 |
| Oxygen efficiency (%) | 99 |
| Compositions (wt %) | Cu | Fe | S | Pb | Zn | As | Sb | Bi | SiO2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Plant data | matte | 70.77 | 5.52 | 20.22 | 1.73 | 1.07 | 0.07 | 0.04 | 0.06 | 0.51 |
| slag | 3.16 | 42.58 | 0.86 | 0.43 | 2.19 | 0.08 | 0.13 | 0.02 | 25.24 | |
| This Work | matte | 70.31 | 4.80 | 20.38 | 1.69 | 1.02 | 0.07 | 0.04 | 0.06 | 0.82 |
| slag | 2.93 | 42.07 | 0.73 | 0.37 | 2.08 | 0.07 | 0.12 | 0.02 | 25.18 | |
| Phases | Plant Data (%) | This Work (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| As | Sb | Bi | Pb | Zn | As | Sb | Bi | Pb | Zn | |
| Matte | 5.91 | 12.31 | 19.10 | 55.61 | 17.76 | 6.23 | 12.58 | 18.74 | 56.71 | 17.35 |
| Slag | 12.08 | 71.05 | 11.40 | 24.91 | 64.86 | 11.06 | 72.30 | 11.13 | 23.47 | 66.46 |
| Gas | 82.01 | 16.64 | 69.50 | 19.48 | 17.38 | 82.71 | 15.12 | 70.136 | 19.82 | 16.19 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Guo, X.; Tian, Q.; Jiang, T.; Chen, M.; Zhao, B. Effects of Matte Grade on the Distribution of Minor Elements (Pb, Zn, As, Sb, and Bi) in the Bottom Blown Copper Smelting Process. Metals 2017, 7, 502. https://doi.org/10.3390/met7110502
Wang Q, Guo X, Tian Q, Jiang T, Chen M, Zhao B. Effects of Matte Grade on the Distribution of Minor Elements (Pb, Zn, As, Sb, and Bi) in the Bottom Blown Copper Smelting Process. Metals. 2017; 7(11):502. https://doi.org/10.3390/met7110502
Chicago/Turabian StyleWang, Qinmeng, Xueyi Guo, Qinghua Tian, Tao Jiang, Mao Chen, and Baojun Zhao. 2017. "Effects of Matte Grade on the Distribution of Minor Elements (Pb, Zn, As, Sb, and Bi) in the Bottom Blown Copper Smelting Process" Metals 7, no. 11: 502. https://doi.org/10.3390/met7110502





