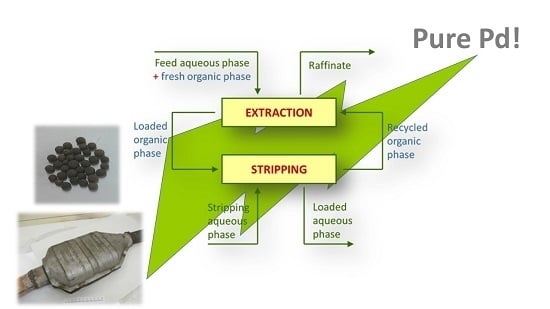
Recycling of Palladium from Spent Catalysts Using Solvent Extraction—Some Critical Points
Abstract
:1. Introduction
2. Solvent Extraction of Palladium(II)
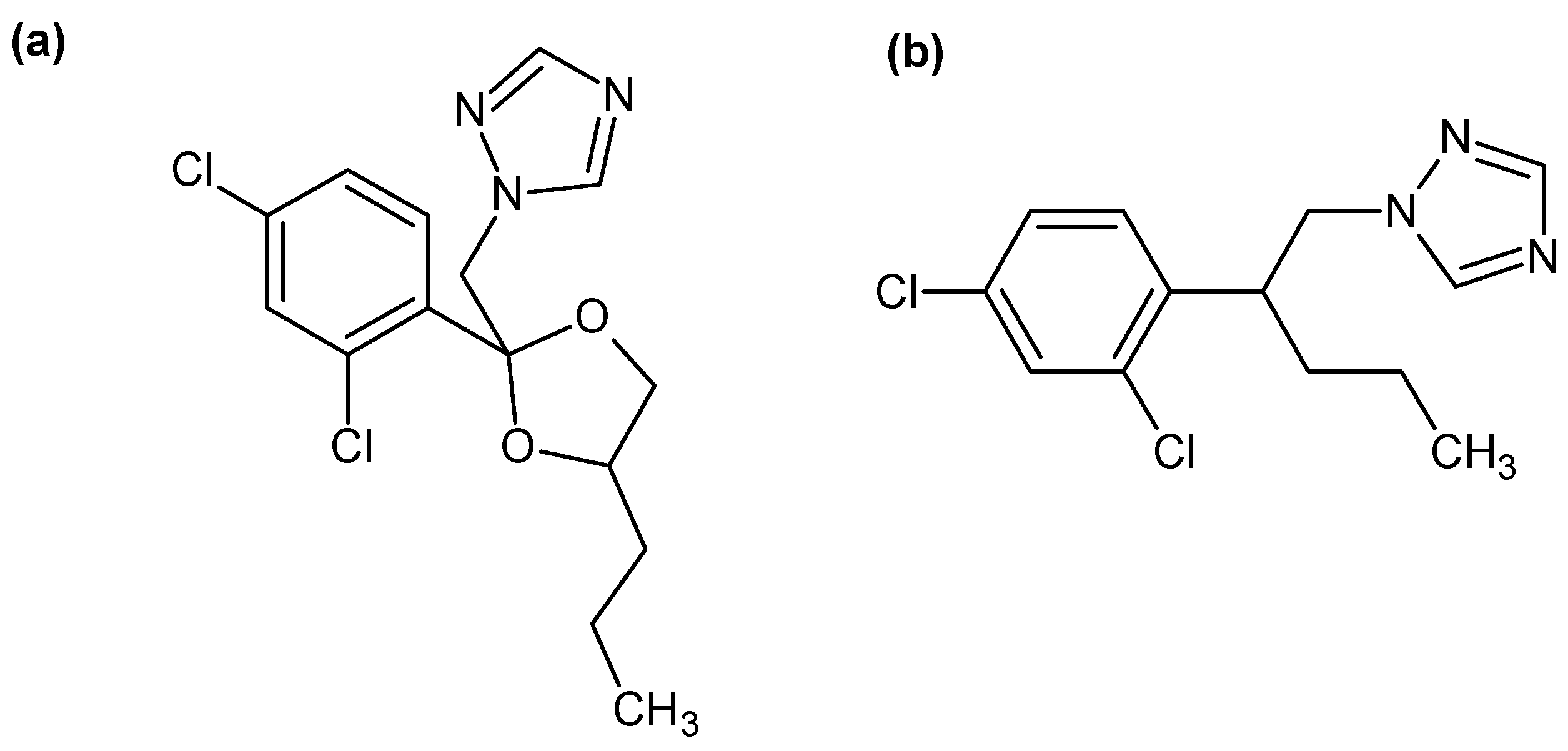
2.1. Commercial Extractants
2.2. Synthesized Extractants
2.2.1. Extractants Containing Amide Functions
2.2.2. Extractants Containing Other Functional Groups
3. Final Summary Tables
4. Concluding Remarks
Acknowledgments
Conflicts of Interest
References
- Ndlovu, J. Anglo American Platinum—Precious Metals Supply. In Proceedings of the Exchange of Good Practices on Metal By-Products Recovery—Technology and Policy Challenges, Brussels, Belgium, 12–13 November 2015; Available online: https://ec.europa.eu/growth/tools-databases/eip-raw-materials/en/content/international-conference-%E2%80%9Cexchange-good-practices-metal-products-recovery-technology-and (accessed on 6 July 2017).
