Thermal Growth Cu1.2Mn1.8O4 Spinel Coatings on Metal Interconnects for Solid Oxide Fuel Cell Applications
Abstract
:1. Introduction
2. Experiments
3. Results and Discussion
3.1. Structure and Morphology of Deposited Coatings
3.2. Characteristics of Thermal Growth Coatings
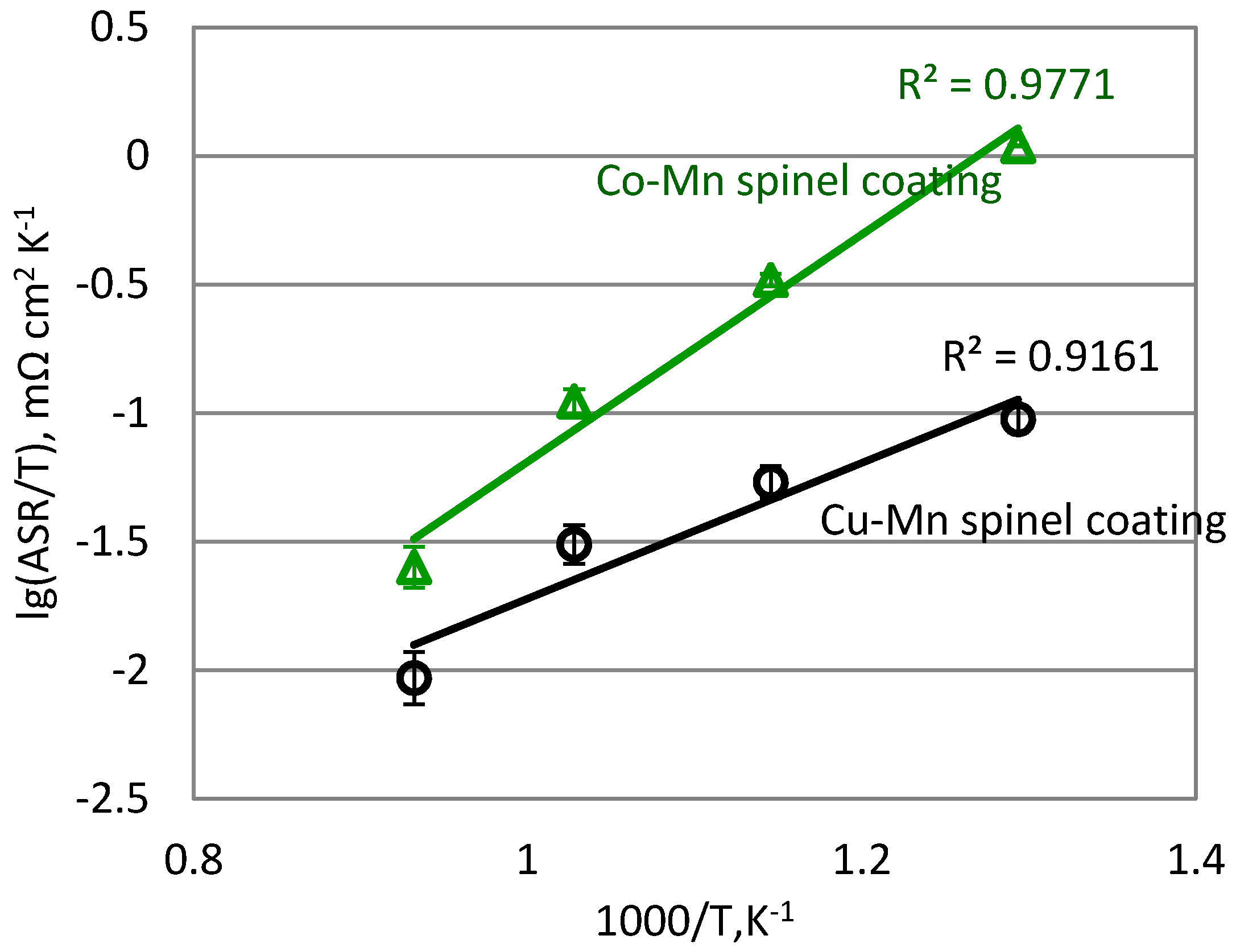
3.3. Electrical Characterization
3.4. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Windisch, H.F.; Sattari, M.; Svensson, J.E.; Froitzheim, J. Chromium vaporization from mechanically deformed pre-coated interconnects in solid oxide fuel cells. J. Power Sources 2015, 297, 217–223. [Google Scholar] [CrossRef]
- Stanislowski, M.; Froitzheim, J.; Niewolak, L.; Quadakkers, W.J.; Hilpert, K.; Markus, T.; Singheiser, L. Reduction of chromium vaporization from SOFC interconnectors by highly effective coatings. J. Power Sources 2007, 164, 578–589. [Google Scholar] [CrossRef]
