Effect of Ni-Content on the Transformation Temperatures in NiTi-20 at. % Zr High Temperature Shape Memory Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results
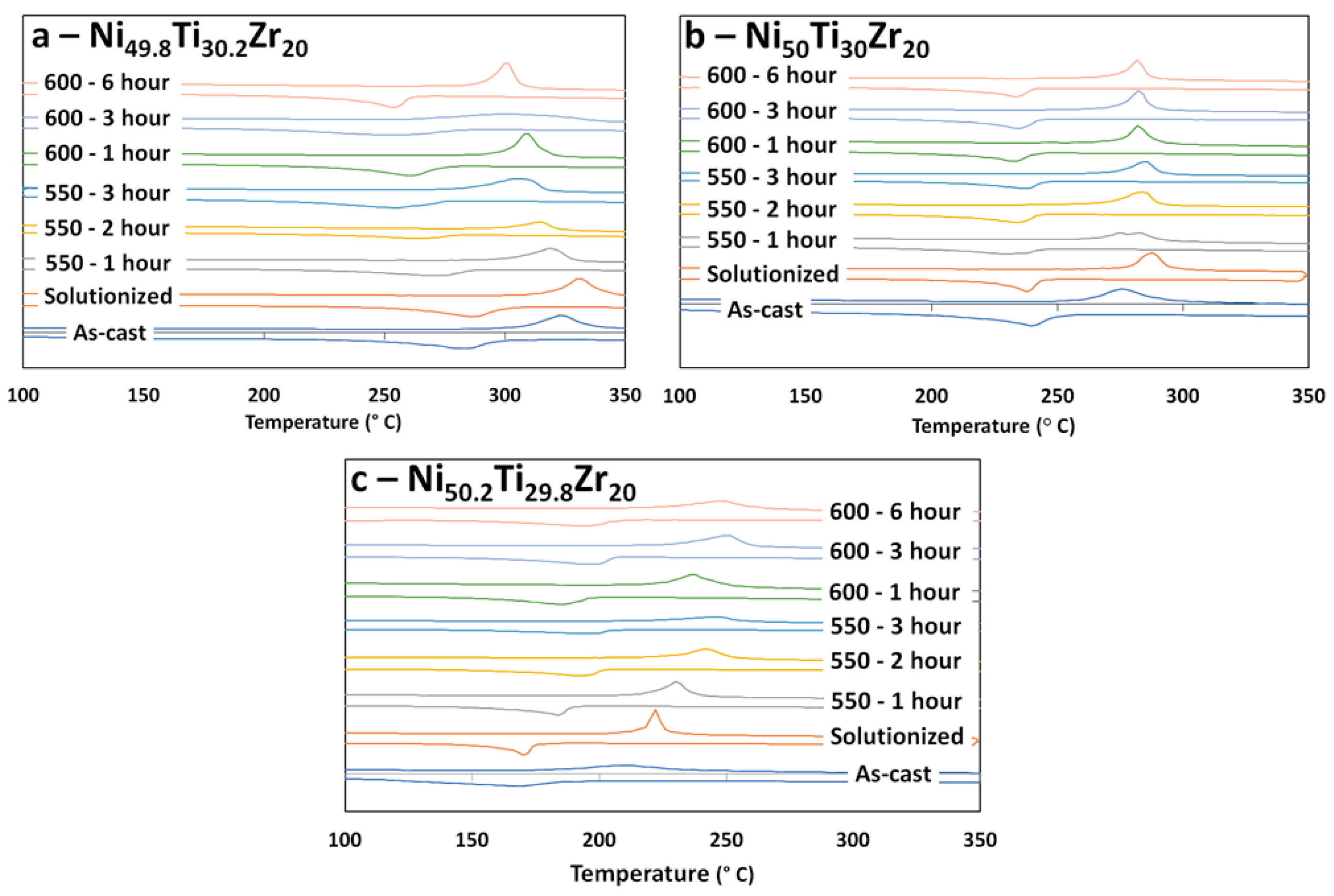
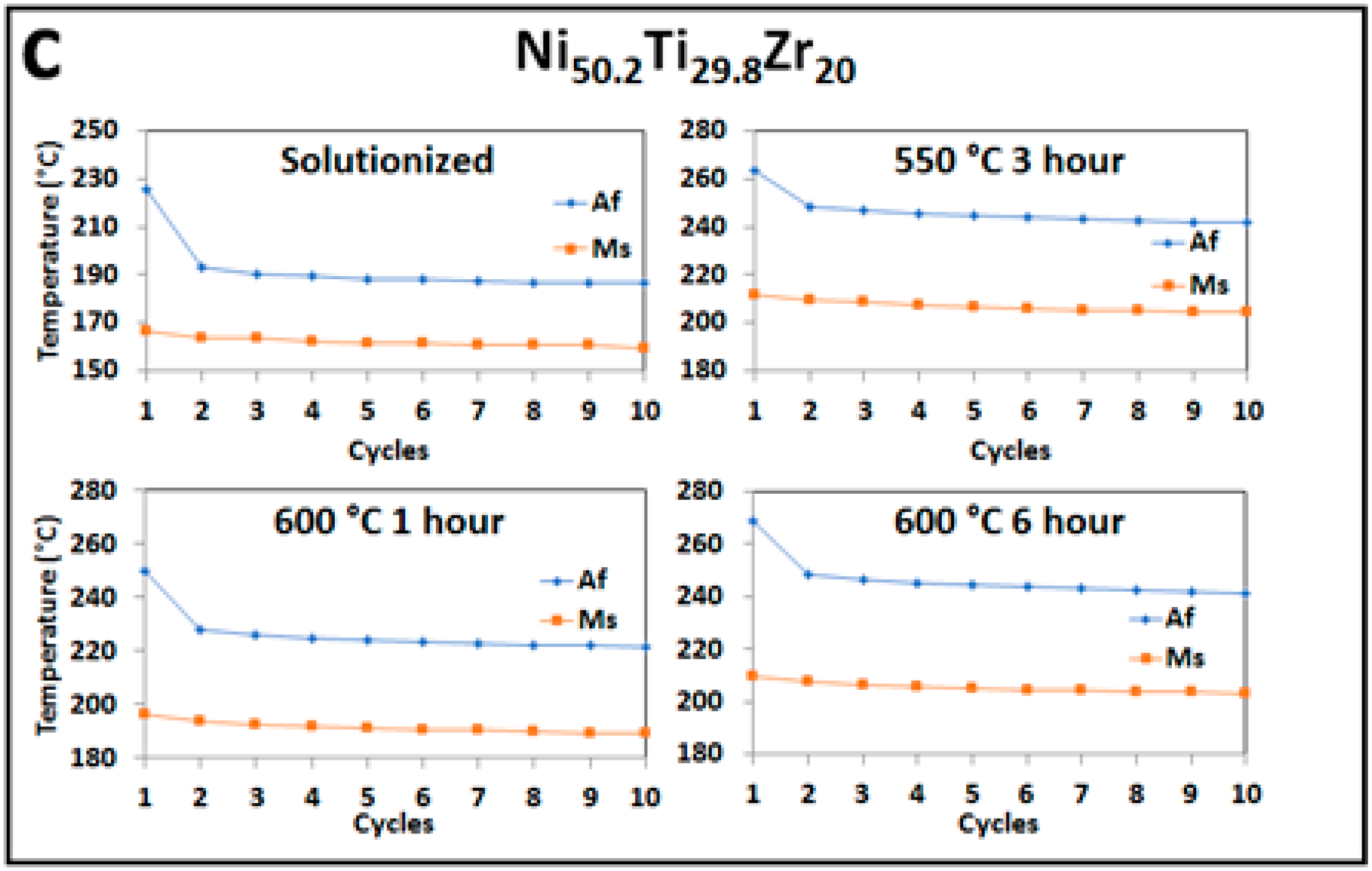
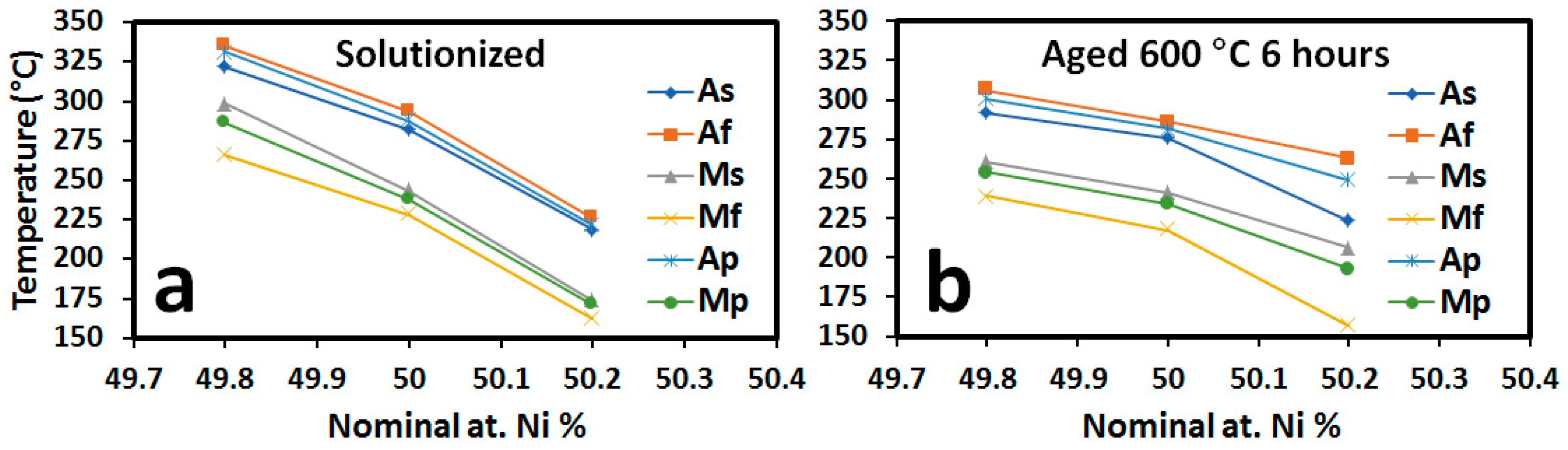
3.1. Differential Scanning Calorimetry (DSC) for Transformation Temperatures