- Ad-Hoc Working Group on Defining Critical Raw Materials (European Commission). Report on Critical Raw Materials for the EU. May 2014. Available online: http://ec.europa.eu/DocsRoom/documents/10010/attachments/1/translations (accessed on 6 July 2017).
- Matthey, J. Precious Metals Management. Available online: http://www.platinum.matthey.com/about-pgm/applications (accessed on 6 July 2017).
- Steinlechner, S.; Antrekowitsch, J. Potential of a hydrometallurgical recycling process for catalysts to cover the demand for critical metals, like PGMs and cerium. JOM 2015, 67, 406–411. [Google Scholar] [CrossRef]
- Cox, M. Solvent extraction in hydrometallurgy. In Solvent Extraction Principles and Practice, 2nd ed.; Rydberg, J., Cox, M., Musikas, C., Choppin, G.R., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 455–505. ISBN 0-8247-5063-2. [Google Scholar]
- Cleare, M.J.; Charlesworth, P.; Bryson, D.J. Solvent extraction in platinum-group metal processing. J. Chem. Technol. Biotechnol. 1979, 29, 210–224. [Google Scholar] [CrossRef]
- Bernardis, F.L.; Grant, R.A.; Sherrington, D.C. A review of separation of the platinum-group metals through their chloro-complexes. React. Funct. Polym. 2005, 65, 205–217. [Google Scholar] [CrossRef]
- Malik, P.; Paiva, A.P. Solvent extraction of rhodium from chloride media by N,N′-dimethyl-N,N′-diphenyltetradecylmalonamide. Solvent Extr. Ion Exch. 2008, 26, 25–40. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Kim, S.-K.; Lee, J.-C. Separation of platinum and palladium from chloride solution by solvent extraction using Alamine 300. Hydrometallurgy 2010, 104, 1–7. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kumar, B.N.; Lee, M.S. Selective recovery of Fe(III), Pd(II), Pt(IV), Rh(III) and Ce(III) from simulated leach liquors of spent automobile catalyst by solvent extraction and cementation. Korean J. Chem. Eng. 2016, 33, 2684–2690. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Tsai, T.-H. Recovery of palladium(II) from acidic chloride solution using kerosene containing tri-n-octyl/decyl amine (Alamine 336). Desalin. Water Treat. 2012, 47, 105–111. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Tsai, T.-H. Solvent extraction of palladium(II) from acidic chloride solutions using tri-octyl/decyl ammonium chloride (Aliquat 336). Desalin. Water Treat. 2014, 52, 1101–1121. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sonu, C.H.; Lee, M.S. Separation of platinum(IV) and palladium(II) from concentrated hydrochloric acid solutions by mixtures of amines with neutral extractants. J. Ind. Eng. Chem. 2015, 32, 238–245. [Google Scholar] [CrossRef]
- Zhidkova, T.I.; Belova, V.V.; Brenno, Y.Y.; Zhidkov, L.L.; Khol’kin, A.I. Palladium extraction by a Cyanex 301-based binary extractant from chloride solutions. Russ. J. Inorg. Chem. 2009, 54, 1502–1506. [Google Scholar] [CrossRef]
