Effect of Different Welding Processes on Electrochemical and Corrosion Behavior of Pure Nickel in 1 M NaCl Solution
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Material
2.2. Welding Procedure
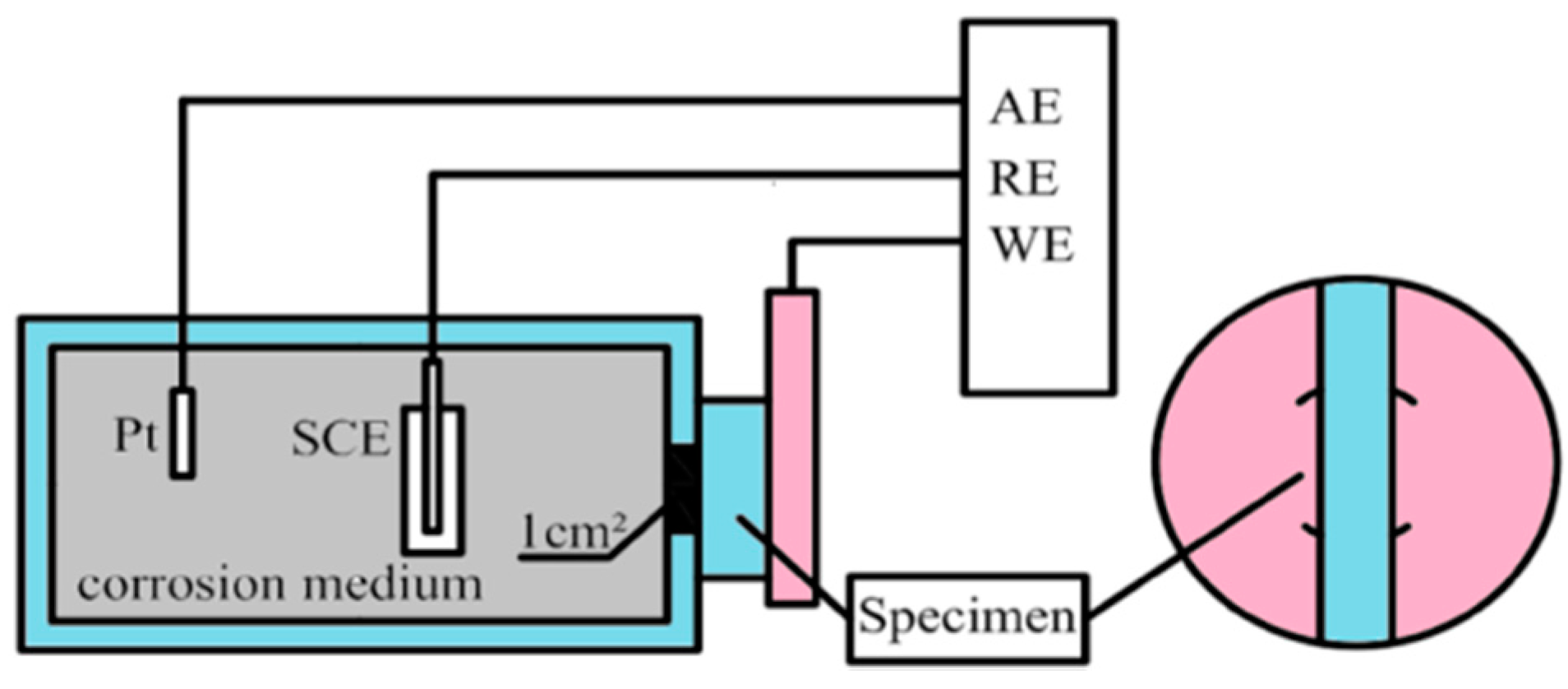
2.3. Electrochemical Testing
3. Results and Discussion
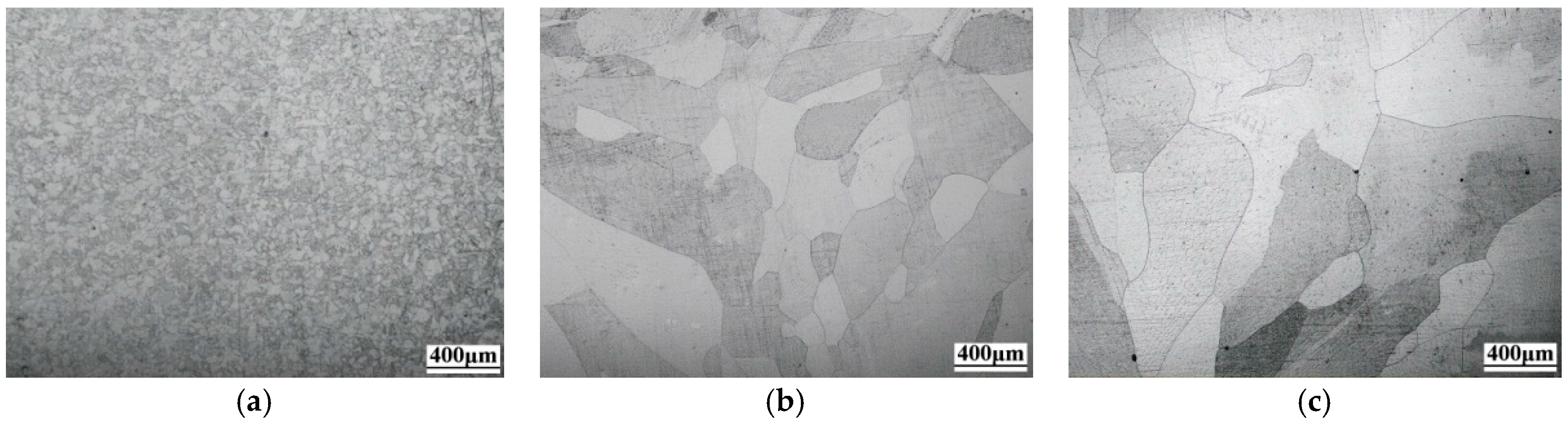
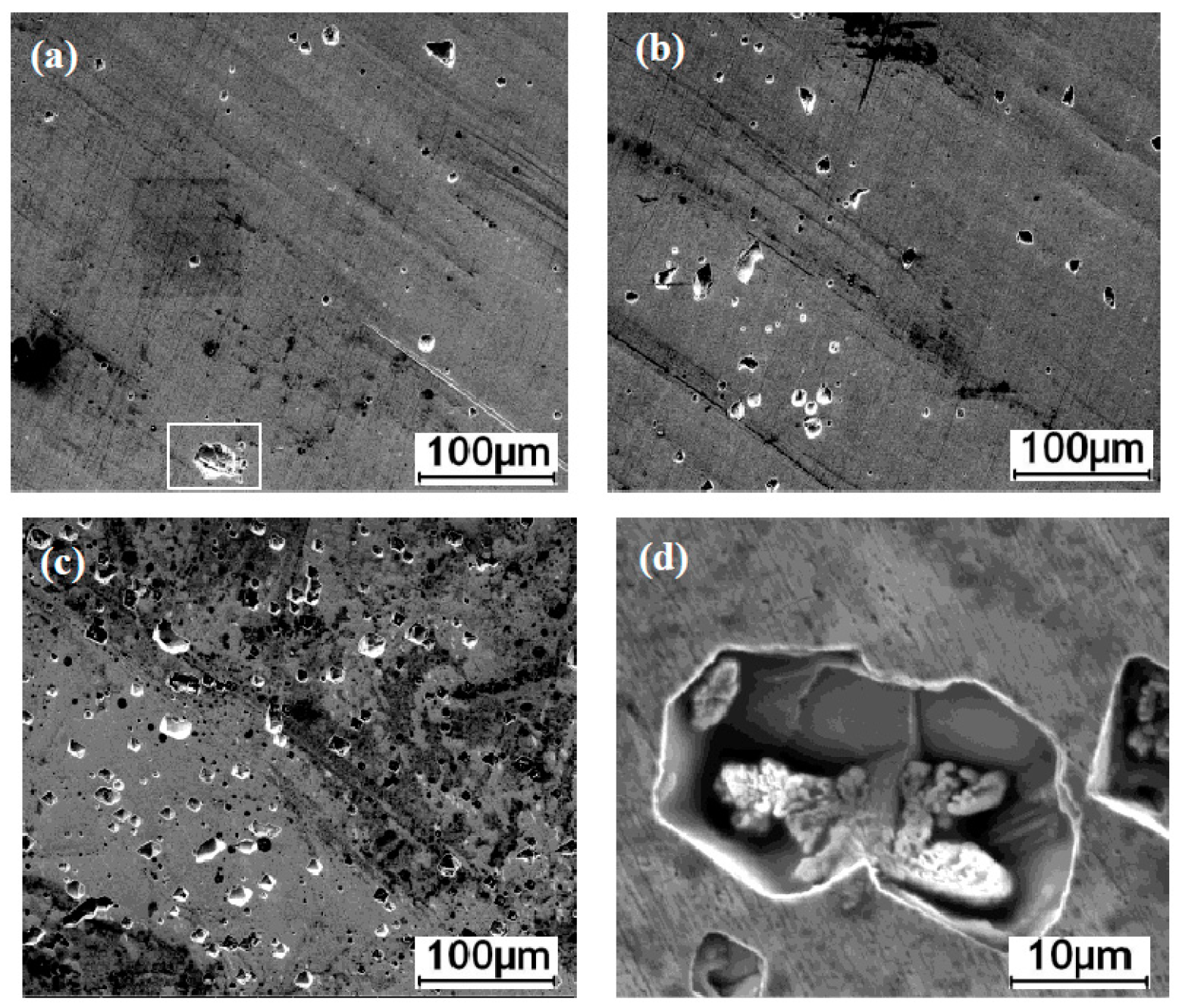
3.1. Microstructures
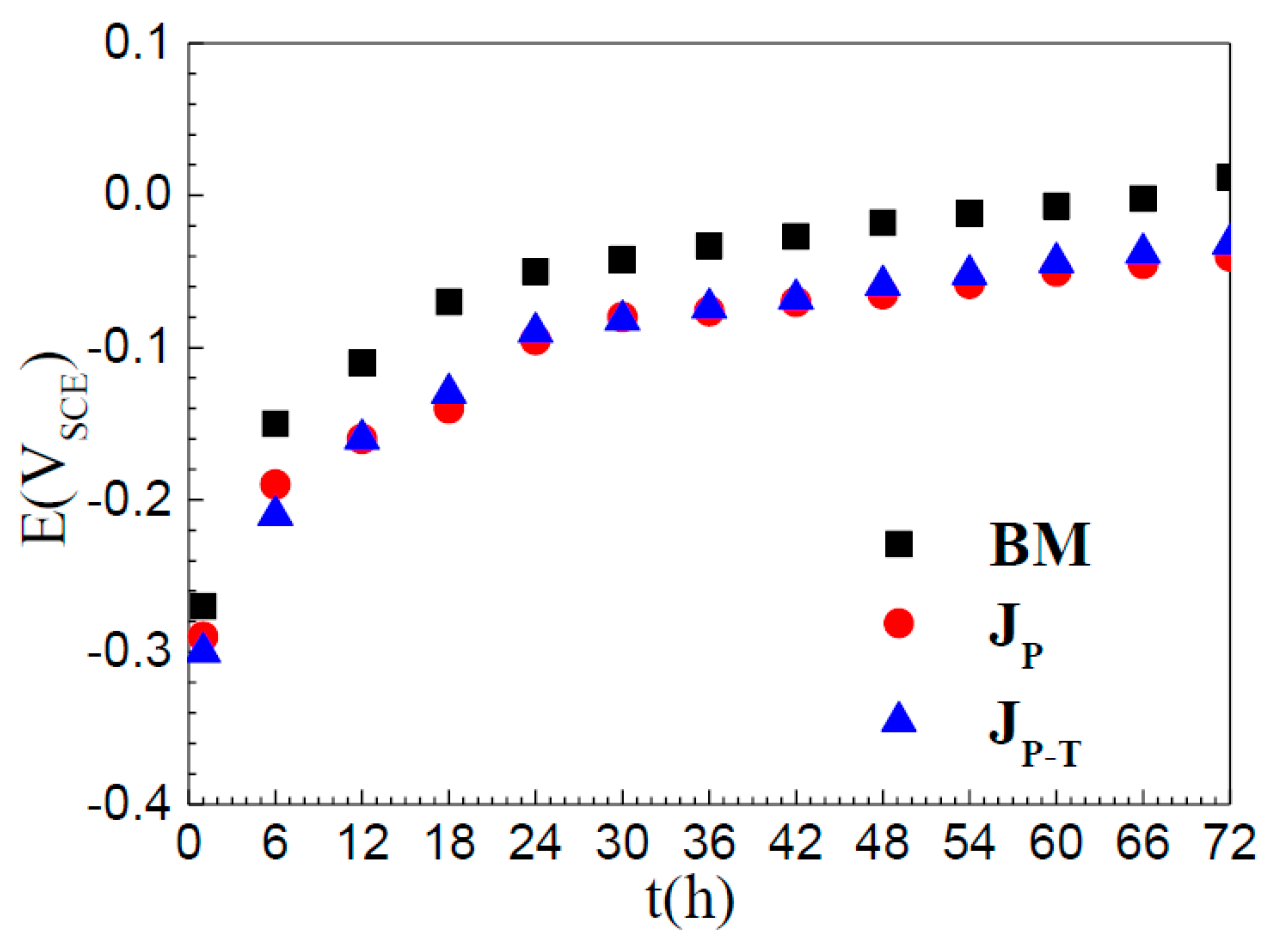
3.2. Open Circuit Potential
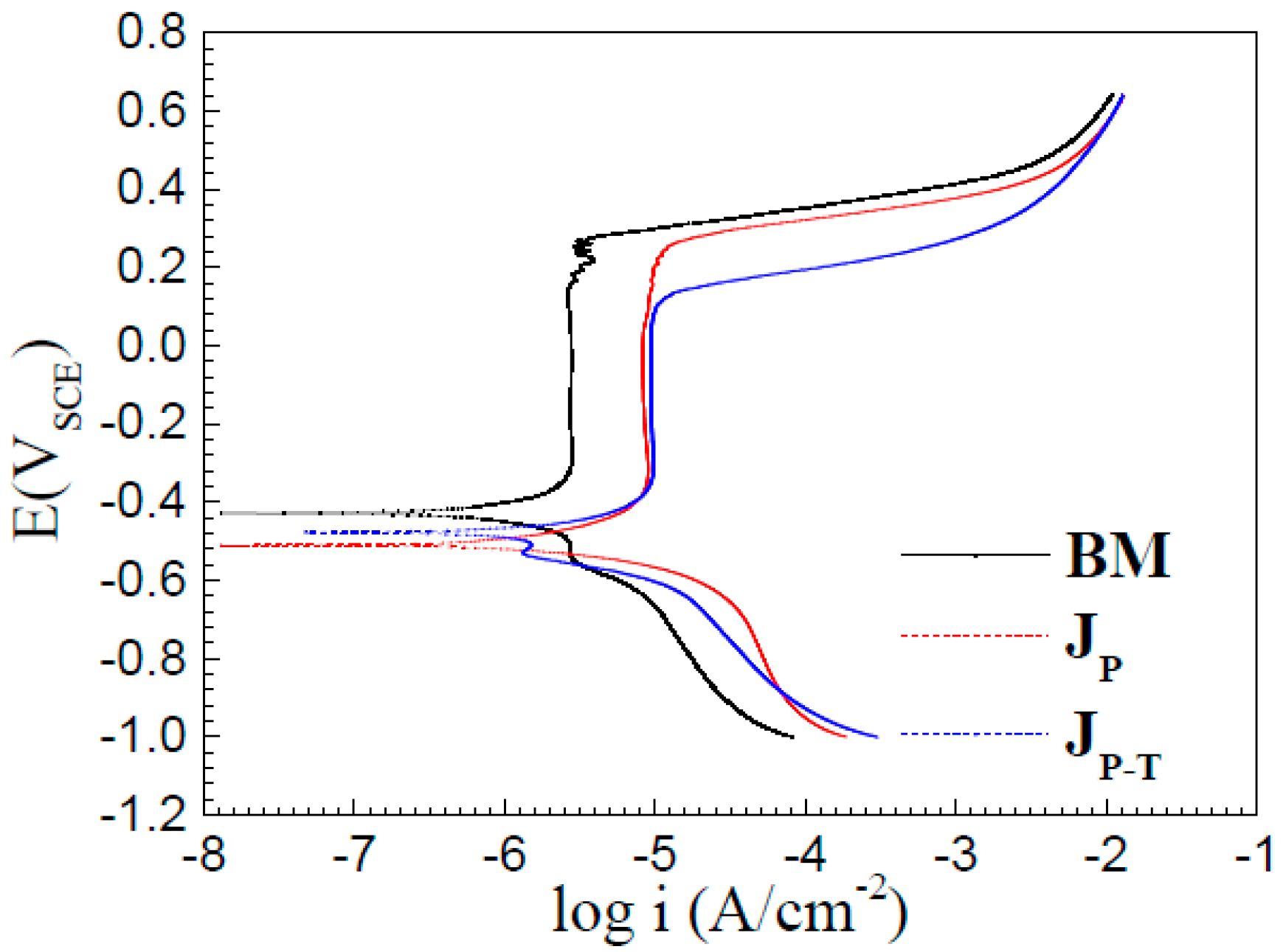
3.3. Polarization Measurements
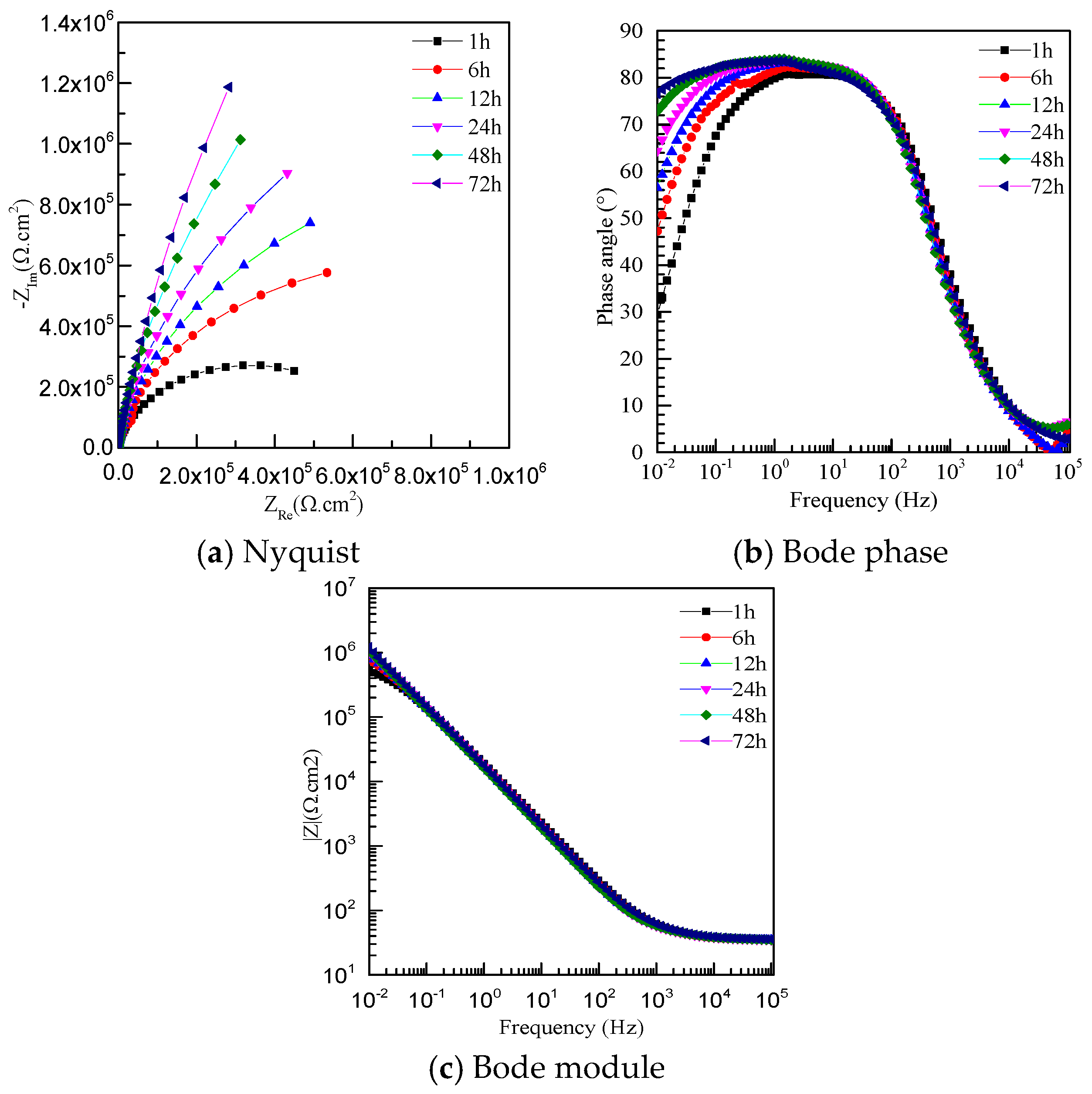
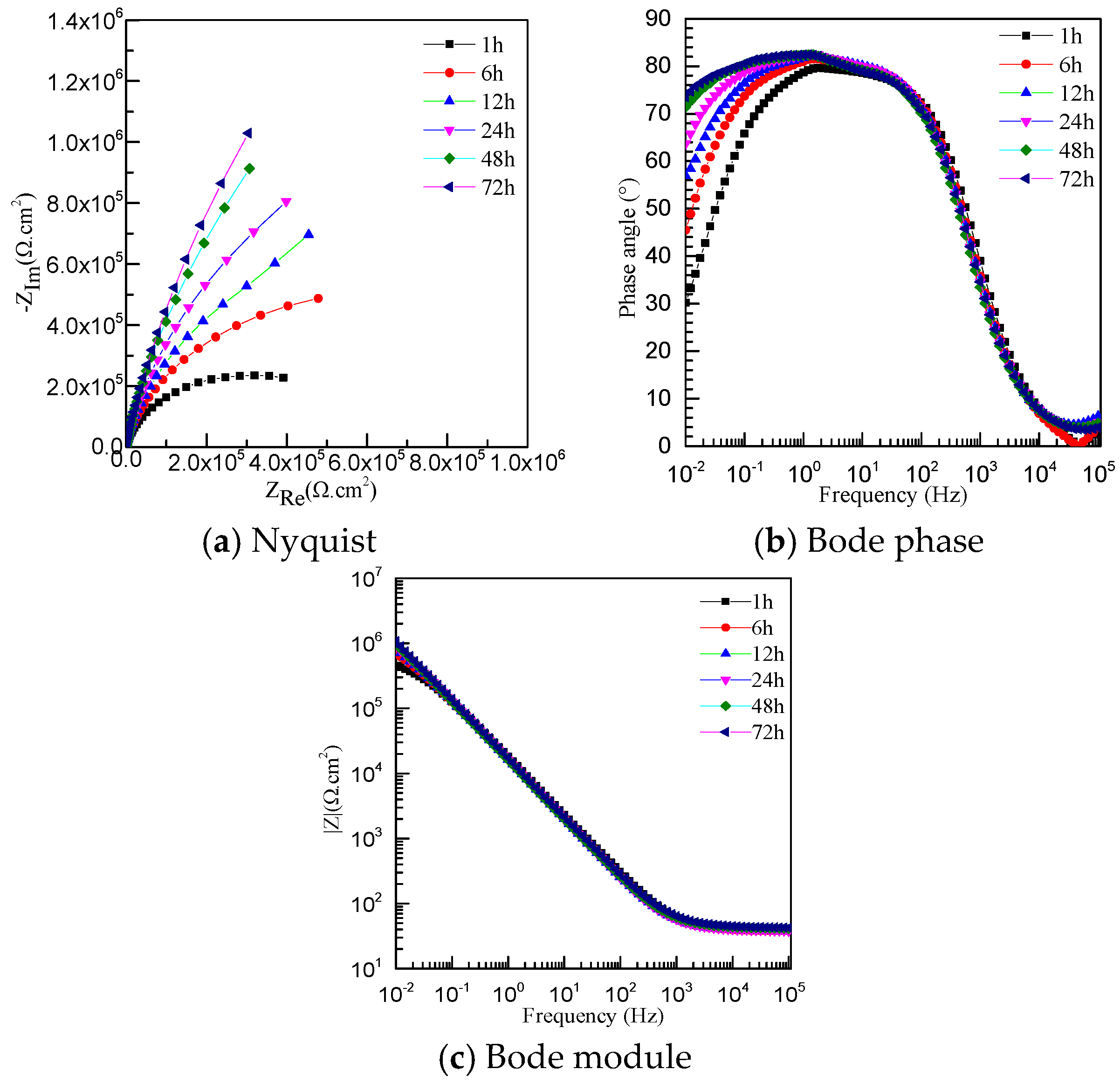
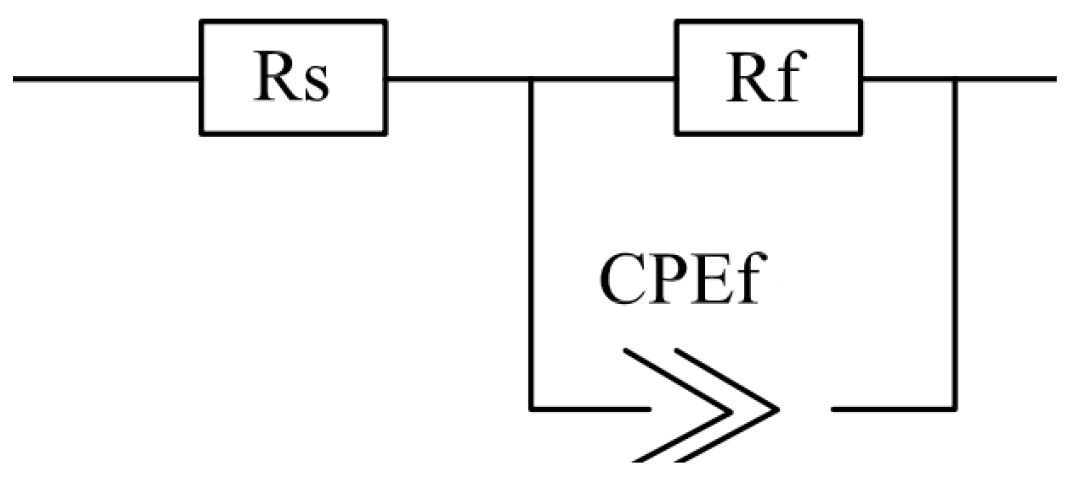
3.4. Electrochemical Impedance Spectroscopy
3.5. Corrosion Morphology
4. Conclusions
- The microstructure of JP is a coarse austenite-cellular structure with an average grain size of 463.57 μm. By comparison, the JP-T is composed of a coarse austenite-columnar structure with a grain size of 547.32 μm, which is due to the increase of total heat input by the extra TIG arc, while BM has an average grain size of 47.32 μm.
- The time profiles of OCP obtained for all three samples are quite similar and all specimens in NaCl solution were gradually passivated until the OCP finally reached relatively constant values at 30 h.