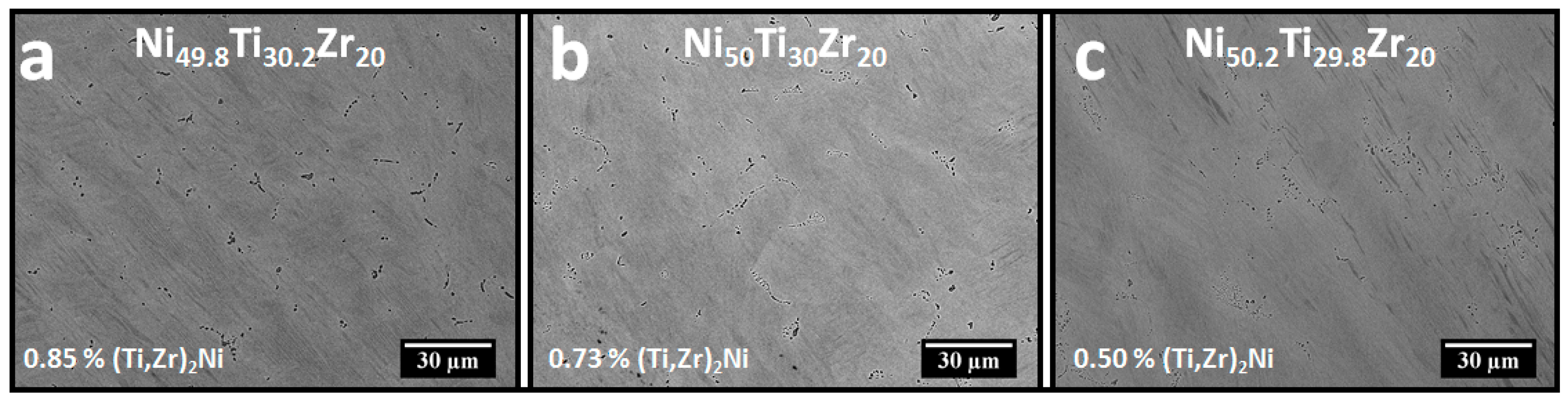
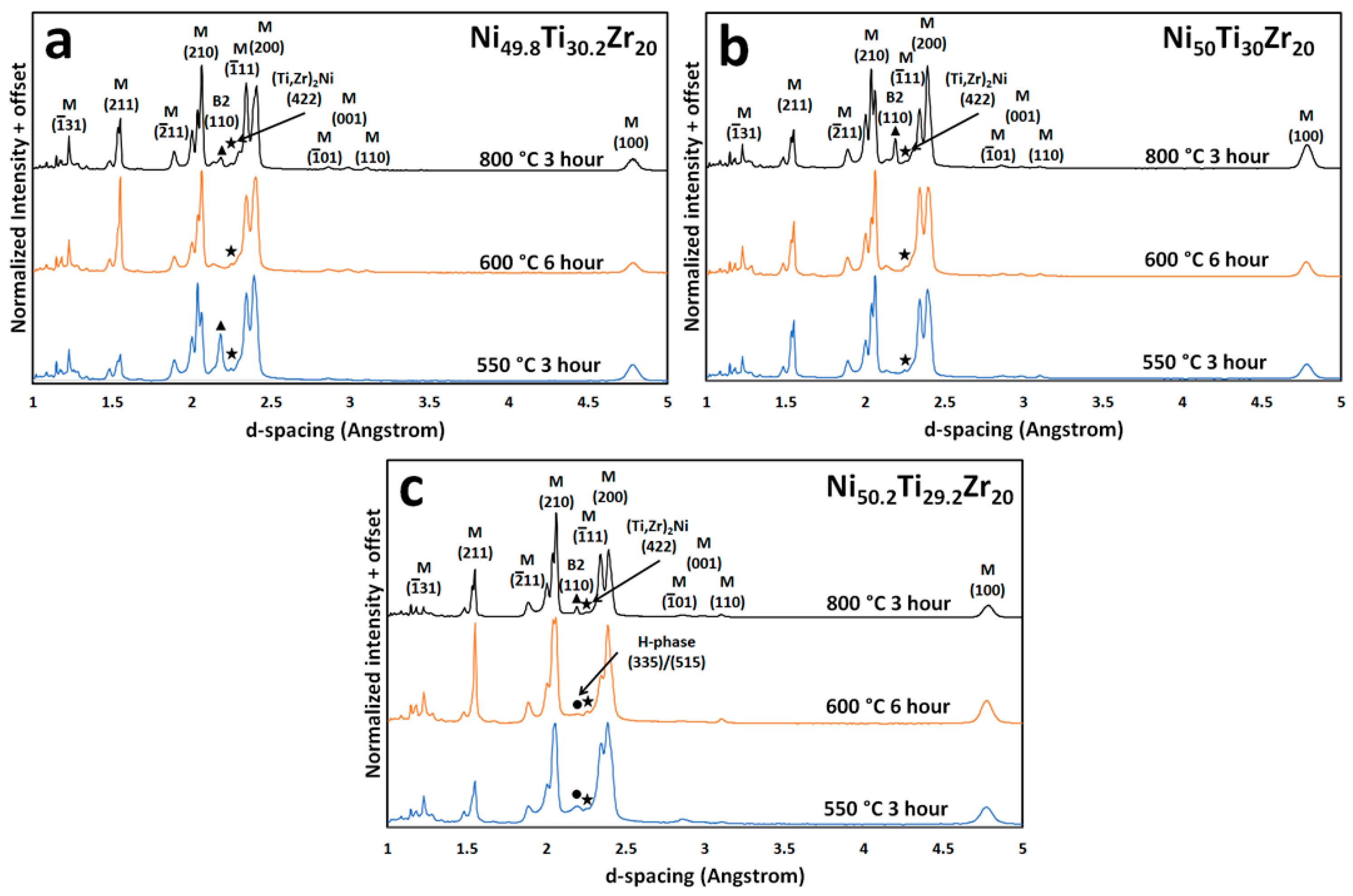
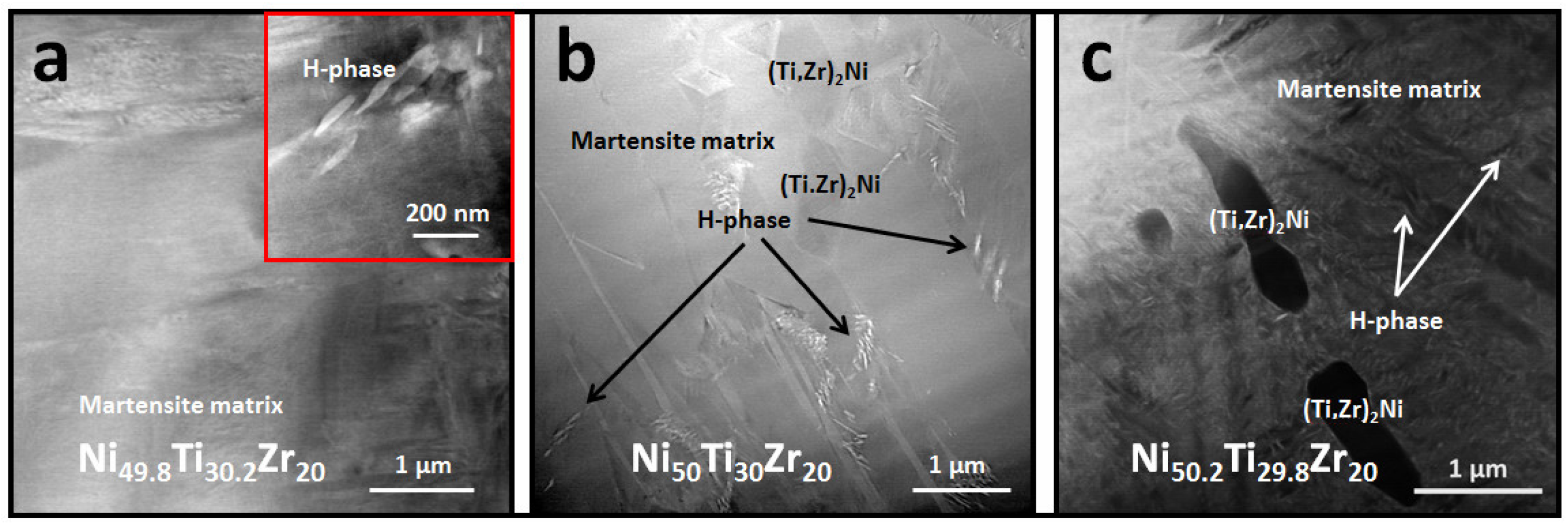
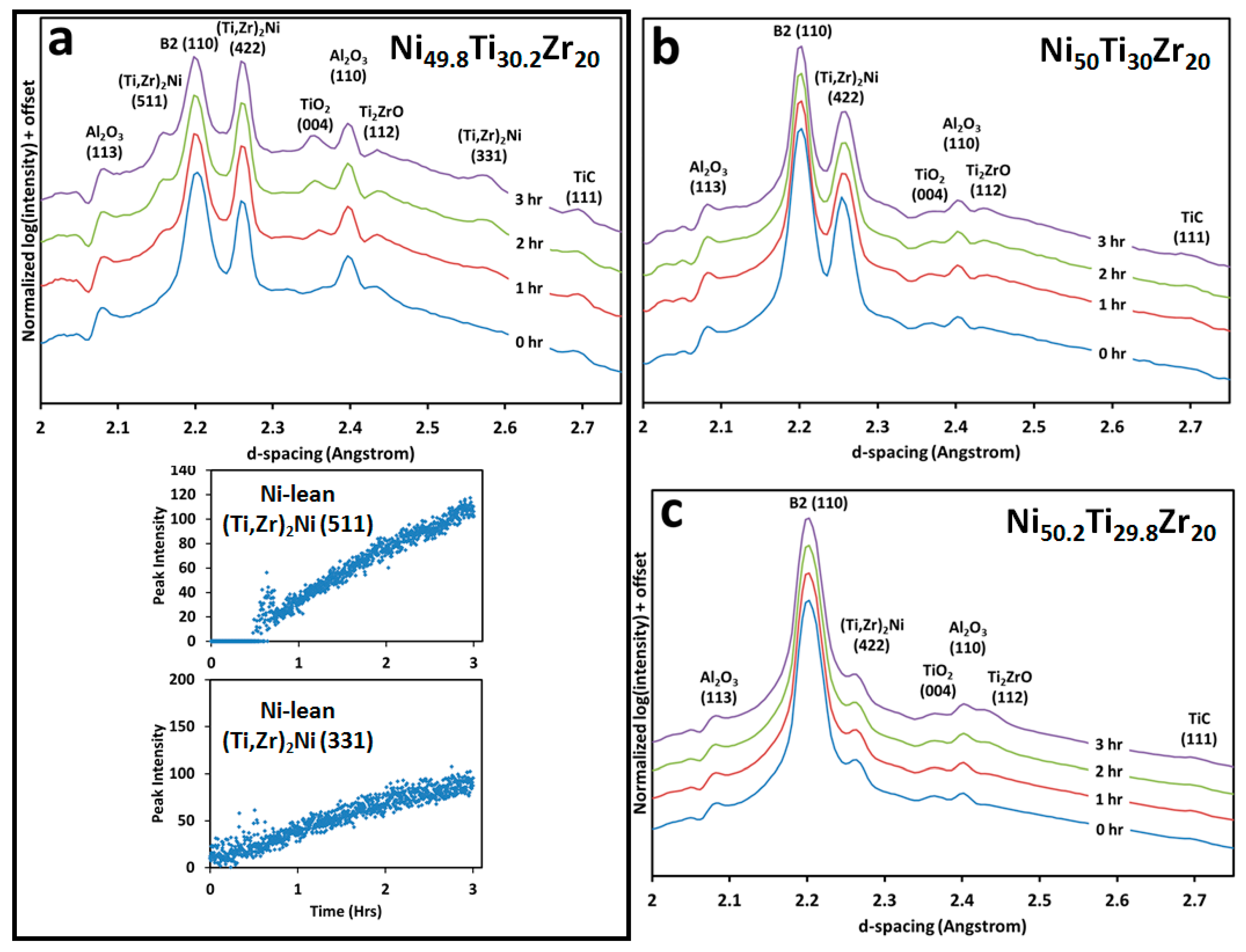
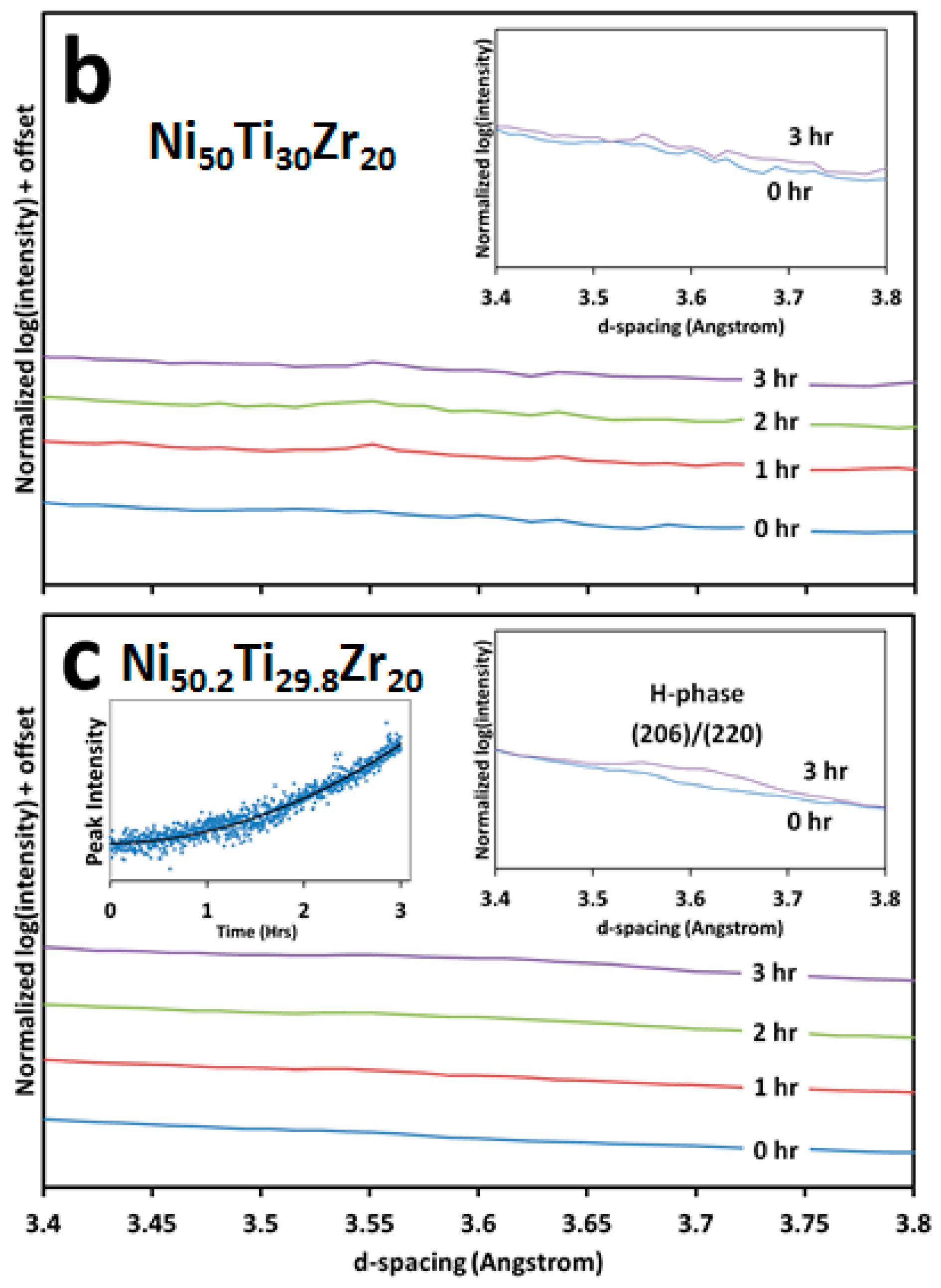
3.2. Microstructural and High Energy Synchrotron Radiation X-ray Diffraction (SR-XRD) Analysis
4. Discussion