- Zhidkova, T.I.; Belova, V.V.; Brenno, Yu.; Zhidkov, L.L.; Khol’kin, A.I. Extraction of platinum and palladium from hydrochloric solutions by trioctymethylammonium dinonylnaphthalenesulfonate. Theor. Found. Chem. Eng. 2009, 43, 826–830. [Google Scholar] [CrossRef]
- Rane, M.V.; Venugopal, V. Study on the extraction of palladium(II) and platinum(IV) using LIX 84I. Hydrometallurgy 2006, 84, 54–59. [Google Scholar] [CrossRef]
- Ramachandra Reddy, B.; Raju, B.; Lee, J.Y.; Park, H.K. Process for the separation and recovery of palladium and platinum from spent automobile catalyst leach liquor using LIX 84I and Alamine 336. J. Hazard. Mat. 2010, 180, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Mhaske, A.A.; Dhadke, P.M. Extraction separation studies of Rh, Pt and Pd using Cyanex 921 in toluene—A possible application to recovery from spent catalysts. Hydrometallurgy 2001, 61, 143–150. [Google Scholar] [CrossRef]
- Gupta, B.; Singh, I. Extraction and separation of platinum, palladium and rhodium using Cyanex 923 and their recovery from real samples. Hydrometallurgy 2013, 134–135, 11–18. [Google Scholar] [CrossRef]
- Nowottny, C.; Halwachs, W.; Schügerl, K. Recovery of platinum, palladium and rhodium from industrial process leaching solutions by reactive extraction. Sep. Purif. Technol. 1997, 12, 135–144. [Google Scholar] [CrossRef]
- Lee, J.Y.; Raju, B.; Kumar, B.N.; Kumar, J.R.; Park, H.K.; Ramachandra Reddy, B. Solvent extraction separation and recovery of palladium and platinum from chloride leach liquors of spent automobile catalyst. Sep. Purif. Technol. 2010, 73, 213–218. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wisniewski, M. Extraction of palladium(II) from chloride solutions with Cyphos®IL 101/toluene mixtures as novel extractant. Sep. Purif. Technol. 2010, 73, 202–207. [Google Scholar] [CrossRef]
- Cieszynska, A.; Wisniewski, M. Selective extraction of palladium(II) from hydrochloric acid solutions with phosphonium extractants. Sep. Purif. Technol. 2011, 80, 385–389. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Rzelewska, M.; Baczynska, M.; Janus, M.; Wisniewski, M. Removal of palladium(II) from aqueous chloride solutions with cyphos phosphonium ionic liquids as metal ion carriers for liquid-liquid extraction and transport across polymer inclusion membranes. Physicochem. Probl. Miner. Process. 2015, 51, 621–631. [Google Scholar]
- Nguyen, V.T.; Lee, J.C.; Chagnes, A.; Kim, M.S.; Jeong, J.; Cote, G. Highly selective separation of individual platinum group metals (Pd, Pt, Rh) from acidic chloride media using phosphonium-based ionic liquid in aromatic diluent. RSC Adv. 2016, 6, 62717–62728. [Google Scholar] [CrossRef]
- Svecova, L.; Papaiconomou, N.; Billard, I. Quantitative extraction of Rh(III) using ionic liquids and its simple separation from Pd(II). Dalton Trans. 2016, 45, 15162–15169. [Google Scholar] [CrossRef] [PubMed]
