Microstructure and Microhardness of Laser Metal Deposition Shaping K465/Stellite-6 Laminated Material
Abstract
:1. Background
2. Experiment
3. Results and Discussion
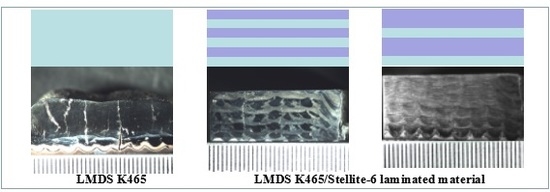
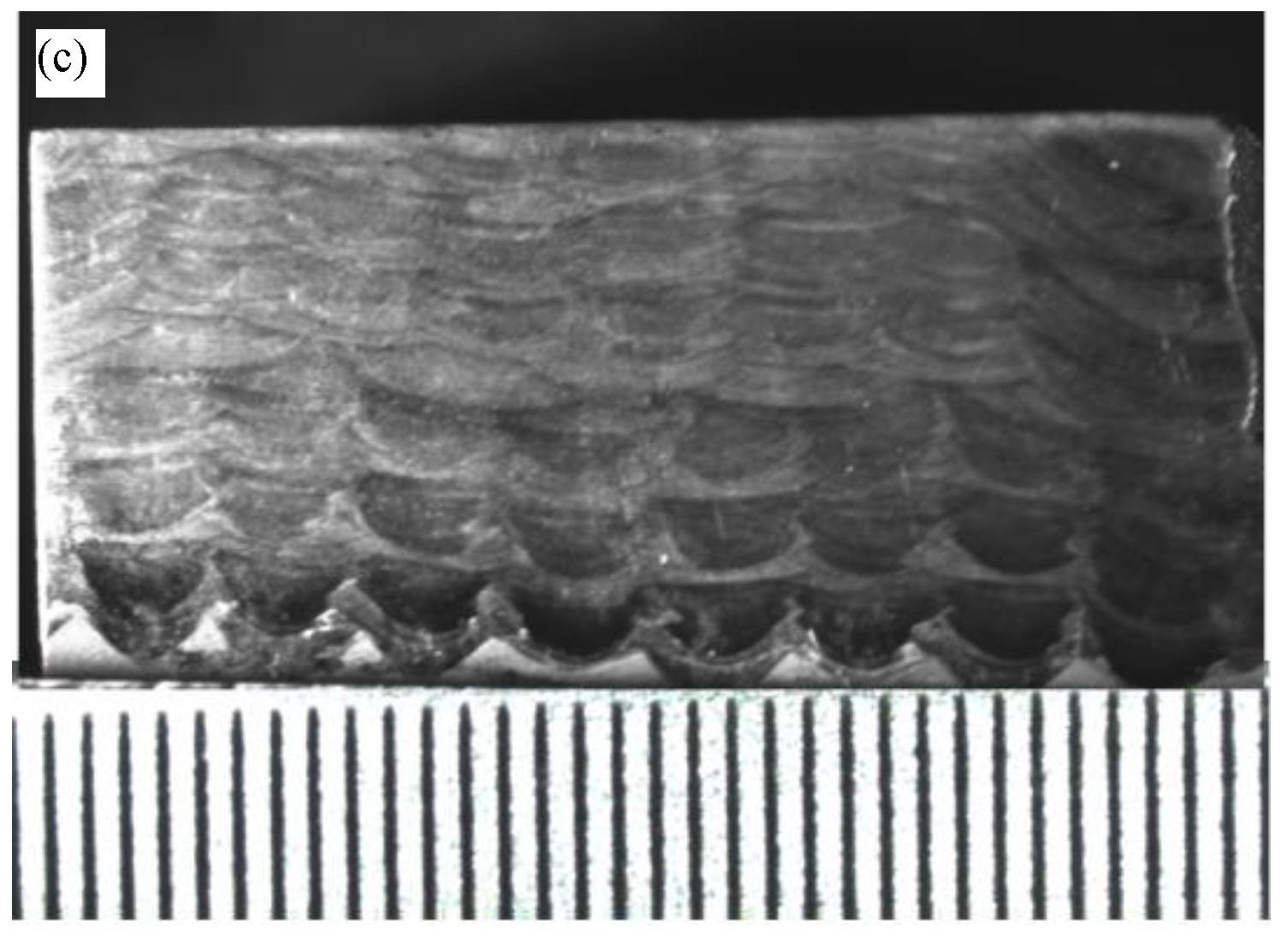
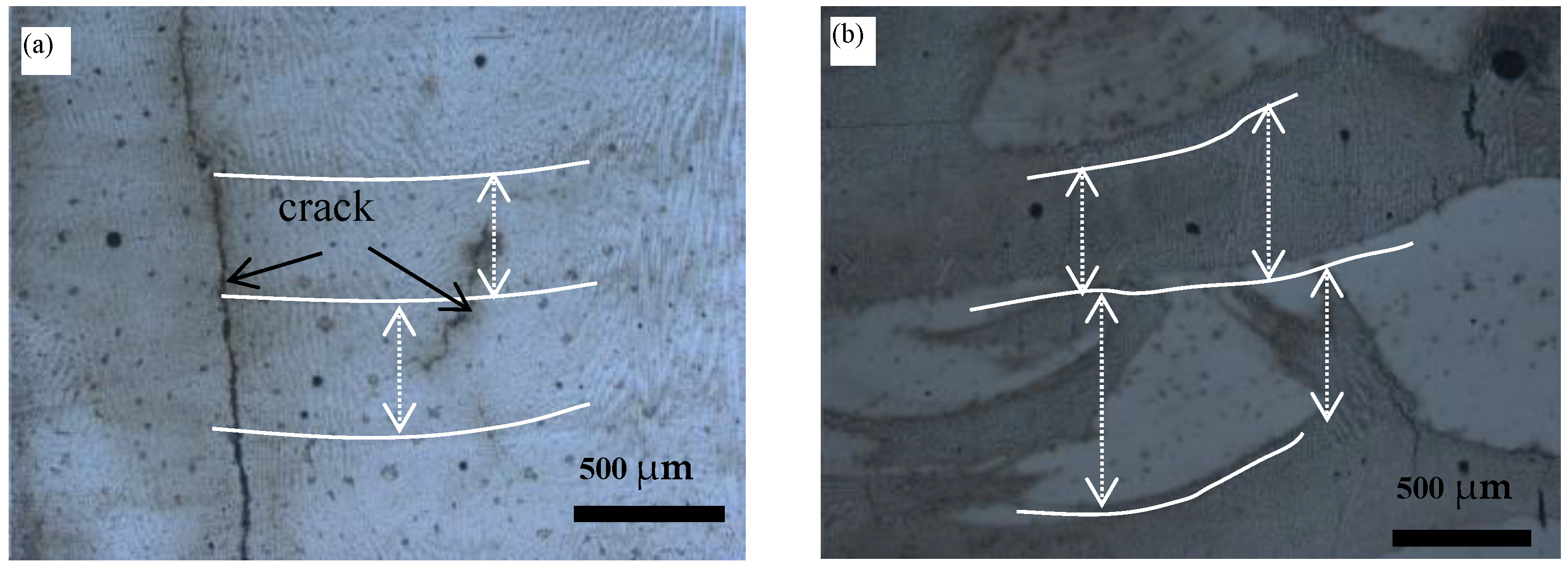
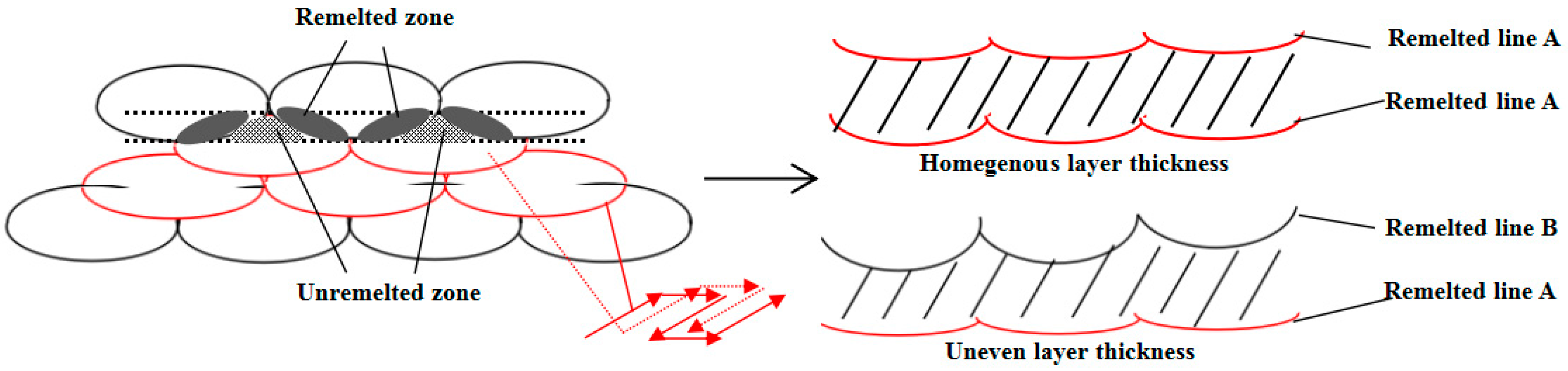
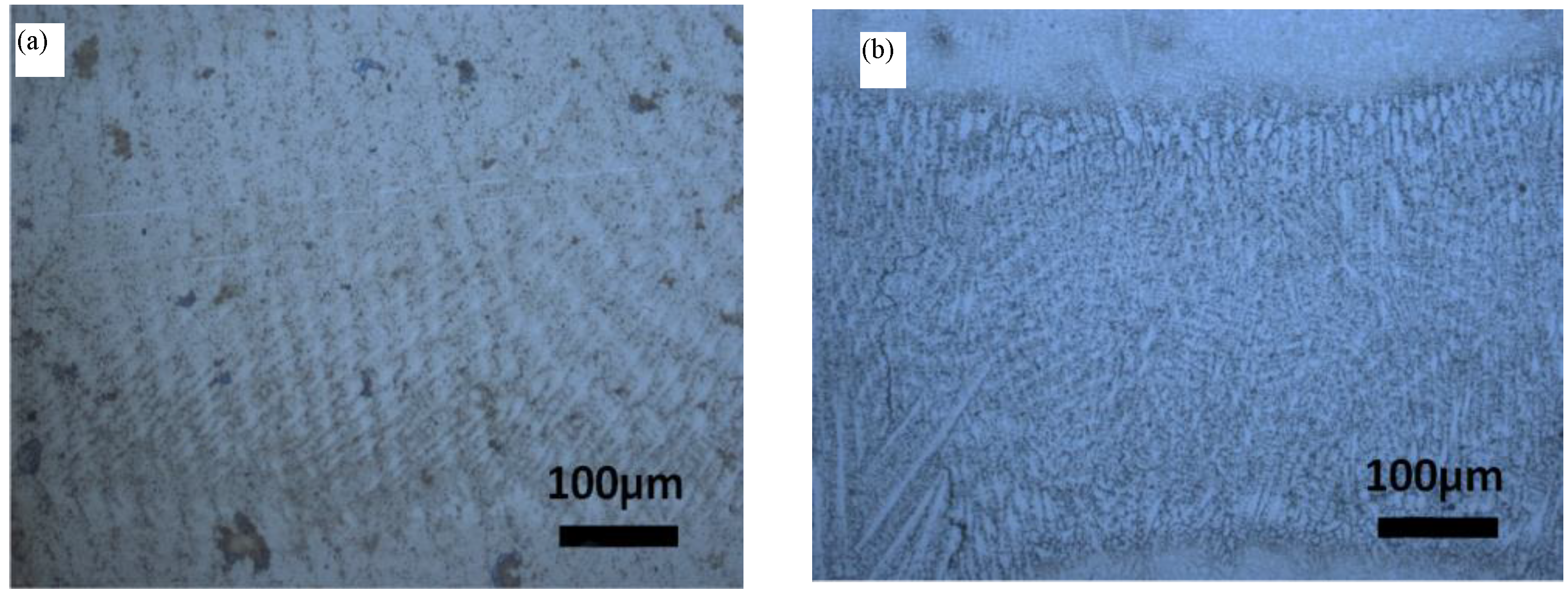
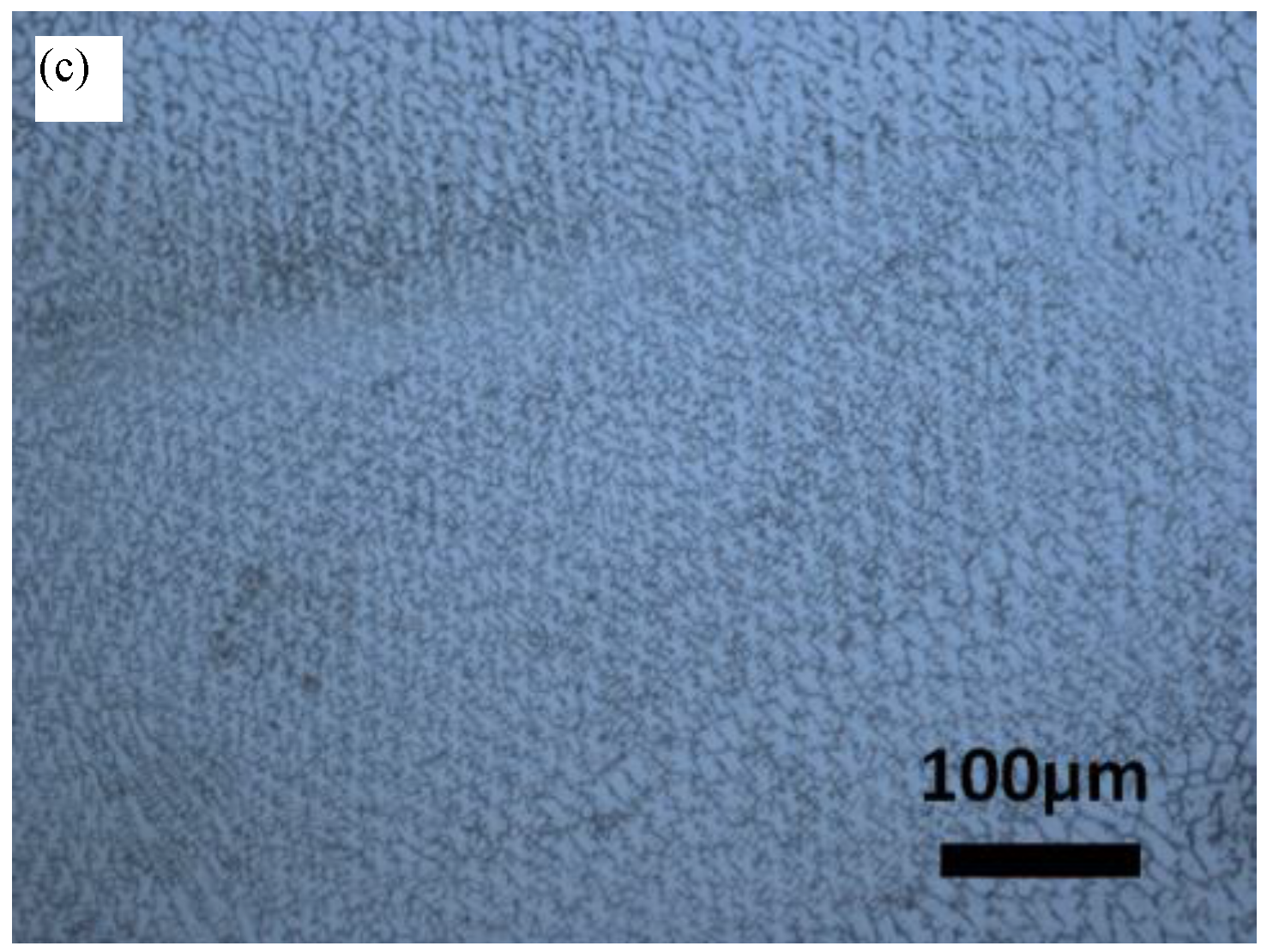
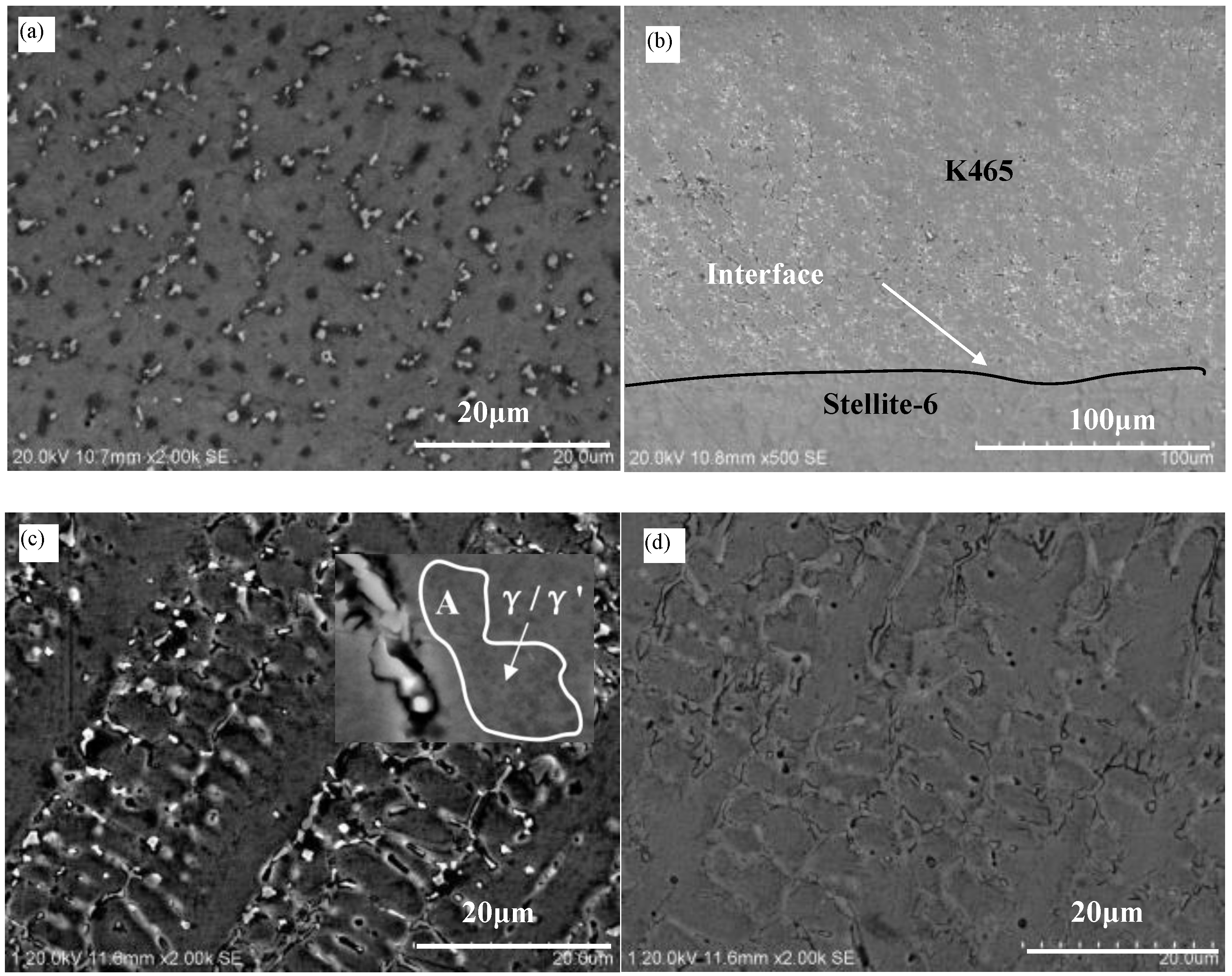
3.1. Microstructure
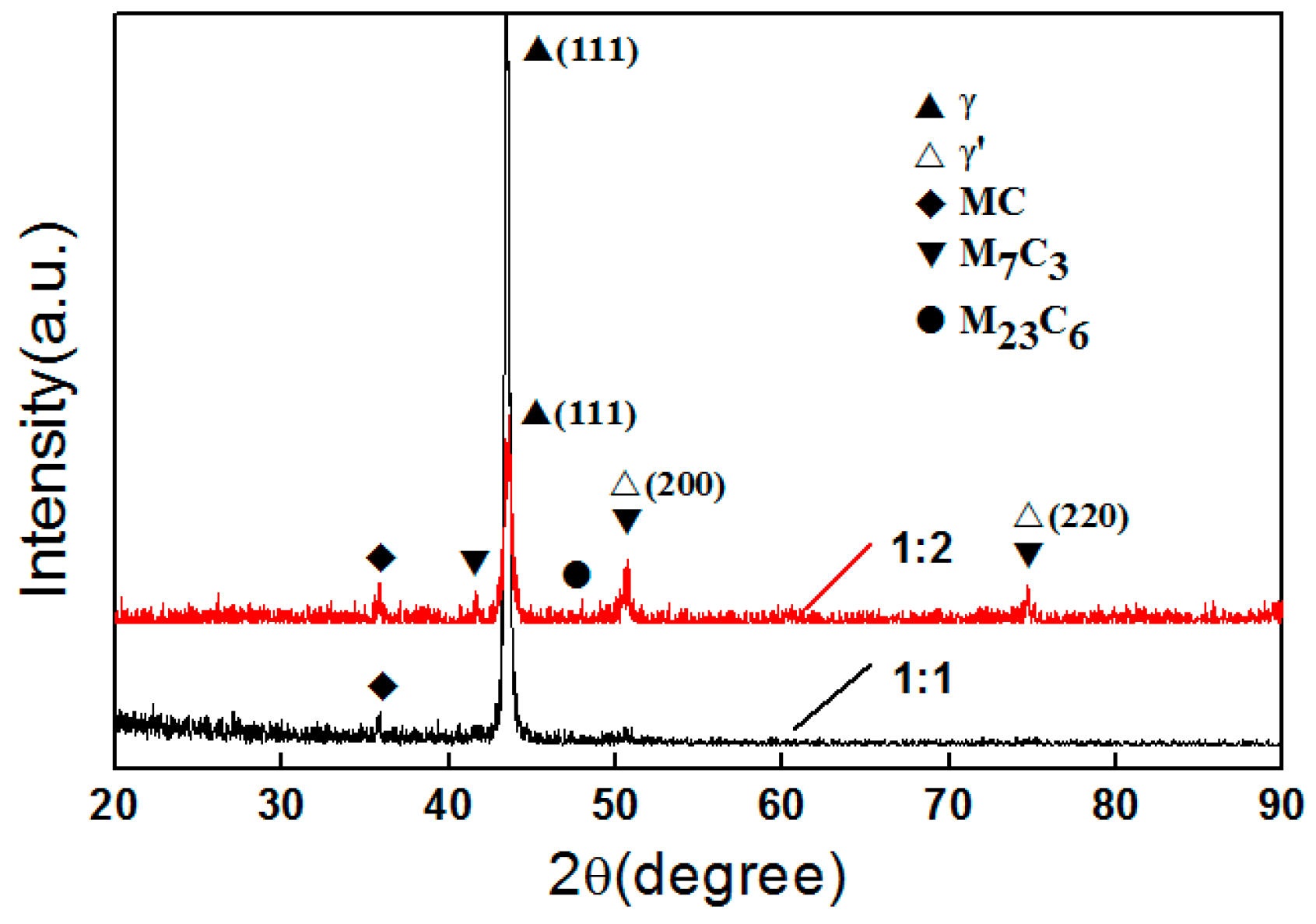
3.2. Phase Compositions
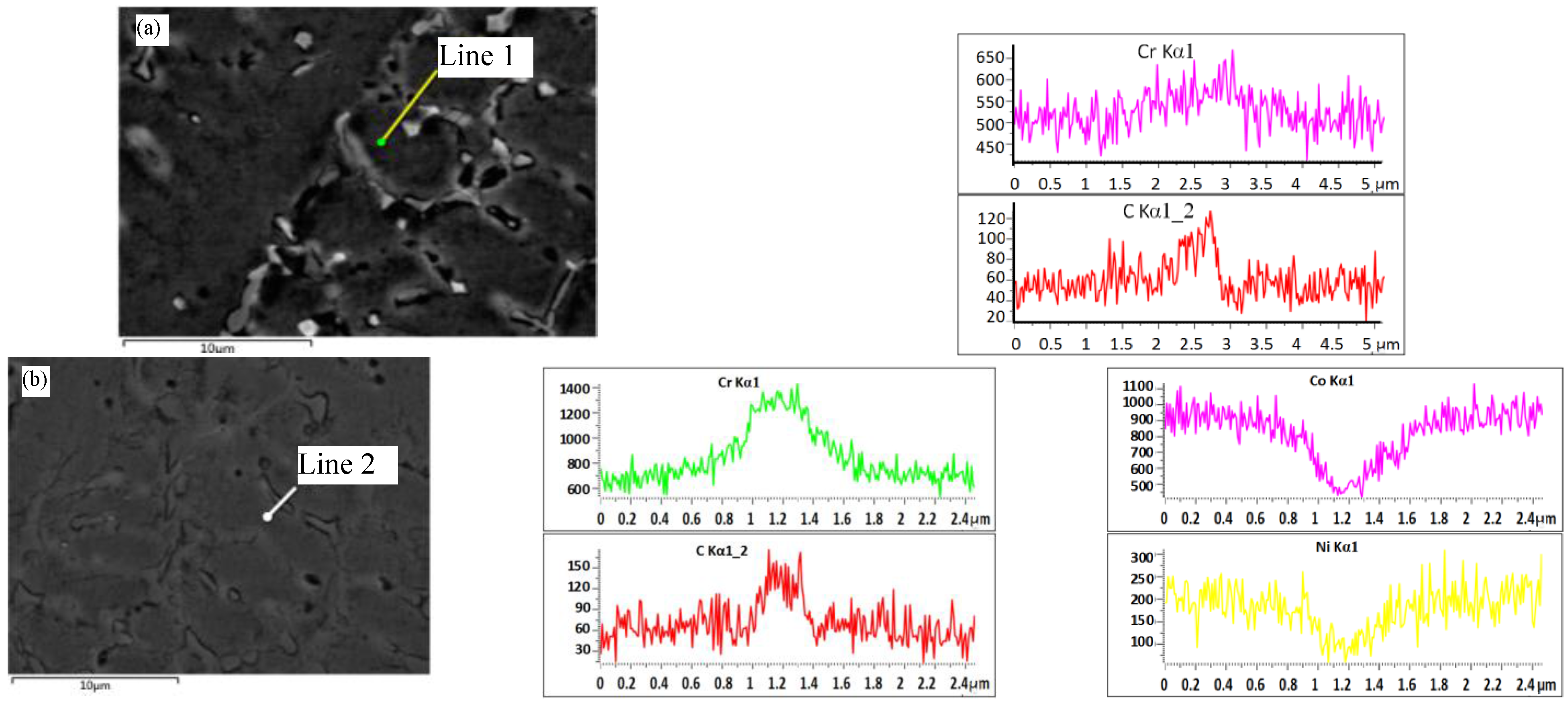
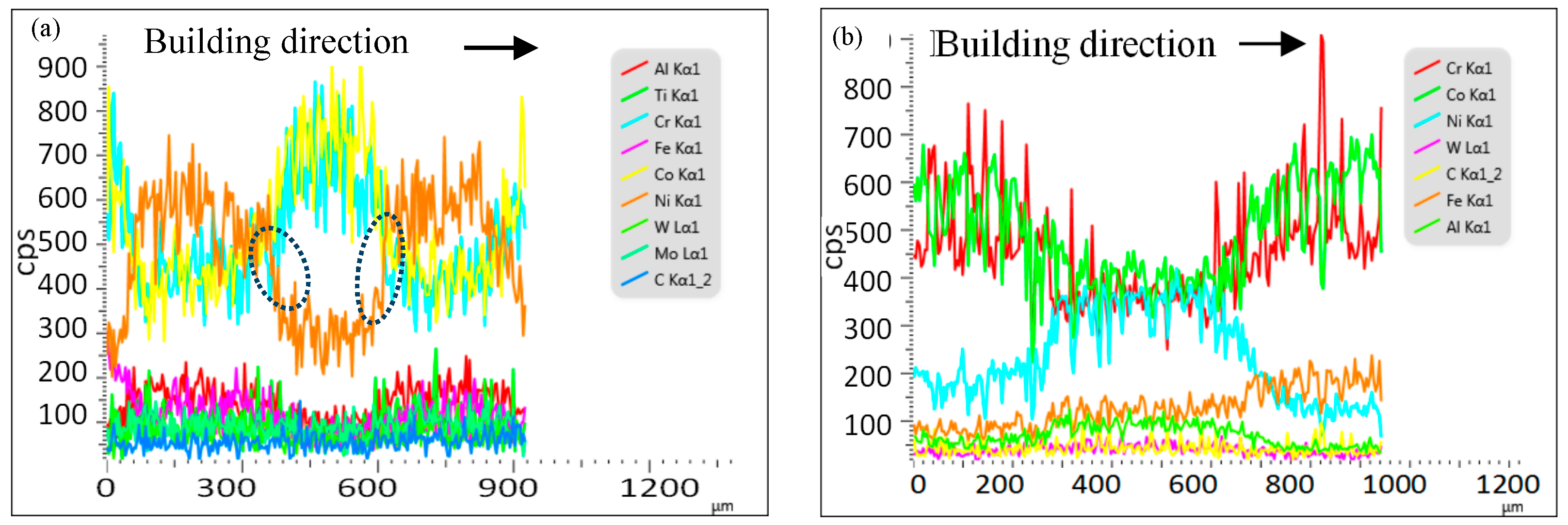
3.3. Composition Distribution
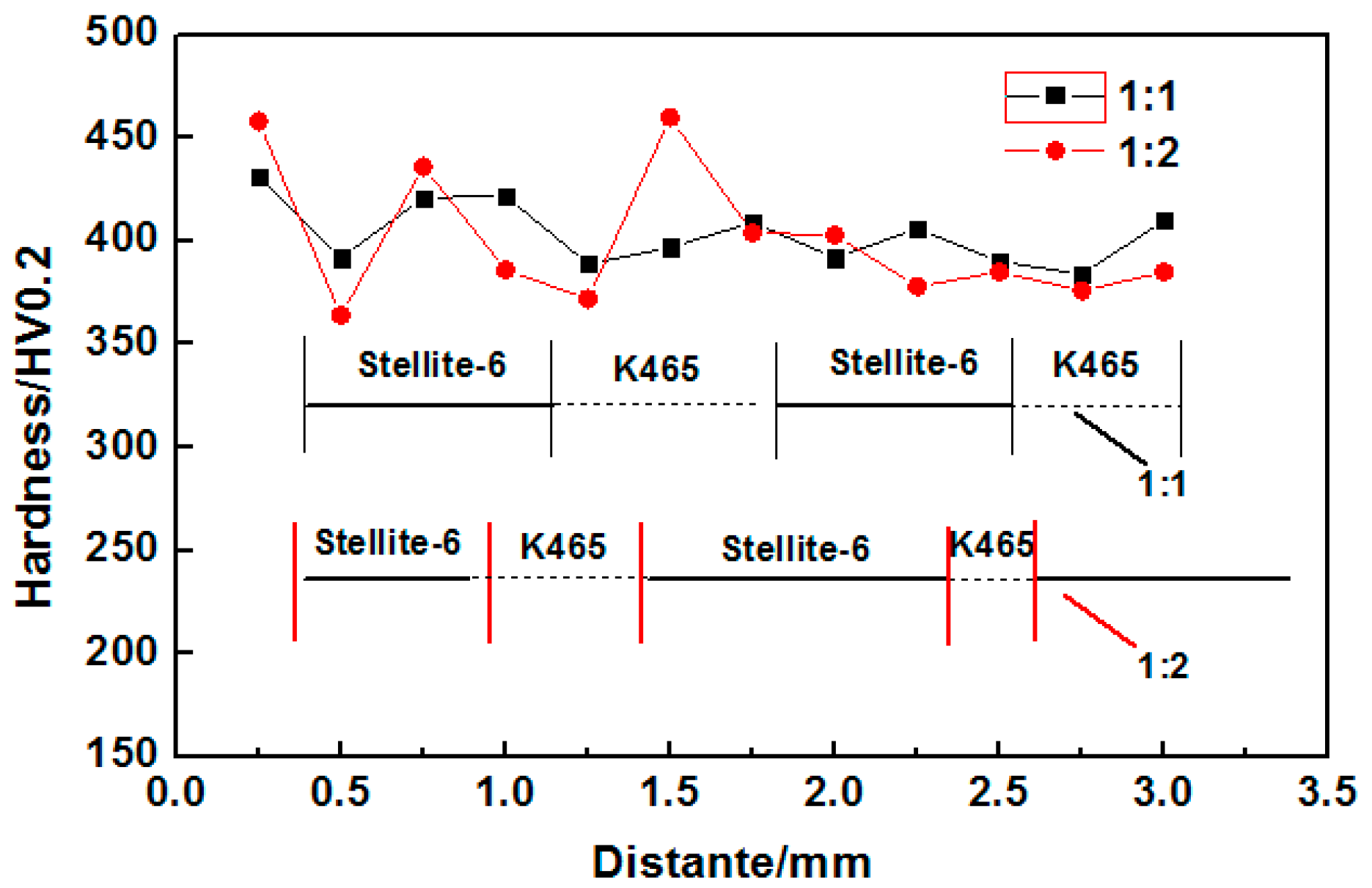
3.4. Microhardness Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, J.X.; Zheng, Q.; Sun, X.F.; Guan, H.R.; Hu, Z.Q. Relative stability of carbides and their effects on the properties of K465 superalloy. Mater. Sci. Eng. A 2006, 429, 341–347. [Google Scholar] [CrossRef]