- Potentiodynamic polarization curves and electrochemical impedance spectroscopy of base metal, JP, and JP-T show that JP had a relatively better corrosion resistance than JP-T, but they were lower than BM. The corrosion morphology observations are consistent with the consequence of the electrochemical experiments.
- Fine grain causes the acquisition of fine-grained samples with a large fraction of grain boundaries. The increase of the grains’ dimensions due to heat treatment decreased the metal stability to pitting corrosion.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Davis, J.R. Nickel, Cobalt, and Their Alloys; ASM International: New York, NY, USA, 2000. [Google Scholar]
- Lou, D.C.; Solberg, J.K.; Akselsen, O.M. Microstructure and property investigation of paste boronized pure nickel and Nimonic 90 superalloy. Mater. Chem. Phys. 2009, 115, 239–244. [Google Scholar] [CrossRef]
- Manoj, K. Hot corrosion of nickel in anhydrous sodium hydroxide. Mater. Chem. Phys. 1996, 45, 171–175. [Google Scholar]
- Benoît, T.O.; Catherine, A.D. Electronic and transport properties of passive films grown on different Ni-Cr binary alloys in relation to the pitting susceptibility. Electrochim. Acta 2014, 133, 373–381. [Google Scholar]
- Barbosa, M.R.; Bastos, J.A. Chloride role in the surface of nickel electrode. Electrochim. Acta 1998, 44, 957–965. [Google Scholar] [CrossRef]