- Magrasó, A.; Windisch, H.F.; Froitzheim, J.; Svensson, J.E.; Haugsrud, R. Reduced long term electrical resistance in Ce/Co coated ferritic stainless steel for solid oxide fuel cell metallic interconnects. Int. J. Hydrogen Energy 2015, 40, 8579–8585. [Google Scholar] [CrossRef]
- Feng, Z.J.; Xu, Y.X.; Zeng, C.L. Preparation and high temperature performances of DyCrO3-based coatings on a ferritic stainless steel interconnect material. J. Power Sources 2013, 235, 54–61. [Google Scholar] [CrossRef]
- Yang, X.L.; Tu, H.Y.; Yu, Q.C. Fabrication of Co3O4 and La0.6Sr0.4CoO3-δCe0.8Gd0.2O2-δ dual layer coatings on SUS430 steel by in-situ phase formation for solid oxide fuel cell interconnects. Int. J. Hydrogen Energy 2015, 40, 607–614. [Google Scholar] [CrossRef]
- Zhu, J.H.; Lewis, M.J.; Du, S.W.; Li, Y.T. CeO2-doped (Co,Mn)3O4 coatings for protecting solid oxide fuel cell interconnect alloys. Thin Solid Films 2015, 596, 179–184. [Google Scholar] [CrossRef]