- Bi, G.J.; Sun, C.N.; Chen, H.C.; Ng, F.L.; Ma, C.C.K. Microstructure and tensile properties of superalloy IN100 fabricated by micro-laser aided additive manufacturing. Mater. Des. 2014, 60, 401–408. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, W.J.; Shang, X.F. Research on the processing experiments of laser metal deposition shaping. Opt. Laser Technol. 2007, 39, 549–557. [Google Scholar] [CrossRef]
- Dinda, G.P.; Dasgupta, A.K.; Mazumder, J. Texture control during laser deposition of nickel-based superalloy. Scr. Mater. 2012, 67, 503–506. [Google Scholar] [CrossRef]
- Zhong, C.; Pirch, N.; Gasser, A.; Gasser, A.; Poprawe, R.; Schleifenbaum, J.H. The Influence of the Powder Stream on High-Deposition-Rate Laser Metal Deposition with Inconel 718. Metals 2017, 7, 443. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, K.; Huang, J.; Hosseini, S.R.E.; Li, Z.G. Characterization of heat affected zone liquation cracking in laser additive manufacturing of Inconel 718. Mater. Des. 2016, 90, 586–594. [Google Scholar] [CrossRef]
- Angelastro, A.; Campanelli, S.L.; Casalino, G. Statistical analysis and optimization of direct metal laser deposition of 227-F Colmonoy nickel alloy. Opt. Laser Technol. 2017, 94, 138–145. [Google Scholar] [CrossRef]
- Acharya, R.; Bansal, R.; Gambone, J.J.; Kaplan, M.A.; Fuchs, G.E.; Rudawski, N.G.; Das, S. Additive Manufacturing and Characterization of René 80 Superalloy Processed Through Scanning Laser Epitaxy for Turbine Engine Hot-Section Component Repair. Adv. Eng. Mater. 2015, 17, 942–950. [Google Scholar] [CrossRef]
- Acharya, R.; Das, S. Additive manufacturing of IN100 superalloy through scanning laser epitaxy for turbine engine hot-section component repair: process development, modeling, microstructural characterization, and process control. Metall. Mater. Trans. A 2015, 46, 3864–3875. [Google Scholar] [CrossRef]
- Yang, J.J.; Li, F.Z.; Wang, Z.M.; Zeng, X.Y. Cracking behavior and control of Rene 104 superalloy produced by direct laser fabrication. J. Mate. Process. Technol. 2015, 225, 229–239. [Google Scholar] [CrossRef]