- Papaiconomou, N.; Svecova, L.; Bonnaud, C.; Cathelin, L.; Billard, I.; Chainet, E. Possibilities and limitations in separating Pt(IV) from Pd(II) combining imidazolium and phosphonium ionic liquids. Dalton Trans. 2015, 44, 20131–20138. [Google Scholar] [CrossRef] [PubMed]
- Cieszynska, A.; Wisniewski, M. Extractive recovery of palladium(II) from hydrochloric acid solutions with Cyphos®IL 104. Hydrometallurgy 2012, 113–114, 79–85. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, C.; Li, J.; Yang, Y. Extraction mechanism, behavior and stripping of Pd(II) by pyridinium-based ionic liquid from hydrochloric acid medium. Hydrometallurgy 2014, 147–148, 164–169. [Google Scholar] [CrossRef]
- Anpilogova, G.R.; Khisamutdinov, R.A.; Golubyatnikova, L.G.; Murinov, Y.I. Propiconazole and penconazole as effective extractants for selective recovery and concentration of platinum(IV) and palladium(II) from hydrochloric acid solutions formed in leaching of spent aluminoplatinum and aluminopalladium catalysts. Russ. J. Appl. Chem. 2016, 89, 206–211. [Google Scholar] [CrossRef]
- Szczepańska, I.; Borowiak-Resterna, A.; Wiśniewski, M. New pyridinecarboxamides for rapid extraction of palladium from acidic chloride media. Hydrometallurgy 2003, 68, 159–170. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Wisniewski, M.; Borowiak-Resterna, A.; Cieszynska, A.; Sastre, A.M. Selective extraction of palladium(II) from hydrochloric acid solutions with pyridinecarboxamides and ACORGA® CLX50. Sep. Purif. Technol. 2007, 53, 337–341. [Google Scholar] [CrossRef]
- Anpilogova, G.R.; Bondareva, S.O.; Khisamutdinov, R.A.; Murinov, Y.I. Fatty imidazolines: A novel extractant for the recovery of palladium(II) from hydrochloric acid solutions. Solvent Extr. Ion Exch. 2014, 32, 206–220. [Google Scholar] [CrossRef]
- Narita, H.; Tanaka, M.; Tamura, K. Extractants for Palladium and Method of Rapidly Separating and Recovering Palladium Using the Same. U.S. Patent 2009/0178513 A1, 16 July 2009. [Google Scholar]
- Narita, H.; Morisaku, K.; Tamura, K.; Tanaka, M.; Shiwaku, H.; Okamoto, Y.; Suzuki, S.; Yaita, T. Extraction properties of palladium(II) in HCl solution with sulfide-containing monoamide compounds. Ind. Eng. Chem. Res. 2014, 53, 3636–3640. [Google Scholar] [CrossRef]
- Narita, H.; Tanaka, M.; Morisaku, K. Extraction properties of platinum group metals with diamide compounds. In Proceedings of the International Solvent Extraction Conference, Beijing, China, 19–23 September 2005; Conference Proceeding Editorial Department: Beijing, China, 2005; pp. 227–232. [Google Scholar]
- Costa, M.C.; Assunção, A.; Costa, A.M.R.; Nogueira, C.; Paiva, A.P. Liquid-liquid extraction of platinum from chloride media by N,N′-dimethyl-N,N′-dicyclohexyltetradecylmalonamide. Solvent Extr. Ion Exch. 2013, 31, 12–23. [Google Scholar] [CrossRef]