- Lai, Y.B.; Guo, P.Y.; Shao, Y.; Zhang, Y.; Liu, N. The Role of Dy Doping on Oxidation Behavior of Co-40Mn/Co Coating for Solid Oxide Fuel Cell Metal Interconnects. J. Alloys Compd. 2017, 694, 383–393. [Google Scholar] [CrossRef]
- Wu, J.W.; Johnson, C.D.; Gemmen, R.S.; Liu, X.B. The performance of solid oxide fuel cells with Mn-Co electroplated interconnect as cathode current collector. J. Power Sources 2009, 189, 1106–1113. [Google Scholar] [CrossRef]
- Zhen, S.Y.; Sun, W.; Li, P.Q.; Tang, G.Z.; Rooney, D.; Sun, K.N.; Ma, X.X. High performance cobalt-free Cu1.4Mn1.6O4 spinel oxide as an intermediate temperature solid oxide fuel cell cathode. J. Power Sources 2016, 315, 140–144. [Google Scholar] [CrossRef]
- Sun, Z.H.; Gopalan, S.; Pal, U.B.; Basu, S.N. Cu1.3Mn1.7O4 spinel coatings deposited by electrophoretic deposition on Crofer 22 APU substrates for solid oxide fuel cell applications. Surf. Coat. Technol. 2017, 323, 49–57. [Google Scholar] [CrossRef]