- Meng, G.Z.; Yang, L.; Shao, Y.W. Effect of microstructures on corrosion behavior of nickel coatings: (II) competitive effect of grain size and twins density on corrosion behavior. J. Mater. Sci. Technol. 2016, 32, 465–469. [Google Scholar] [CrossRef]
- Meng, G.Z.; Yang, L.; Shao, Y.W. Effect of microstructures on corrosion behavior of nickel coatings: (I) abnormal grain size effect on corrosion behavior. J. Mater. Sci. Technol. 2015, 31, 1186–1192. [Google Scholar] [CrossRef]
- Li, L.; Ying, L.; Wang, F.H. Influence of grain size on the corrosion behavior of a Ni-based superalloy nanocrystalline coating in NaCl acidic solution. Electrochim. Acta 2008, 53, 2453–2462. [Google Scholar] [CrossRef]
- Tan, X.; Peng, X.; Wang, F. The effect of grain refinement on the adhesion of an alumina scale on an aluminide coating. Corros. Sci. 2014, 85, 280–286. [Google Scholar] [CrossRef]
- Aghuy, A.A.; Zakeri, M.; Moayed, M.H. Effect of grain size on pitting corrosion of 304L austenitic stainless steel. Corros. Sci. 2015, 94, 368–376. [Google Scholar] [CrossRef]
- Freire, L.; Nóvoa, X.R.; Montemor, M.F.; Carmezim, M.J. Study of passive films formed on mild steel in alkaline media by the application of anodic potentials. Mater. Chem. Phys. 2009, 114, 962–972. [Google Scholar] [CrossRef]