- Li, Q.G.; Lin, X.; Wang, X.H.; Yang, H.O.; Song, M.; Huang, W.D. Research on the Grain Boundary Liquation Mechanism in Heat Affected Zones of Laser Forming Repaired K465 Nickel-Based Superalloy. Metals 2016, 6, 64. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lin, X.; Song, K.; Jiang, X.Y.; Yang, H.O.; Huang, W.D. Effect of heat input on cracking in laser solid formed DZ4125 superalloy. Opt. Laser Technol. 2016, 86, 1–7. [Google Scholar] [CrossRef]
- Carter, L.N.; Martin, C.; Withers, P.J.; Attallah, M.M. The influence of the laser scan strategy on grain structure and cracking behaviour in SLM powder-bed fabricated nickel superalloy. J. Alloys Compd. 2014, 615, 338–347. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lin, X.; Yu, X.B.; Xu, J.J.; Lei, M.; Huang, W.D. Effect of Ti addition on cracking and microhardness of Inconel 625 during the laser solid forming processing. J. Alloys Compd. 2017, 711, 267–277. [Google Scholar] [CrossRef]
- Harrison, N.J.; Todd, I.; Mumtaz, K. Reduction of micro-cracking in nickel superalloys processed by selective laser melting: A fundamental alloy design approach. Acta Mater. 2015, 94, 59–68. [Google Scholar] [CrossRef]