4.1. Transformation Temperatures and Precipitation with Respect to Ni-Content
4.2. Aging at 550 °C and 600 °C vs. Annealing at 800 °C
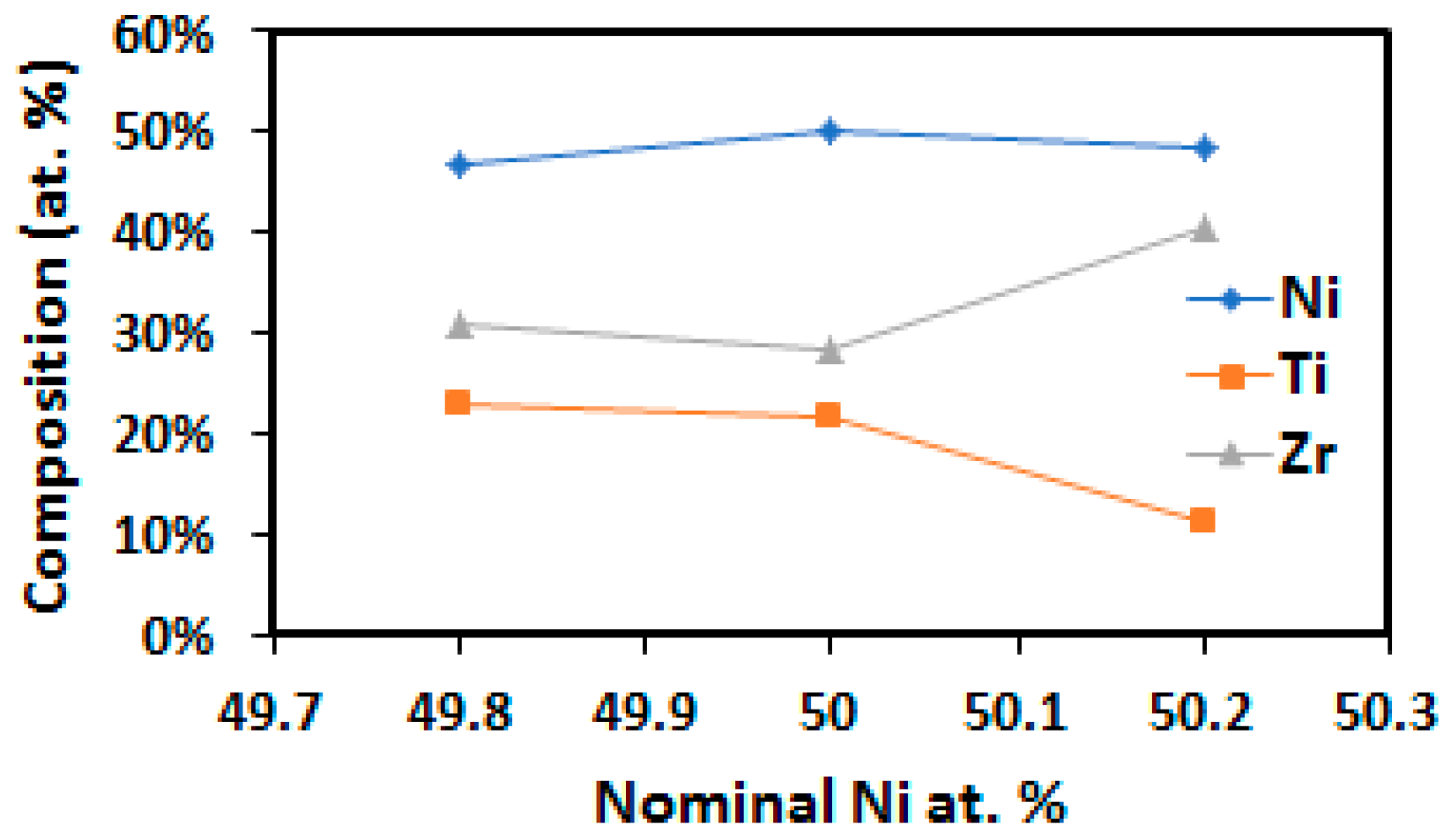
4.3. Composition of the H-Phase
5. Conclusions
- Small changes in at. % Ni have a dramatic effect on the TTs of the NiTi-20 at. % Zr system, even more so than in binary NiTi. From 49.8–50.2 at. % Ni, a drop of the 110 °C of the Af is observed while in binary the same composition range only changes the Af by approximately 36 °C, meaning composition control is even more crucial in the NiTi-20 at. % Zr system.
- TTs can be tuned for a given application through aging treatments. The Ni-rich alloy, 50.2 at. %, can be shifted to higher transformation temperatures due to the formation of nanoscale H-phase precipitates, while the Ni-lean alloy can be shifted to lower transformation temperatures due to the formation of (Ti,Zr)2Ni precipitates. In both Ni-lean and Ni-rich cases, precipitation results in moving the martensite matrix composition toward the equimolar composition. The equimolar alloy, however, shows little response to aging.
- The Ni-rich alloy forms fine nano-scale H-phase precipitates when aging at lower temperatures, i.e., 550 and 600 °C, but these precipitates no longer form when temperature is increased, i.e., 800 °C. Although equimolar and Ni-lean alloys do not show an appreciable amount of H-phase, some local pockets exist along martensitic grain boundaries.