- Paiva, A.P.; Carvalho, G.I.; Costa, M.C.; Costa, A.M.R.; Nogueira, C. The solvent extraction performance of N,N′-dimethyl-N,N′-dibutylmalonamide towards platinum and palladium in chloride media. Sep. Sci. Technol. 2014, 49, 966–973. [Google Scholar] [CrossRef]
- Assunção, A.; Matos, A.; Costa, A.M.R.; Candeias, A.; Costa, M.C. A bridge between liquid-liquid extraction and the use of bacterial communities for palladium and platinum recovery as nanosized metal sulphides. Hydrometallurgy 2016, 163, 40–48. [Google Scholar] [CrossRef]
- Narita, H.; Tanaka, M.; Morisaku, K. Palladium extraction with N,N,N′,N′-tetra-n-octyl-thiodiglycolamide. Min. Eng. 2008, 21, 483–488. [Google Scholar] [CrossRef]
- Sasaki, Y.; Morita, K.; Saeki, M.; Hisamatsu, S.; Yoshizuka, K. Precious metal extraction by N,N,N′,N′-tetraoctyl-thiodiglycolamide and its comparison with N,N,N′,N′-tetraoctyl-diglycolamide and methylimino-N,N′-dioctylacetamide. Hydrometallurgy 2017, 169, 576–584. [Google Scholar] [CrossRef]
- Huang, Y.; Li, N.; Li, Y.; Wu, J.; Li, S.; Chen, S.; Zhu, L. Extraction of precious metals with a new amide extractant. Adv. Mater. Res. 2014, 878, 399–405. [Google Scholar] [CrossRef]
- Paiva, A.P.; Carvalho, G.I.; Costa, M.C.; Costa, A.M.R.; Nogueira, C. Recovery of platinum and palladium from chloride solutions by a thiodiglycolamide derivative. Solvent Ext. Ion Exch. 2014, 32, 78–94. [Google Scholar] [CrossRef]
- Ortet, O.; Paiva, A.P. Liquid-liquid extraction of palladium(II) from chloride media by N,N′-dimethyl-N,N′-dicyclohexylthiodiglycolamide. Sep. Purif. Technol. 2015, 156, 363–368. [Google Scholar] [CrossRef]
- Paiva, A.P.; Ortet, O.; Carvalho, G.I.; Nogueira, C.A. Recovery of palladium from a spent industrial catalyst through leaching and solvent extraction. Hydrometallurgy 2017, 171, 394–401. [Google Scholar] [CrossRef]
- Ortet, O.; Santos, M.S.C.S.; Paiva, A.P. Palladium(II) and N,N′-dimethyl-N,N′ dicyclohexylthiodiglycolamide—The extracted species from concentrated chloride solutions. Sep. Purif. Technol. 2016, 170, 1–9. [Google Scholar] [CrossRef]
- Das, A.; Ruhela, R.; Singh, A.K.; Hubli, R.C. Evaluation of novel ligand dithiodiglycolamide (DTDGA) for separation and recovery of palladium from simulated spent catalyst dissolver solution. Sep. Purif. Technol. 2014, 125, 151–155. [Google Scholar] [CrossRef]
- Mohamed, D. Evaluation of unsymmetrical dithiodiglycolamide as novel extractant for application in selective separation of palladium(II) from aqueous solutions. Russ. J. Appl. Chem. 2016, 89, 1322–1327. [Google Scholar] [CrossRef]
- Narita, H.; Tanaka, M.; Morisaku, K.; Abe, T. Rapid separation of palladium(II) from platinum(IV) in hydrochloric acid solution with thiodiglycolamide. Chem. Lett. 2004, 33, 1144–1145. [Google Scholar] [CrossRef]