- Angelastro, A.; Campanelli, S.L.; Casalino, G.; Ludovico, A.D. Optimization of Ni-based WC/Co/Cr composite coatings produced by multilayer laser cladding. Adv. Mater. Sci. Eng. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Abe, T.; Sasahara, H. Dissimilar metal deposition with a stainless steel and nickel-based alloy using wire and arc-based additive manufacturing. Precis. Eng. 2016, 45, 387–395. [Google Scholar] [CrossRef]
- Shah, K.; Haq, I.U.; Khan, A.; Shah, S.A.; Khan, M.; Pinkerton, A.J. Parametric study of development of Inconel-steel functionally graded materials by laser direct metal deposition. Mater. Des. 2014, 54, 531–538. [Google Scholar] [CrossRef]
- Liu, B.X.; Huang, L.J.; Rong, X.D.; Geng, L.; Yin, F.X. Bending behaviors and fracture characteristics of laminated ductile-tough composites under different modes. Compos. Sci. Technol. 2016, 126, 94–105. [Google Scholar] [CrossRef]
- Soodi, M.; Masood, S.H.; Brandt, M. Thermal expansion of functionally graded and wafer-layered structures produced by laser direct metal deposition. Int. J. Adv. Manuf. Technol. 2013, 69, 2011–2018. [Google Scholar] [CrossRef]
- Kolednik, O.; Predan, J.; Fisher, F.D.; Fratzl, P. Improvements of strength and fracture resistance by spatial material property variations. Acta Mater. 2014, 68, 279–294. [Google Scholar] [CrossRef]
- Yan, F.; Liu, S.; Hu, C.; Wang, C.M.; Hu, X.Y. Liquation cracking behavior and control in the heat affected zone of GH909 alloy during Nd: YAG laser welding. J. Mater. Process. Technol. 2017, 244, 44–50. [Google Scholar] [CrossRef]
- Yin, H.; Felicelli, S.D. Dendrite growth simulation during solidification in the LENS process. Acta Mater. 2010, 58, 1455–1465. [Google Scholar] [CrossRef]
- Farshidianfar, M.H.; Khajepour, A.; Gerlich, A.P. Effect of real-time cooling rate on microstructure in laser additive manufacturing. J. Mater. Process. Technol. 2016, 231, 468–478. [Google Scholar] [CrossRef]