- H-phase is shown to shift compositionally with changes in Ni-content. Specifically by increasing the Zr content in the precipitate from 30 at. % to 40 at. % Zr in the equimolar and Ni-rich conditions, respectively. This behavior helps explain the wide range of H-phase compositions reported in the literature in both NiTi-Hf and -Zr alloys.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buehler, W.; Wiley, R. Tini-ductile intermetallic compound. Trans. Am. Soc. Met. 1962, 55, 269–276. [Google Scholar]
- Otsuka, K.; Ren, X. Physical metallurgy of Ti–Ni-based shape memory alloys. Prog. Mater. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef]
- Mohd Jani, J.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. (1980–2015) 2014, 56, 1078–1113. [Google Scholar] [CrossRef]
- Van Humbeeck, J. Non-medical applications of shape memory alloys. Mater. Sci. Eng. A 1999, 273–275, 134–148. [Google Scholar] [CrossRef]
- Pelton, A.; Duerig, T.; Stöckel, D. A guide to shape memory and superelasticity in nitinol medical devices. Minim. Invasive Ther. Allied Technol. 2004, 13, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Benafan, O.; Brown, J.; Calkins, F.; Kumar, P.; Stebner, A.; Turner, T.; Vaidyanathan, R.; Webster, J.; Young, M. Shape memory alloy actuator design: Casmart collaborative best practices and case studies. Int. J. Mech. Mater. Des. 2014, 10, 1–42. [Google Scholar] [CrossRef]
- Benafan, O.; Noebe, R.; Padula, S., II; Vaidyanathan, R. Microstructural response during isothermal and isobaric loading of a precipitation-strengthened Ni-29.7Ti-20Hf high-temperature shape memory alloy. Metall. Mater. Trans. A 2012, 43, 4539–4552. [Google Scholar] [CrossRef]
- Hartl, D.J.; Lagoudas, D.C. Aerospace applications of shape memory alloys. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2007, 221, 535–552. [Google Scholar] [CrossRef]
- Calkins, F.; Mabe, J.; Ruggeri, R. Overview of boeing’s shape memory alloy based morphing aerostructures. In Proceedings of the SMASIS08: ASME Conference on Smart Materials, Adaptive Structures and Intelligent Systems, Ellicott City, MD, USA, 28–30 October 2008; pp. 1–11. [Google Scholar]
- Eckelmeyer, K. The effect of alloying on the shape memory phenomenon in nitinol. Scr. Metall. 1976, 10, 667–672. [Google Scholar] [CrossRef]
- David, N.A.I.; Thoma, P.E.; Kao, M.Y.; Angst, D.R. High Transformation Temperature Shape Memory Alloy. U.S. Patents 5114504, 19 May 1992. [Google Scholar]
- Hsieh, S.; Wu, S. A study on lattice parameters of martensite in Ti50.5−xNi49.5Zrx shape memory alloys. J. Alloys Compd. 1998, 270, 237–241. [Google Scholar] [CrossRef]
- Hsieh, S.F.; Wu, S.K. Room-temperature phases observed in Ti53−xNi47Zrx high-temperature shape memory alloys. J. Alloys Compd. 1998, 266, 276–282. [Google Scholar] [CrossRef]
- Pu, Z.J.; Tseng, H.-K.; Wu, K.-H. Martensite transformation and shape memory effect of NiTi-Zr high-temperature shape memory alloys. Proc. SPIE 1995, 2441, 171–178. [Google Scholar]
- Sandu, A.M.; Tsuchiya, K.; Yamamoto, S.; Todaka, Y.; Umemoto, M. Influence of isothermal ageing on mechanical behaviour in Ni-rich Ti–Zr–Ni shape memory alloy. Scr. Mater. 2006, 55, 1079–1082. [Google Scholar] [CrossRef]
- Sandu, A.M.; Tsuchiya, K.; Tabuchi, M.; Yamamoto, S.; Todaka, Y.; Umemoto, M. Microstructural evolution during isothermal aging in Ni-rich Ti-Zr-Ni shape memory alloys. Mater. Trans. 2007, 48, 432–438. [Google Scholar] [CrossRef]
- Han, X.D.; Wang, R.; Zhang, Z.; Yang, D.Z. A new precipitate phase in a tinihf high temperature shape memory alloy. Acta Mater. 1998, 46, 273–281. [Google Scholar] [CrossRef]
- Evirgen, A.; Karaman, I.; Noebe, R.; Santamarta, R.; Pons, J. Effect of precipitation on the microstructure and the shape memory response of the Ni50.3Ti29.7Zr20 high temperature shape memory alloy. Scr. Mater. 2013, 69, 354–357. [Google Scholar] [CrossRef]
- Evirgen, A.; Karaman, I.; Santamarta, R.; Pons, J.; Hayrettin, C.; Noebe, R.D. Relationship between crystallographic compatibility and thermal hysteresis in Ni-rich NiTiHf and NiTiZr high temperature shape memory alloys. Acta Mater. 2016, 121, 374–383. [Google Scholar] [CrossRef]
- Karaca, H.; Acar, E.; Tobe, H.; Saghaian, S. NiTiHf-based shape memory alloys. Mater. Sci. Technol. 2014, 30, 1530–1544. [Google Scholar] [CrossRef]