- Joshi, S.; Petric, A. Nickel substituted CuMn2O4 spinel coatings for solid oxide fuel cell interconnects. Int. J. Hydrogen Energy 2017, 42, 5584–5589. [Google Scholar] [CrossRef]
- Guo, P.Y.; Shao, Y.; Zeng, C.L.; Wu, M.F.; Li, W.L. Oxidation characterization of FeAl coated 316 stainless steel interconnects by high-energy micro-arc alloying technique for SOFC. Mater. Lett. 2011, 65, 3180–3183. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.Y.; Shao, Y.; Lai, Y.B.; Zhang, J.Q. Preparation and high-temperature performance of Co-10Mn and Co-40Mn alloy coatings for solid oxide fuel cell metal interconnects. J. Alloys Compd. 2016, 680, 685–693. [Google Scholar] [CrossRef]
- Lai, Y.B.; Guo, P.Y.; Shao, Y.; Tang, P.J.; Zhang, Y.; Zhang, J.F. Formation and performances of spinel reaction layers on Co-40Mn coatings under an oxygen pressure of 105 Pa for solid oxide fuel cell interconnect application. Vacuum 2016, 130, 14–24. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, X.F.; Ouyang, C.; Wu, J.; Gao, Y.Q.; Huang, Z. Annealing effect on the structural, electrical and 1/f noise roperties of Mn-Co-Ni-O thin films. J. Mater. Sci. Mater. Electron. 2014, 25, 1959–1964. [Google Scholar] [CrossRef]
- Yokoyama, T.; Meguro, T.; Kato, K.; Okazaki, S.; Ito, D.; Tatami, J.; Wakihara, T.; Kome, K. Preparation and electrical properties of sintered oxide composed of MnFeNiO4 with a cubic spinel structure. J. Electroceram. 2013, 31, 353–359. [Google Scholar] [CrossRef]
- Bateni, M.R.; Wei, P.; Deng, X.H.; Petric, A. Spinel coatings for UNS 430 stainless steel interconnects. Surf. Coat. Technol. 2007, 201, 4677–4684. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.W.; Liu, X.; Baker, A. Studies on elements diffusion of Mn/Co coated ferritic stainless steel for solid oxide fuel cell interconnects application. Int. J. Hydrogen Energy 2013, 38, 5075–5083. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Bahlawane, N.; Vannier, V.; Hoinghaus, K.K. Structure sensitivity of propene oxidation over Co-Mn spinels. Proc. Combust. Inst. 2013, 34, 2261–2268. [Google Scholar] [CrossRef]
- Fu, Q.X.; Tietz, F.; Sebold, D.; Wessel, E.; Buchkremer, H.P. Magnetron-sputtered cobalt-based protective coatings on ferritic steels for solid oxide fuel cell interconnect applications. Corros. Sci. 2012, 54, 68–76. [Google Scholar] [CrossRef]
- Young, D.J. High Temperature Oxidation and Corrosion of Metals, 1st ed.; Elsevier Ltd.: Cambridge, UK, 2008; pp. 29–47. ISBN 978-0-08-044587-8. [Google Scholar]
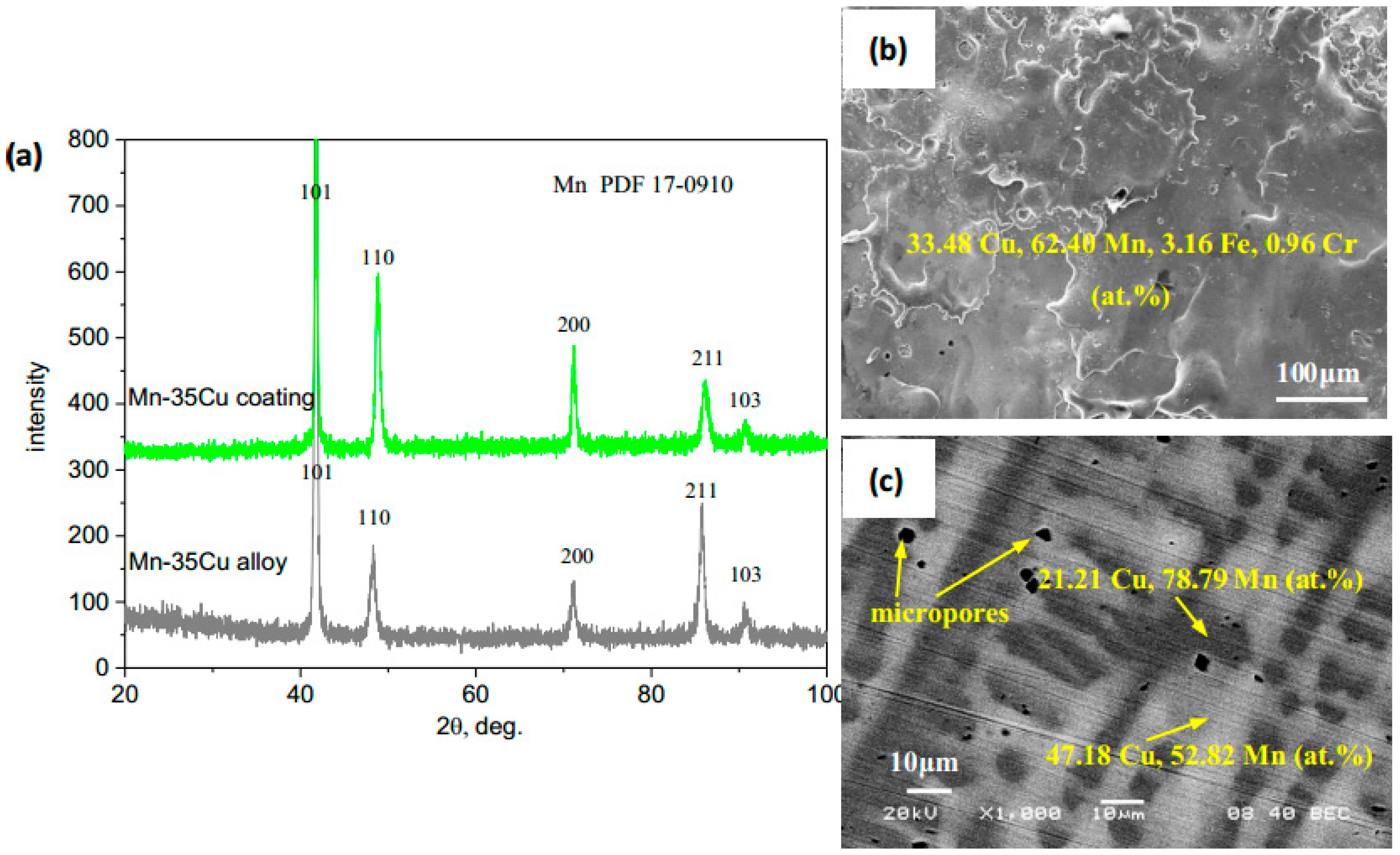
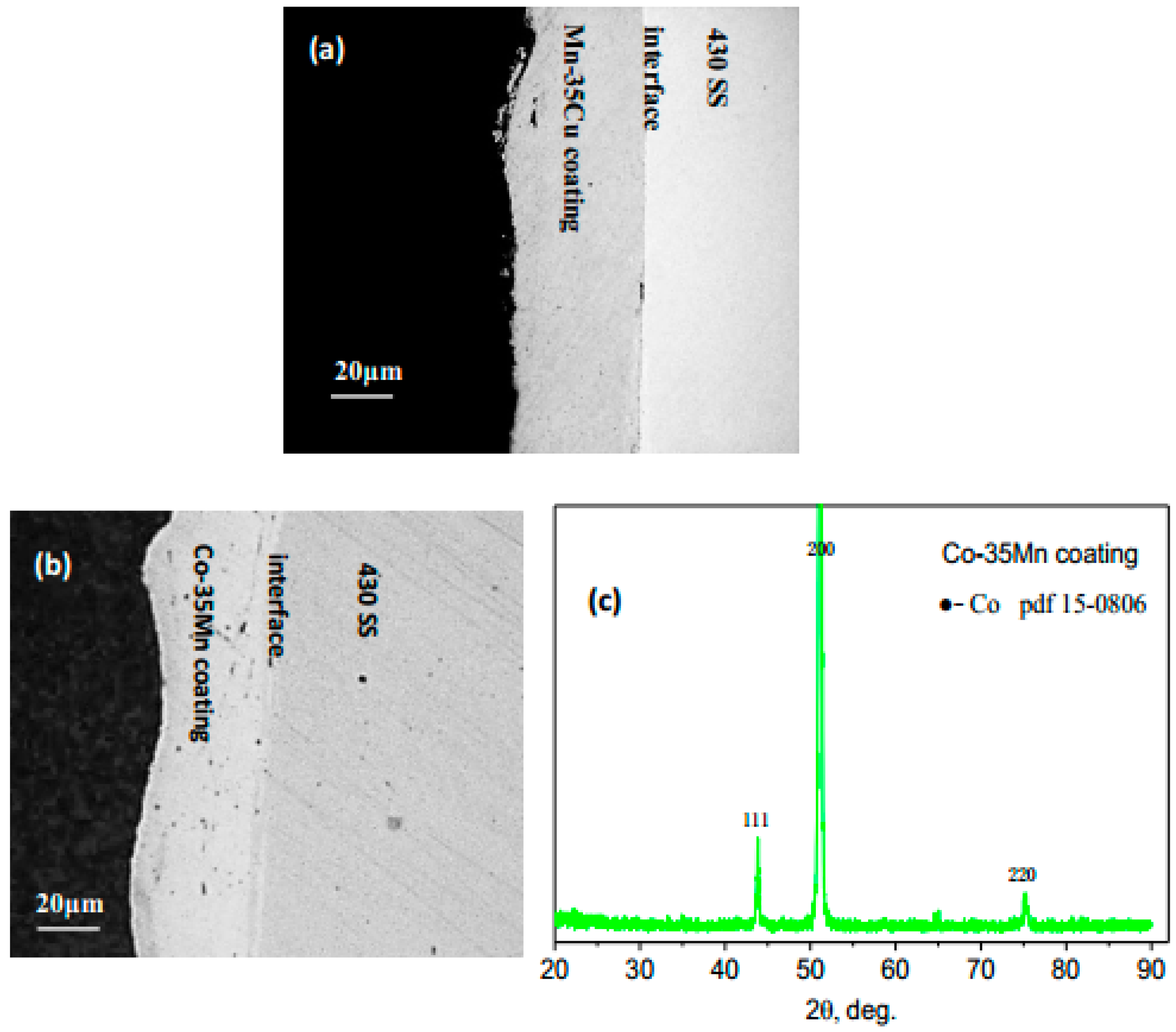

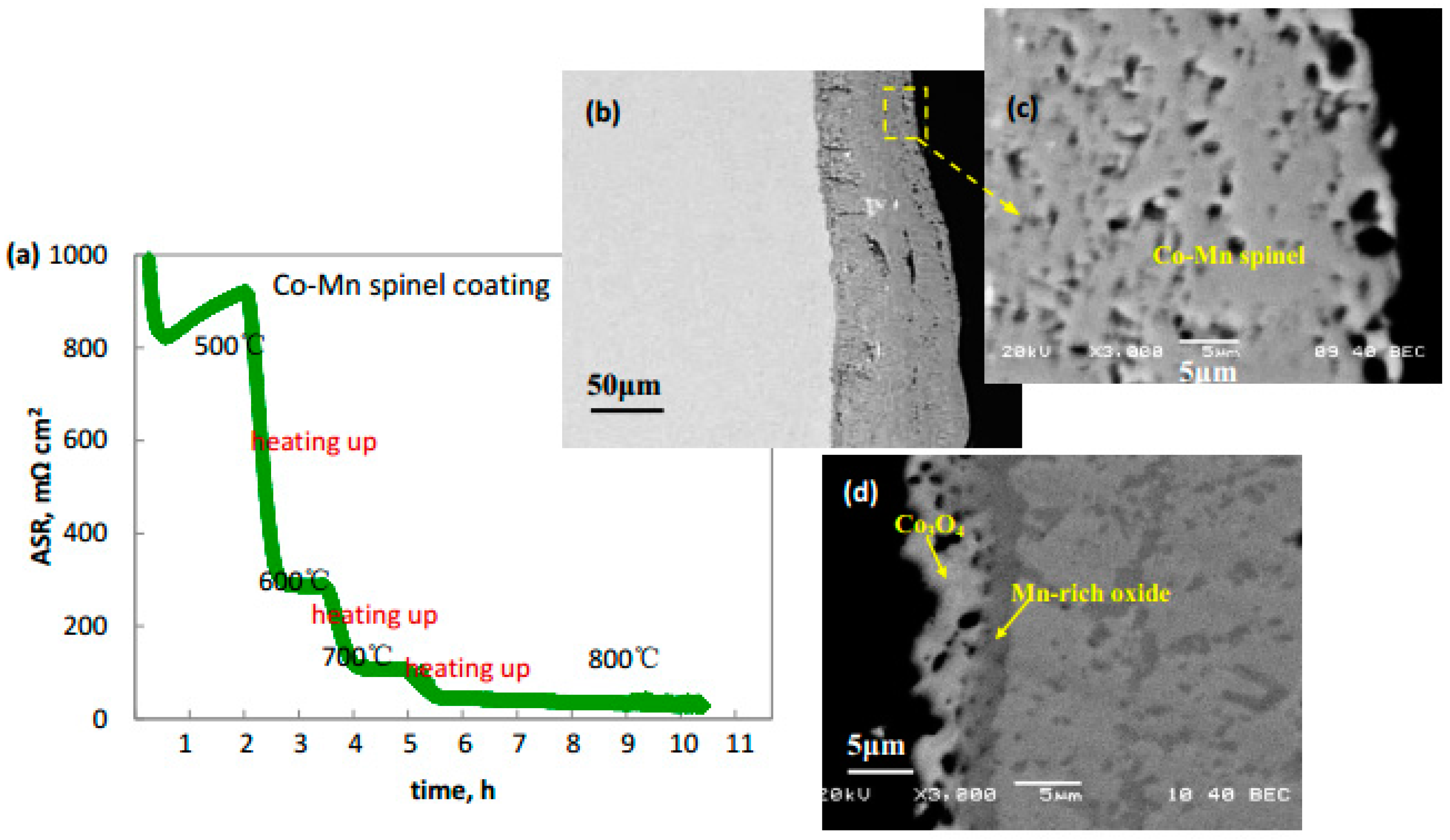
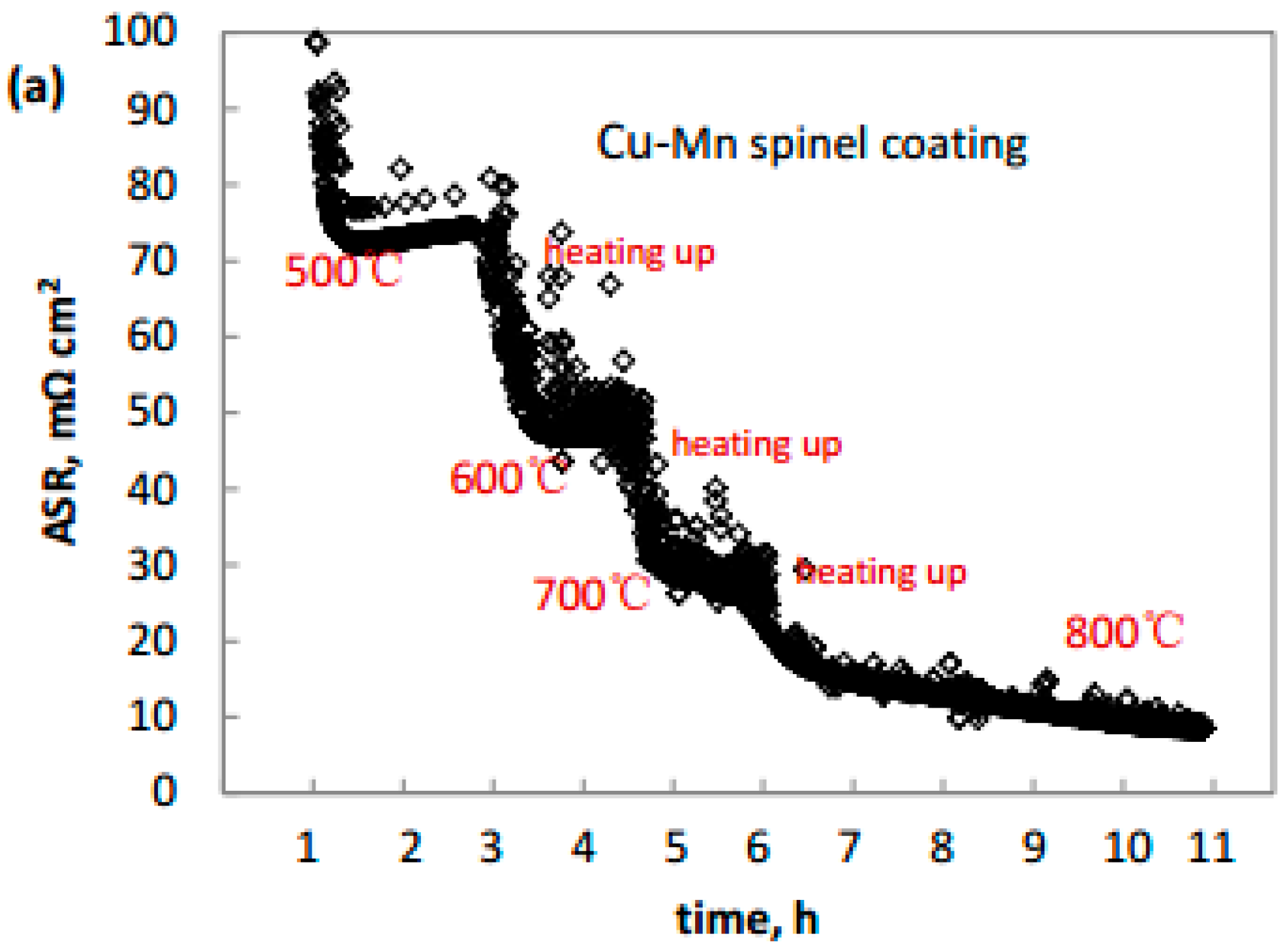
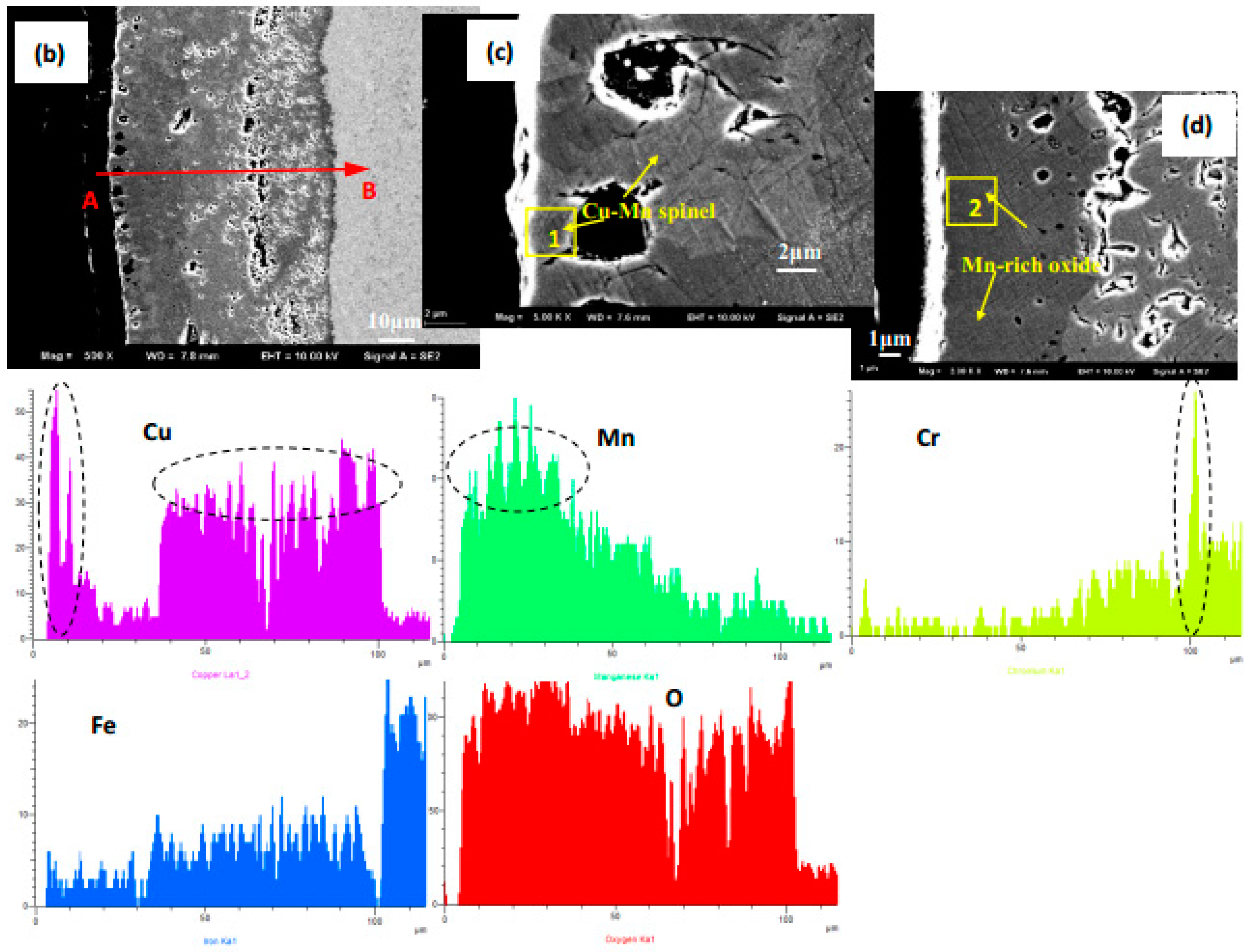
| Elements, at. % | Co | Mn | Cu | Fe | Cr | O | Notes |
|---|---|---|---|---|---|---|---|