- Paiva, A.P.; Martins, M.E.; Ortet, O. Palladium(II) recovery from hydrochloric acid solutions by N,N′-dimethyl-N,N′-dibutylthiodiglycolamide. Metals 2015, 5, 2303–2315. [Google Scholar] [CrossRef]
- Paiva, A.P.; Ortet, O. Solvent extraction of palladium from hydrochloric acid media by N,N-disubstituted thioamides. In Proceedings of the International Solvent Extraction Conference, Würzburg, Germany, 7–11 September 2014; Gesellschaft für Chemische Technik und Biotechnologie e.V.—DECHEMA: Würzburg, Germany, 2014; pp. 820–825. [Google Scholar]
- Ortet, O.; Paiva, A.P. Development of tertiary thioamide derivatives to recover palladium(II) from simulated complex chloride solutions. Hydrometallurgy 2015, 151, 33–41. [Google Scholar] [CrossRef]
- Ortet, O.; Santos, M.S.C.S.; Paiva, A.P. Palladium(II) extraction from concentrated chloride media: Reactions involving thioamide derivatives. Sep. Sci. Technol. 2016, 51, 1461–1471. [Google Scholar] [CrossRef]
- Yamada, M.; Gandhi, M.R.; Sato, D.; Kaneta, Y.; Kimura, N. Comparative study on palladium(II) extraction using thioamide-modified acyclic and cyclic extractants. Ind. Eng. Chem. Res. 2016, 55, 8914–8921. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Yamada, M.; Kondo, Y.; Shibayama, A.; Hamada, F. Rapid and selective extraction of Pd(II) ions using the SCS type pincer ligand 1,3-bis(dimethylthiocarbamoyloxy)benzene, and its Pd(II) extraction mechanism. RSC Adv. 2016, 6, 1243–1252. [Google Scholar] [CrossRef]
- Yoshida, W.; Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Extraction and stripping behavior of platinum group metals using an amic-acid-type extractant. J. Chem. Eng. Jpn. 2017, 50, 521–526. [Google Scholar] [CrossRef]
- Narita, H.; Suzuki, T.; Motokawa, R. Recent research in solvent extraction of platinum group metals. J. Jpn. Inst. Met. Mater. 2017, 81, 1–10. [Google Scholar] [CrossRef]
- Preston, J.S.; du Preez, A.C. Solvent extraction of platinum-group metals from hydrochloric acid solutions by dialkyl sulphoxides. Solvent Ext. Ion Exch. 2002, 20, 359–374. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, Z. Solvent extraction and separation of palladium(II) and platinum(IV) from hydrochloric acid medium with dibutyl sulfoxide. Min. Eng. 2009, 22, 1271–1276. [Google Scholar] [CrossRef]
- Pan, L.; Bao, X.; Gu, G. Solvent extraction of palladium(II) and effective separation of palladium(II) and platinum(IV) with synthetic sulfoxide MSO. J. Min. Metall. Sect. B-Metall. 2013, 49, 57–63. [Google Scholar] [CrossRef]
- Traeger, J.; Tillmann, K.; Kelling, A.; Lubahn, S.; Cleve, E.; Mickler, W.; Heydenreich, M.; Müller, H.; Holdt, H.-J. Complexation of palladium(II) with unsaturated dithioethers—A systematic development of highly selective ligands for solvent extraction. Eur. J. Inorg. Chem. 2012, 14, 2341–2352. [Google Scholar] [CrossRef]