- Qiao, Y.; Zheng, Y.; Ke, W.; Okafor, P.C. Electrochemical behavior of high nitrogen stainless steel in acidic solution. Corros. Sci. 2009, 51, 979–986. [Google Scholar] [CrossRef]
- Marcelin, S.; Pébère, N.; Régnier, S. Electrochemical characterization of a martensitic stainless steel in a neutral chloride solution. Electrochim. Acta 2013, 87, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Wu, X.; Han, E. Effects of temperature on the protective property, structure and composition of the oxide film on Alloy 625. Corros. Sci. 2009, 51, 2565–2567. [Google Scholar] [CrossRef]
- Rybalka, K.V.; Beketaeva, L.A.; Davydov, A.D. Effect of self-passivation on the electrochemical and corrosion behavior of alloy C-22 in NaCl solution. Corros. Sci. 2012, 54, 161–166. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; Carranza, R.M. Properties of the passive film on alloy 22 in chloride solutions obtained by electrochemical impedance. J. Electrochem. Soc. 2011, 156, 221–230. [Google Scholar] [CrossRef]
- Abd El-Haleen, S.M.; Abd El-Waness, S. Chloride induced pitting corrosion of nickel in alkaline solutions and its inhibition by organic amines. Mater. Chem. Phys. 2011, 128, 418–426. [Google Scholar] [CrossRef]