| Mn-35Cu coating, 100 h | - | 25.67 | 14.30 | 1.36 | - | 58.67 | area 1 |
| - | 30.67 | 1.78 | 1.29 | - | 66.26 | area 2 | |
| - | 7.97 | 37.19 | 0.54 | - | 54.30 | area 3 | |
| Co-35Mn coating, 100 h | 36.54 | 5.32 | - | - | - | 58.15 | area 4 |
| 15.23 | 21.96 | - | - | - | 62.80 | area 5 |
| Elements, at. % | Co | Mn | Cu | Fe | Cr | O | Notes |
|---|---|---|---|---|---|---|---|
| Mn-35Cu coating, 100 h | - | 21.13 | 20.02 | - | - | 58.85 | area 1 |
| - | 32.70 | 2.83 | 4.92 | - | 59.55 | area 2 |
| Number | Reaction | T, °C | ΔGo, J mol−1 | PO2, atm |
|---|---|---|---|---|
| 1 | Mn(s) + 1/2O2(g) = MnO(s) | 750 | −337,829.6 | 3.15 × 10−35 |
| 2 | 2/3Cr(s) + 1/2O2(g) = 1/3Cr2O3(s) | 750 | −285,442.0 | 7.07 × 10−30 |
| 3 | 3Mn(s) + 2O2(g) = Mn3O4(s) | 750 | −259,818.1 | 2.92 × 10−27 |
| 4 | Fe(s) + 1/2O2(g) = FeO(s) | 750 | −197,985.8 | 6.03 × 10−21 |
| 5 | 3FeO(s) + 1/2O2(g) = Fe3O4(s) | 750 | −184,232.7 | 1.53 × 10−19 |