- Traeger, J.; König, J.; Städtke, A.; Holdt, H.-J. Development of a solvent extraction system with 1,2-bis(2-methoxyethylthio)benzene for the selective separation of palladium(II) from secondary raw materials. Hydrometallurgy 2012, 127–128, 30–38. [Google Scholar] [CrossRef]
- Senthil, K.; Akiba, U.; Fujiwara, K.; Hamada, F.; Kondo, Y. New heterocyclic dithioether ligands for highly selective separation and recovery of Pd(II) from acidic leach liquors of spent automobile catalyst. Ind. Eng. Chem. Res. 2017, 56, 1036–1047. [Google Scholar] [CrossRef]
- Gandhi, R.M.; Yamada, M.; Haga, K.; Shibayama, A. Synthesis of pincer-type extractants for selective extraction of palladium from PGMs: An improved liquid-liquid extraction approach to current refining processes. Sci. Rep. 2017, 7, 8709–8722. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Gandhi, M.R.; Kunda, U.M.R.; Hamada, F. Thiacalixarenes: Emergent supramolecules in crystal engineering and molecular recognition. J. Incl. Phenom. Macrocycl. Chem. 2016, 85, 1–18. [Google Scholar] [CrossRef]
- Yamada, M.; Gandhi, M.R.; Kaneta, Y.; Hu, Y.; Shibayama, A. Calix[4]arene-based n-dialkylamino extractants for selective platinum group metal separation from automotive catalysts. ChemistrySelect 2017, 2, 1052–1057. [Google Scholar] [CrossRef]

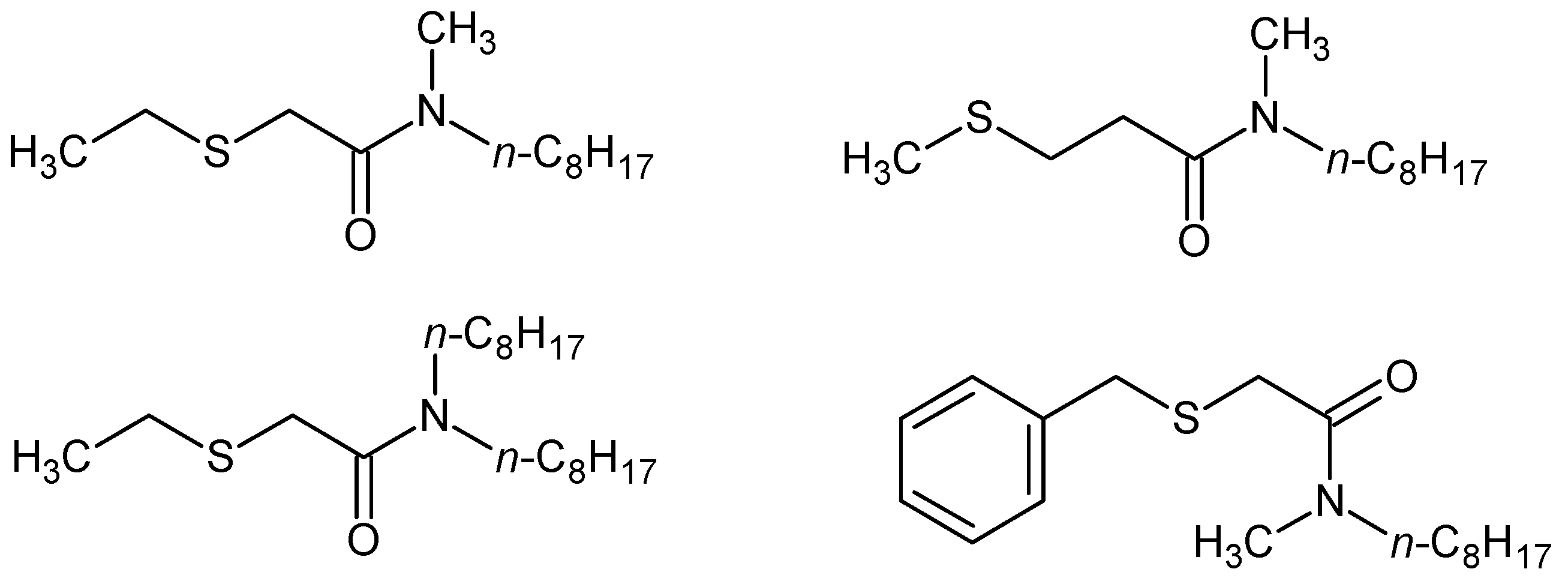
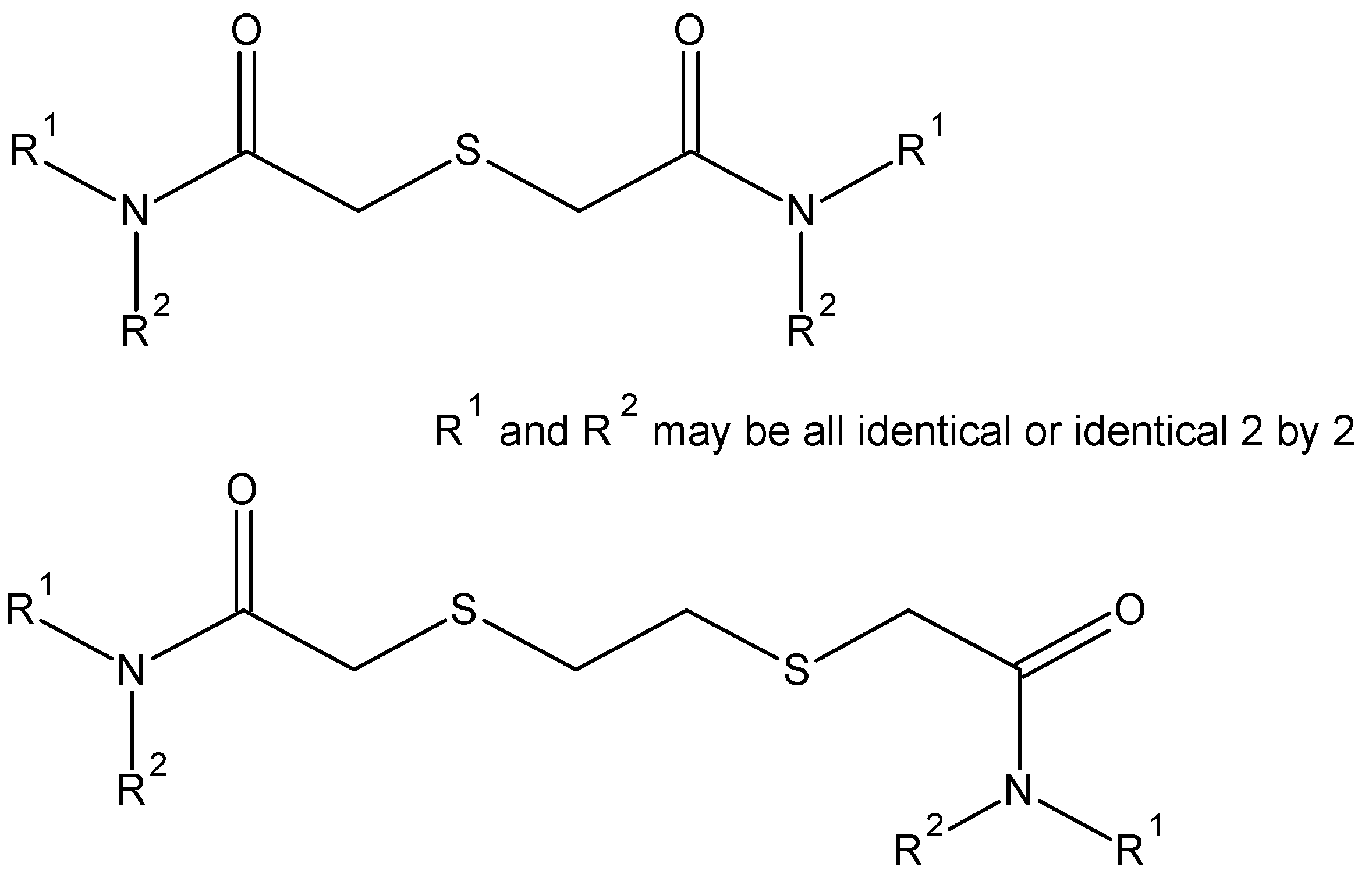
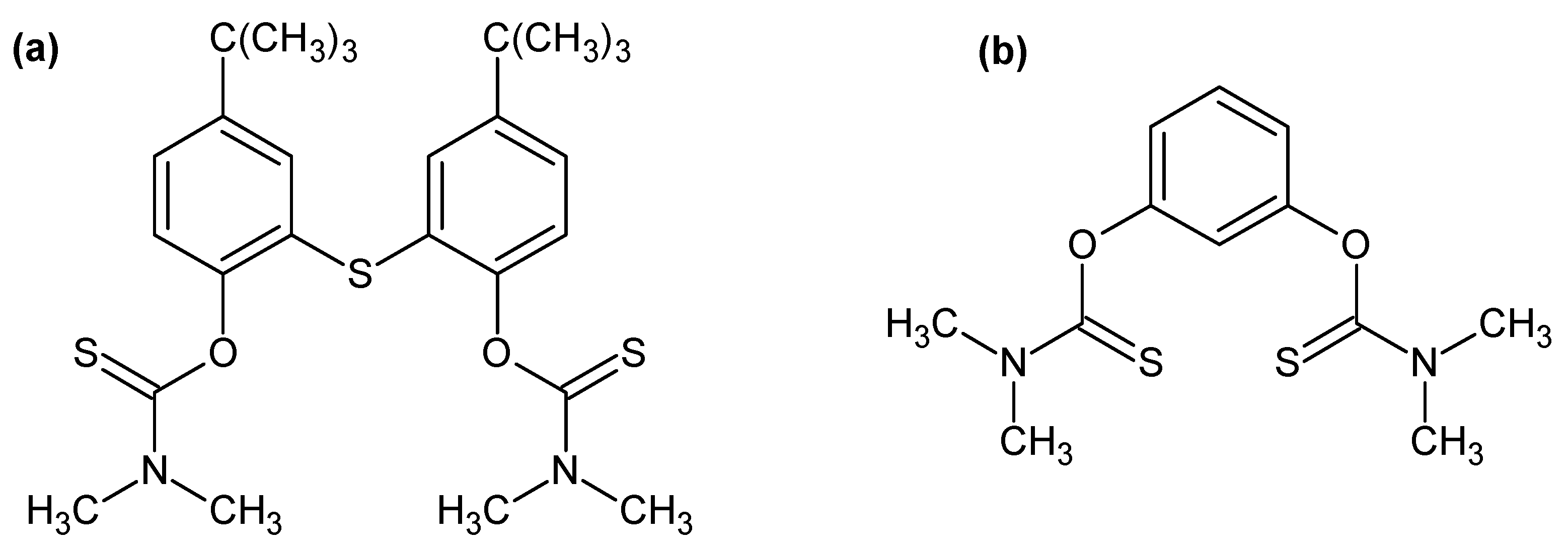
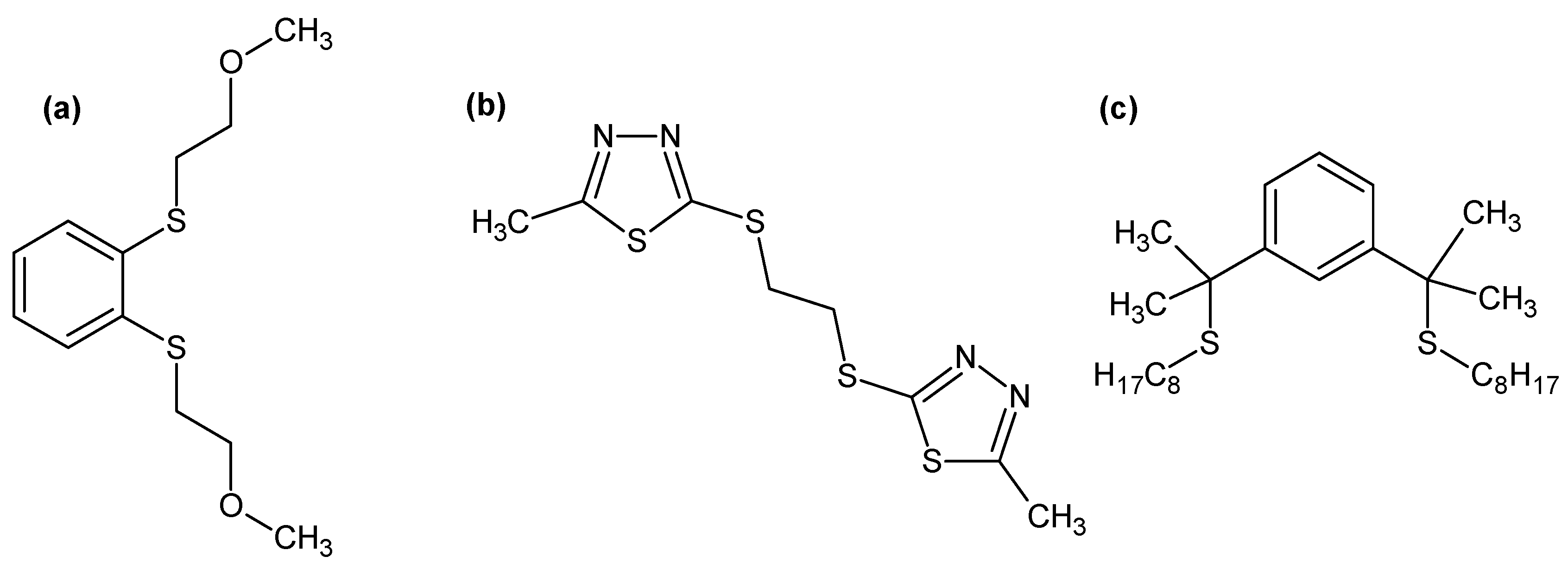
| Extractants | Scope of the Study | Selectivity/Stripping | Extraction Reactions/Organic Species | References |
|---|---|---|---|---|
| N,N′-dimethyl-N,N′-diphenyl-TDGA | Diluent chloroform; Influence of [HCl] | Pt(IV), Ru(III), Rh(III), Ir/no stripping | Ion-pair and complexation | [36] |
| N,N,N′,N′-tetra-n-octyl-TDGA | Diluent n-dodecane [41] + 2-ethylhexanol [40]; Influence of [HCl] [40,41], [HNO3] and [H2SO4] [41] | Pd(II), Pt(IV), Rh(III), Fe(III), Cu(II), Ni(II) Zn(II)/stripping with ammonia [40] Ag(I), Au(III), Hg(II)/no stripping [41] | [PdCl2(TDGA)2] [40]; 1H NMR and IR indicate determinant role of S in complexation [41] | [40,41] |
| N,N′-dimethyl-N,N′-didecyl-TDGA | Diluent dodecane + 2-ethylhexanol; Influence of [HCl] | Pt(IV), Au(III), Fe(III)/no stripping | [PdCl2(TDGA)2] | [42] |
| N,N′-dimethyl-N,N′-dicyclohexyl-TDGA | Diluent 1,2-dichloroethane [43], toluene; Influence of [HCl] | Pt(IV) [43]; Pt(IV), Rh(III), Fe(III), Al(III) [44]; Cr, Al(III) [45]/thiourea in HCl | [PdCl2(TDGA)2] with HCl co-extraction [46] | [43,44,45,46] |
| N,N,N′,N′-tetra-(2-ethylhexyl)-DTDGA | Diluent n-dodecane; Influence of [HCl] | Cr, Fe, Mn, Ni, Pt/thiourea in HCl | [PdCl2(DTDGA)] | [47] |
| N,N′-dimethyl-N,N′-didecyl-DTDGA | Diluent n-dodecane; Influence of [HCl] | Pt(IV), Rh(III), Cr(II), Ni(II), Fe(III), Nd(III), Zr(II), Sr(II), Mn(II)/thiourea in HCl | [PdCl2(DTDGA)] | [48] |