- Toni, M.; Vincent, M.; Lorena, H.K. Intergranular effects on the local electronic properties of the passive film on nickel. Corros. Sci. 2013, 69, 245–251. [Google Scholar]
- Chen, T.; John, H.; Xu, J. Influence of surface modifications on pitting corrosion behavior of nickel-base alloy 718. Part 1: Effect of machine hammer peening. Corros. Sci. 2013, 77, 230–245. [Google Scholar] [CrossRef]
- Rofagha, R.; Splonter, S.J.; Erd, U.; Mcintyre, N.S. XPS characterization of the passive film formed on nanocrystalline nickel in sulphuric acid. Nanostruct. Mater. 1994, 4, 69–78. [Google Scholar] [CrossRef]
- Rofagha, R.; Langer, R.; El-Sheerik, A.M.; Erb, G.; Palumbo, K.T. The corrosion behavior of nanocrystalline nickel. Scr. Mater. 1991, 25, 2867–2872. [Google Scholar] [CrossRef]
- Mishra, R.; Balasubramaniam, R. Effect of nanocrystalline grain size on the electrochemical and corrosion behavior of nickel. Corros. Sci. 2004, 46, 3019–3029. [Google Scholar] [CrossRef]
- Qin, L.Y.; Lian, J.S.; Jiang, Q. Effect of grain size on corrosion behavior of electrodeposited bulk nanocrystalline Ni. Trans. Nonferrous Met. Soc. China 2010, 20, 82–89. [Google Scholar] [CrossRef]
- Beverskog, B.; Bojinov, M.; Englund, A. A mixed-conduction model for oxide films on Fe, Cr and Fe-Cr alloys in high-temperature aqueous electrolytes—I. Comparison of the electrochemical behavior at room temperature and at 200 °C. Corros. Sci. 2002, 44, 1901–1921. [Google Scholar] [CrossRef]