| 6 | Co(s) + 1/2O2(g) = CoO(s) | 750 | −161,559.9 | 3.16 × 10−17 |
| 7 | Cu(s) + 1/2O2(g) = CuO(s) | 750 | −64,946.9 | 2.32 × 10−7 |
| 8 | 3CoO(s) + 1/2O2(g) = Co3O4(s) | 750 | −31753.7 | 5.71 × 10−4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, P.; Lai, Y.; Shao, Y.; Zhang, Y.; Wang, Y. Thermal Growth Cu1.2Mn1.8O4 Spinel Coatings on Metal Interconnects for Solid Oxide Fuel Cell Applications. Metals 2017, 7, 522. https://doi.org/10.3390/met7120522
Guo P, Lai Y, Shao Y, Zhang Y, Wang Y. Thermal Growth Cu1.2Mn1.8O4 Spinel Coatings on Metal Interconnects for Solid Oxide Fuel Cell Applications. Metals. 2017; 7(12):522. https://doi.org/10.3390/met7120522
Chicago/Turabian StyleGuo, Pingyi, Yongbiao Lai, Yong Shao, Yu Zhang, and Yuxin Wang. 2017. "Thermal Growth Cu1.2Mn1.8O4 Spinel Coatings on Metal Interconnects for Solid Oxide Fuel Cell Applications" Metals 7, no. 12: 522. https://doi.org/10.3390/met7120522