| Extractants | Application to Real Leaching Solutions | Comments | References |
|---|---|---|---|
| Alamine 308 | No | Poorly selective over Pt(IV) (and other metals) | [10] |
| LIX84I | Yes | Selective for Pd(II) for leaches at pH 2 | [17] |
| Cyanex 921 | No | Pd(II), Pt(IV) and Rh(III) co-extracted, but separated in the presence of Sn | [18] |
| Cyanex 923 | Yes | Applied to sulfuric acid leaches | [19] |
| Cyanex 471X | Yes | Selective Pd(II) separation from low concentrated HCl | [20] |
| TBP | No | Pd(II) and Pt(IV) co-extracted | [21] |
| Cyphos 101 IL | No | Selective Pd(II) separation from low concentrated HCl [22,23,24,25]; in pure form, able to extract Pd(II) from 1–8 M HCl [26]; more fundamental research necessary | [22,23,24,25,26] |
| Cyphos 102 IL | No | Able to extract Pd(II) from 1–8 M HCl [26], with Pt(IV) co-extraction [27]; more fundamental research necessary | [24,26,27] |
| Propiconazole and penconazole | No | Selective for Pd(II) over Al(III) from 3–4 M HCl; more fundamental and applied research necessary | [30] |
| Extractants | Application to Real Leaching Solutions | Comments | References |
|---|---|---|---|
| Sulfur-functionalized amides | No | Selective Pd(II) separation from HCl until 5 M; more applied research necessary | [34,35] |
| Thiodiglycolamides | Yes | Selective Pd(II) separation within a wide HCl range; already tested in practical conditions | [40,41,44,45,50] |
| Dithiodiglycolamides | No | Efficient and selective for Pd(II) extraction above 3 M HCl; more applied research necessary | [47,48] |
| Tertiary thioamides | Yes | Selective Pd(II) separation within a wide HCl range; already tested in practical conditions; elaborated syntheses | [51,52] |
| Thiocarbamates | Yes | Selective Pd(II) separation from low concentrated HCl; more applied research necessary | [54,55] |
| Dialkyl sulfoxides | No | Selective extraction of Pd(II) over Pt(IV) optimized; more applied research necessary | [58,59,60] |
| Dithioethers | Yes | Versatile selective extraction of Pd(II) by structurally different compounds; more fundamental and applied research necessary | [62,63,64] |
| Functionalized thiacalix[4/6]arenes | Yes | Selective Pd(II) separation from low concentrated HCl; elaborated syntheses | [65,66] |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, A.P. Recycling of Palladium from Spent Catalysts Using Solvent Extraction—Some Critical Points. Metals 2017, 7, 505. https://doi.org/10.3390/met7110505
Paiva AP. Recycling of Palladium from Spent Catalysts Using Solvent Extraction—Some Critical Points. Metals. 2017; 7(11):505. https://doi.org/10.3390/met7110505
Chicago/Turabian StylePaiva, Ana Paula. 2017. "Recycling of Palladium from Spent Catalysts Using Solvent Extraction—Some Critical Points" Metals 7, no. 11: 505. https://doi.org/10.3390/met7110505