- Elzbieta, S.; Digby, D.M. Nature of the passive film on nickel. Electrochim. Acta 2002, 48, 69–76. [Google Scholar]
- Bru, G.J.; Van, D.E.; Sluyters, R.M. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275. [Google Scholar]
- Chao, C.Y.; Lin, L.F.; Macdonald, D.D. A point defect model for anodic passive films. J. Electrochem. Soc. 1982, 129, 1874–1879. [Google Scholar] [CrossRef]
- Chen, T.; John, H.; Xu, J. Influence of surface modifications on pitting corrosion behavior of nickel-base alloy 718. Part 2: Effect of aging treatment. Corros. Sci. 2014, 78, 151–161. [Google Scholar] [CrossRef]
- Musiani, M.; Orazem, M.E.; Pébère, N. Constant-phase element behavior caused by coupled resistivity and permittivity distributions in films. J. Electrochem. Soc. 2011, 158, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Murat, D. The corrosion behavior of nanocrystalline nickel based thin films. Mater. Chem. Phys. 2016, 17, 276–280. [Google Scholar]
- Herrasti, P.; Ponce, C.; Walsh, F.C. The corrosion behavior of nano-grained metals and alloys. Rev. Metal. 2012, 48, 377–394. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Gao, Y.; Xue, L.; Hu, T.; Xu, T. Grain size effect in corrosion behavior of electrodeposited nanocrystalline Ni coating in alkaline solution. Scr. Mater. 2006, 55, 657–660. [Google Scholar] [CrossRef]