- Meng, X.; Cai, W.; Zheng, Y.; Zhao, L. Phase transformation and precipitation in aged Ti–Ni–Hf high-temperature shape memory alloys. Mater. Sci. Eng. A 2006, 438, 666–670. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.M.; Pons, J.; Santamarta, R.; Karaman, I.; Noebe, R.D. Stability of a Ni-rich Ni-Ti-Zr high temperature shape memory alloy upon low temperature aging and thermal cycling. Scr. Mater. 2016, 124, 47–50. [Google Scholar] [CrossRef]
- Prasher, M.; Sen, D. Influence of aging on phase transformation and microstructure of Ni50.3Ti29.7Zr20 high temperature shape memory alloy. J. Alloys Compd. 2014, 615, 469–474. [Google Scholar] [CrossRef]
- Yang, F.; Coughlin, D.R.; Phillips, P.J.; Yang, L.; Devaraj, A.; Kovarik, L.; Noebe, R.D.; Mills, M.J. Structure analysis of a precipitate phase in an Ni-rich high-temperature NiTiHf shape memory alloy. Acta Mater. 2013, 61, 3335–3346. [Google Scholar] [CrossRef]
- Stebner, A.P.; Bigelow, G.S.; Yang, J.; Shukla, D.P.; Saghaian, S.M.; Rogers, R.; Garg, A.; Karaca, H.E.; Chumlyakov, Y.; Bhattacharya, K.; et al. Transformation strains and temperatures of a Nickel–Titanium–Hafnium high temperature shape memory alloy. Acta Mater. 2014, 76, 40–53. [Google Scholar] [CrossRef]
- Coughlin, D.R.; Phillips, P.J.; Bigelow, G.S.; Garg, A.; Noebe, R.D.; Mills, M.J. Characterization of the microstructure and mechanical properties of a 50.3Ni–29.7Ti–20Hf shape memory alloy. Scr. Mater. 2012, 67, 112–115. [Google Scholar] [CrossRef]
- Saghaian, S.M.; Karaca, H.E.; Tobe, H.; Pons, J.; Santamarta, R.; Chumlyakov, Y.I.; Noebe, R.D. Effects of Ni content on the shape memory properties and microstructure of Ni-rich NiTi-20Hf alloys. Smart Mater. Struct. 2016, 25, 095029. [Google Scholar] [CrossRef]
- Hornbuckle, B.C.; Sasaki, T.T.; Bigelow, G.S.; Noebe, R.D.; Weaver, M.L.; Thompson, G.B. Structure–property relationships in a precipitation strengthened Ni–29.7Ti–20Hf (at%) shape memory alloy. Mater. Sci. Eng. A 2015, 637, 63–69. [Google Scholar] [CrossRef]
- Hammersley, A. Fit2d: An Introduction and Overview; European Synchrotron Radiation Facility Internal Report ESRF97HA02T; ESRF: Grenoble, France, 1997; Volume 68. [Google Scholar]
- Young, M.; Almer, J.; Daymond, M.; Haeffner, D.; Dunand, D. Load partitioning between ferrite and cementite during elasto-plastic deformation of an ultrahigh-carbon steel. Acta Mater. 2007, 55, 1999–2011. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Lutterotti, L.; Matthies, S.; Wenk, H. Maud: A friendly java program for material analysis using diffraction. IUCr Newslett. CPD 1999, 21, 14–15. [Google Scholar]
- Kudoh, Y.; Tokonami, M.; Miyazaki, S.; Otsuka, K. Crystal structure of the martensite in Ti-49.2 at. % Ni alloy analyzed by the single crystal x-ray diffraction method. Acta Metall. 1985, 33, 2049–2056. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quiros, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography open database (cod): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2011, 40, D420–D427. [Google Scholar] [CrossRef] [PubMed]
- Yurko, G.; Barton, J.; Parr, J. The crystal structure of Ti2Ni (a correction). Acta Crystallogr. 1962, 15, 1309. [Google Scholar] [CrossRef]
- Frenzel, J.; George, E.P.; Dlouhy, A.; Somsen, C.; Wagner, M.F.X.; Eggeler, G. Influence of Ni on martensitic phase transformations in NiTi shape memory alloys. Acta Mater. 2010, 58, 3444–3458. [Google Scholar] [CrossRef]
- Allafi, J.K.; Ren, X.; Eggeler, G. The mechanism of multistage martensitic transformations in aged Ni-rich NiTi shape memory alloys. Acta Mater. 2002, 50, 793–803. [Google Scholar] [CrossRef]
- Moshref-Javadi, M.; Seyedein, S.H.; Salehi, M.T.; Aboutalebi, M.R. Age-induced multi-stage transformation in a Ni-rich NiTiHf alloy. Acta Mater. 2013, 61, 2583–2594. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carl, M.; Smith, J.D.; Van Doren, B.; Young, M.L. Effect of Ni-Content on the Transformation Temperatures in NiTi-20 at. % Zr High Temperature Shape Memory Alloys. Metals 2017, 7, 511. https://doi.org/10.3390/met7110511
Carl M, Smith JD, Van Doren B, Young ML. Effect of Ni-Content on the Transformation Temperatures in NiTi-20 at. % Zr High Temperature Shape Memory Alloys. Metals. 2017; 7(11):511. https://doi.org/10.3390/met7110511
Chicago/Turabian StyleCarl, Matthew, Jesse D. Smith, Brian Van Doren, and Marcus L. Young. 2017. "Effect of Ni-Content on the Transformation Temperatures in NiTi-20 at. % Zr High Temperature Shape Memory Alloys" Metals 7, no. 11: 511. https://doi.org/10.3390/met7110511