- Li, Q.G.; Lin, X.; Liu, F.G.; Liu, F.C.; Huang, W.D. Microstructural characteristics and mechanical properties of laser solid formed K465 superalloy. Mater. Sci. Eng. A 2017, 700, 649–655. [Google Scholar] [CrossRef]
- Bartkowski, D.; Mlynarczak, A.; Piasecki, A.; Dudziak, B.; Goscianski, M.; Bartkowska, A. Microstructure, microhardness and corrosion resistance of Stellite-6 coatings reinforced with WC particles using laser cladding. Opt. Laser Technol. 2015, 68, 191–201. [Google Scholar] [CrossRef]
- Apay, S.; Gulenc, B. Wear properties of AISI 1015 steel coated with Stellite 6 by microlaser welding. Mater. Des. 2014, 55, 1–8. [Google Scholar] [CrossRef]
- Shoja-Razavi, R. Laser Surface Treatment of Stellite 6 Coating Deposited by HVOF on 316L Alloy. J. Mater. Eng. Perform. 2016, 25, 2583–2595. [Google Scholar] [CrossRef]
- Tang, Q.; Pang, S.Y.; Chen, B.B.; Suo, H.B.; Zhou, J.X. A three dimensional transient model for heat transfer and fluid flow of weld pool during electron beam freeform fabrication of Ti-6-Al-4-V alloy. Int. J. Heat Mass Trans. 2014, 78, 203–215. [Google Scholar] [CrossRef]