- Balyanov, A.; Kutnyakova, J.; Amirkhanova, N.A.; Stolyarov, V.V. Corrosion resistance of ultrafine-grained Ti. Scr. Mater. 2004, 51, 225–229. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]

| Ni | Si | Mn | Mg | Fe | C | S | Others |
|---|---|---|---|---|---|---|---|
| 99.85 | 0.071 | 0.038 | <0.001 | 0.02 | 0.0038 | 0.01 | <0.0062 |
| Specimen | PAW Current I (A) | PAW Voltage U (V) | TIG Current I (A) | TIG Voltage U (V) | Welding Speed v (mm·s−1) |
|---|---|---|---|---|---|
| JP | 165 | 24 | - | - | 3 |
| JP-T | 160 | 24 | 195 | 13 | 6 |
| Specimen | EZCP (mV) | icorr (µA/cm2) | βc (V/dec) | ipass (µA/cm2) | Epit (mV) |
|---|---|---|---|---|---|
| BM | −428 | 7.059 | −0.582 | 2.16 | 315 |
| JP | −510 | 70.06 | −6.247 | 6.48 | 286 |
| JP-T | −477 | 71.57 | −13.873 | 7.35 | 174 |
| Specimen | Rs (Ω·cm2) | Rf (Ω·cm2) | Qf (Ω−1·cm−2·sn) | N |
|---|---|---|---|---|
| BM-1 h | 55.51 | 1.196 × 106 | 4.61 | 0.87 |
| BM-6 h | 55.58 | 1.431 × 106 | 6.49 | 0.87 |
| BM-12 h | 57.10 | 2.282 × 106 | 6.75 | 0.88 |
| BM-24 h | 60.34 | 3.846 × 106 | 6.75 | 0.88 |
| BM-48 h | 39.91 | 4.194 × 106 | 9.46 | 0.88 |
| BM-72 h | 41.79 | 8.422 × 106 | 8.97 | 0.88 |
| JP-1 h | 37.42 | 6.339 × 105 | 10.5 | 0.89 |