| Element | C | Mo | Cr | Nb | Fe | Ti | Si | Al | W | Mn | Co | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K465 | 0.17 | 1.80 | 8.75 | 2.00 | - | 2.45 | - | 5.55 | 10.25 | - | 9.75 | Bal. |
| Stellite-6 | 1.09 | 0.22 | 29.27 | - | 1.93 | - | 1.00 | - | 4.29 | 0.13 | Bal. | 2.34 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhao, J.; Zhao, Y.; Zhang, H.; Shi, F. Microstructure and Microhardness of Laser Metal Deposition Shaping K465/Stellite-6 Laminated Material. Metals 2017, 7, 512. https://doi.org/10.3390/met7110512
Wang Z, Zhao J, Zhao Y, Zhang H, Shi F. Microstructure and Microhardness of Laser Metal Deposition Shaping K465/Stellite-6 Laminated Material. Metals. 2017; 7(11):512. https://doi.org/10.3390/met7110512
Chicago/Turabian StyleWang, Zhiguo, Jibin Zhao, Yuhui Zhao, Hongyu Zhang, and Fan Shi. 2017. "Microstructure and Microhardness of Laser Metal Deposition Shaping K465/Stellite-6 Laminated Material" Metals 7, no. 11: 512. https://doi.org/10.3390/met7110512