| JP-6 h | 37.82 | 1.297 × 106 | 11.13 | 0.90 |
| JP-12 h | 38.32 | 1.940 × 106 | 11.33 | 0.91 |
| JP-24 h | 38.57 | 2.880 × 106 | 11.49 | 0.91 |
| JP-48 h | 38.91 | 3.261 × 106 | 11.56 | 0.91 |
| JP-72 h | 39.31 | 6.600 × 106 | 10.44 | 0.90 |
| JP-T-1 h | 39.27 | 5.548 × 105 | 11.17 | 0.88 |
| JP-T-6 h | 38.88 | 1.112 × 106 | 11.85 | 0.89 |
| JP-T-12 h | 39.02 | 1.760 × 106 | 12.01 | 0.90 |
| JP-T-24 h | 39.28 | 2.867 × 106 | 12.03 | 0.90 |
| JP-T-48 h | 42.78 | 3.087 × 106 | 12.00 | 0.89 |
| JP-T-72 h | 44.39 | 6.041 × 106 | 11.09 | 0.89 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, B.; Zhang, L.; Yang, C.; Yang, Y. Effect of Different Welding Processes on Electrochemical and Corrosion Behavior of Pure Nickel in 1 M NaCl Solution. Metals 2017, 7, 532. https://doi.org/10.3390/met7120532
Wang X, Wang B, Zhang L, Yang C, Yang Y. Effect of Different Welding Processes on Electrochemical and Corrosion Behavior of Pure Nickel in 1 M NaCl Solution. Metals. 2017; 7(12):532. https://doi.org/10.3390/met7120532
Chicago/Turabian StyleWang, Xijing, Boshi Wang, Liangliang Zhang, Chao Yang, and Yan Yang. 2017. "Effect of Different Welding Processes on Electrochemical and Corrosion Behavior of Pure Nickel in 1 M NaCl Solution" Metals 7, no. 12: 532. https://doi.org/10.3390/met7120532




